Introduction
During the long-term evolution process, insects have gradually formed complicated chemosensory systems to detect external environment information. One of the chemosensory systems is olfactory system that allows the organism to recognise volatile chemical signals conferring the capacity to detect food, predators and mates (Gong et al., Reference Gong, Chen, Cheng and Zhong2009; Maike et al., Reference Maike, Heinz and Jürgen2009; Sanchez-Garcia et al., Reference Sanchez-Gracia, Vieira and Rozas2009; Zhou et al., Reference Zhou, Wanga and van Loon2009). Olfactory sensation is mediated by specific neurons located in antennal sensilla. These sensilla are cuticular structures with an aqueous lumen surrounding the dendrites of the olfactory neurons and containing high concentrations of odorant-binding proteins (OBPs), as well as chemosensory proteins (CSPs) (Pelosi, Reference Pelosi1996; Mosbah et al., Reference Mosbah, Campanacci, Lartigue, Tegoni, Cambillau and Darbon2003).
The OBPs are small globular proteins with molecular masses of 14–16 kDa. Their typical feature is a set of six cysteine residues with a conserved spacing pattern, paired to form three disulfide bridges (Leal et al., Reference Leal, Nikonova and Peng1999). In contrast to OBPs, CSPs are shorter (12–14 kDa) and have only four conserved cysteines forming two disulfide bridges (Angeli et al., Reference Angeli, Ceron, Scaloni, Monti, Monteforti, Minnocci, Petacchi and Pelosi1999). The X-ray data of MbraCSPA6 from the moth Mamestra brassicae showed that it is comprised of six α-alpha helices connected by α–α loops and reveals a novel fold, different from that of OBPs (Lartigue et al., Reference Lartigue, Campanacci, Roussel, Larsson, Jones and Tegoni2002; Martine et al., Reference Martine, Christine, François, Isabelle, Patrick and Emmanuelle2004). However, they both share some consistent characteristics, such as low isoelectric point, binding affinity to small ligands and very high concentrations in the proximity of chemosensory neurons (Pelosi et al., Reference Pelosi, Zhou, Ban and Calvello2006). Owing to the high concentrations of OBPs and CSPs in antennal olfactory neurons of insects, it has been suggested that such soluble proteins play an important role in transporting hydrophobic odorants to their receptors on dendrite membranes (Bohbot et al., Reference Bohbot, Sobrio, Lucas and Nagnan-Le Meillour1998; Nagnan-Le Meillour et al., Reference Nagnan-Le Meillour, Cain, Jacquin-Joly, Francois, Ramachandran, Maida and Steinbrecht2000; Picimbon et al., Reference Picimbon, Dietrich, Krieger and Breer2001; Wanner et al., Reference Wanner, Willis, Theilmann, Isman, Feng and Plettner2004; Pelletier & Leal, Reference Pelletier and Leal2011).
However, CSPs are broadly expressed in various tissues that lack sensilla, and its function is still uncertain. For example, several Bombyx mori CSP genes are expressed in non-sensory tissues, such as testes, ovaries and compound eyes (Gong et al., Reference Gong, Zhang, Zhao, Lin, Xia and Xiang2007), but CSPs from Chroistoneura fumiferana and Apis mellifera are expressed at specific developmental stages, including egg, embryo and molting stages (Wanner et al., Reference Wanner, Isman, Feng, Plettner and Theilmann2005; Forêt et al., Reference Forêt, Kevin and Wanner2007). The gene expression pattern of CSPs suggests that it may be involved in development and reproduction of insects.
CSPs are associated with insect sensory organs, including the sensillum lymph in some cases (Wanner et al., Reference Wanner, Willis, Theilmann, Isman, Feng and Plettner2004). However, there is no direct evidence of their role in chemoreception in vivo (Gong et al., Reference Gong, Zhang, Zhao, Lin, Xia and Xiang2007), and different studies have been reported on non-chemosensory functions of CSPs in insects. For example, honeybee CSP5 was localized in ovaries and embryos, and its function was suggested to take part in the development of embryonic integument as revealed by RNAi (Maleszka et al., Reference Maleszka, Forê, Saint and Maleszka2007). A cockroach CSP was isolated from regenerating legs where it was associated with the epidermis and may promote the regeneration of legs in cockroach (Kitabayashi et al., Reference Kitabayashi, Arai, Kubo and Natori1998). In Drosophila melanogaster, the transcription levels of DmelpebIII and Dmelphk3 were increased in response to infection by viruses and bacteria, respectively (Sabatier et al., Reference Sabatier, Jouanguy, Dostert, Zachary, Dimarcq, Bulet and Imler2003). Further, Dmelphk3 are shown to be a target of a dorsal transcription factor involved in embryo and tissue development (Stathopoulos et al., Reference Stathopoulos, Van Drenth, Erives, Markstein and Levine2002). In Plutella xylostella, 5′ regulatory region of two CSPs were found to have many transcription factor binding sites that are involved in embryos developmental regulation (Gong et al., Reference Gong, Zhong, Hu, Luo and Ren2010). So, it suggests that some CSPs may perform roles not related to chemoreception. The certain physiological roles of CSPs remain unknown and would be interesting to study.
Spodoptera exigua, generally called as beet armyworm, is a worldwide agricultural pest that has developed resistance to many chemical pesticides. RNA interference has emerged as an effective technique to disrupt the gene functions in S. exigua (Chen et al., Reference Chen, Tian, Zou, Tang, Hu and Zhang2008). Two storage hexamerins (SeHex and SeSP1) from S. exigua have been silenced by RNAi in fifth instar that caused significant death, whereas silencing efficiency was confirmed by RT-PCR (Tang et al., Reference Tang, Wang and Zhang2010). RNAi of soluble trehalase genes (SeTre-1 and SeTre-2) in S. exigua was reached up to 83% efficiency rates at 72 h post injection and caused significantly higher mortality rates during the larva-pupa stage and pupa-adult stage (Chen et al., Reference Chen, Tang, Chen, Yao, Huang, Chen, Zhang and Zhang2010). It suggests a potential of using RNAi in functional genome study of S. exigua.
In the present study, three full-length cDNA of CSPs from S. exigua were cloned and analyzed. The expression profile of these CSPs was confirmed not only in different development stages but also in antennae, head, legs and wings. Furthermore, the genes exhibited different expression patterns in testes and ovaries. We used RNA interference (RNAi) to investigate the functions of the genes in female survival and reproduction.
Materials and methods
Insect culture, developmental staging and collection of tissues
Larvae of the S. exigua were reared at 25±1°C with an L14:D10 photoperiod on artificial diet. The developmental stages of S. exigua whole life history were synchronized at each molt by collecting new larvae or pupae. The samples were collected from all developmental stages at day-1 eggs, 1st to 5th instar day-1 larvae and pupae (day-1, day-7). The antennae, head, legs, wings, testes and ovaries were dissected from virgin male and female adults within two days of eclosion, respectively. All tissues were immediately frozen in liquid nitrogen and stored at −80°C until use.
RNA isolation, cDNA synthesis and rapid amplification of cDNA ends (RACE)
Total RNA was extracted from adults of S. exigua using the Trizol reagent (Invitrogen, www.invitrogen.com) according to the protocol provided by the manufacturer. It served as templates for the first-strand cDNA synthesis using an oligo(dT)18 primer and AMV reverse transcriptase (TaKaRa, www.takara-bio.com).
The first-strand cDNA (1 μl) was used as a template for PCR, and the components of the PCR mix were as: PCR buffer containing 0.2 mM dNTPs, 10 mM of each primer, and 2.5 U of Taq DNA polymerase (Transgene, China) in a total volume of 50 ml. Two degenerate primers, 3RCSP1, 3RCSP2 (table 1), were designed based on multiple alignment of published CSP-like transcripts from nearly related species, such as Bombyx mori and Plutella xylostella. Rapid amplification of cDNA ends (3′ RACE) PCR reactions were performed with primers 3RCSP1 and Oligo(dT)20 at the following conditions: one cycle of 2 min at 94°C; ten cycles of 30 s at 90°C and 60 s at 72°C followed by 25 cycles of 30 s at 94°C, 30 s at 66°C and 60 s at 72°C, and a last cycle 72°C for 10 min. A second PCR was carried out with nested primers 3RCSP2 and Oligo(dT)20 at the following conditions: one cycle (94°C, 2 min); 35 cycles (94°C, 30 s; 68°C, 45 s; 72°C, 1 min) and a last cycle 72°C for 10 min. A strong DNA band corresponding to the expected size of approximately 350 bp was excised from the agarose gel and purified using DNA gel extraction kit (Tiangen, www.tiangen.com). The PCR products were cloned into the pMD18-T vector (Takara) and positive clones were sequenced by invitrogen (Invitrogen, cnservice.invitrogrn.com).
Table 1. Cloning strategy for SexiCSPs cDNA and primers used for Genomic DNA isolation, qRT-PCR and dsSexiCSPs RNA synthesis.

5′ RACE of S. exigua cDNA was prepared by GeneRacer system (Invitrogen Life Technologies, Paisley, UK) following the manufacturer's instructions. Specific primers, for 5′ RACE (table 1), were synthesized based on the cDNA sequence of the 3′ RACE production. 5′ RACE was performed using 5 μl of 5′ ready cDNA with GeneRacer5′ Primer (Invitrogen) and 5 SP; nested PCR was carried out with GeneRacer5′ Nested Primer (Invitrogen) and 5 SP nest. The PCR conditions were as follows: 2 min at 94°C, followed by 30 cycles of 30 s at 94°C, 45 s at 60°C and 120 s at 72°C, 10 min at 72°C.
Genomic DNA sequencing and gene structure
Regions of the CSP genes were amplified from genomic DNA by PCR to confirm exon/intron structure of the genes. Specific primer pairs (table 1) were designed according to cDNA sequence and used for amplification (Invitrogen). For SexiCSPs, the reactions were: 94°C for 3 min; 35 cycles at 94°C for 30 s, 60°C for 45 s, and 72°C for 2 min followed by incubation for 10 min at 72°C. LA Taq polymerase (TaKaRa) was used following the manufacturer's instructions. Exons and introns were identified by comparing and analyzing the cDNA and genomic DNA sequences. The SexiCSPs gene was constructed using LaserGene software.
Sequence analysis of S. exigua CSPs
The SexiCSPs cDNA sequence was confirmed by comparing with other CSPs sequences deposited in GenBank using the ‘Nucleotide Blast’ and ‘Blast-X’ tools at the National Center for Biotechnology Information (NCBI) website. The signal peptide and cleavage sites were predicted using SignalP 3.0 (Emanuelsson et al., Reference Emanuelsson, Brunak, Heijne and Nielsen2007) at http://www.cbs.dtu.dk/services/SignalP-3.0/. The neighbour-joining method was used to construct a phylogenetic tree with MEGA 4.0 software (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007) based on the amino acid sequences of known CSPs. Multiple sequence alignment was carried out with ClustalW2 program at online service http://www.ebi.ac.uk/Tools/clustalw2/index.html (Higgins et al., Reference Higgins, Thompson, Gibson and Thompson1994). Other protein sequence analysis tools used to know MW (molecular weight), pI and N-glycosylation sites) were obtained from the ExPASy Proteomics website (http://expasy.org/).
Determination of expression profile of SexiCSPs by quantitative RT-PCR
Developmental expression of SexiCSPs mRNA was determined in the adult and larval stages by quantitative RT-PCR. In short, 150–200 female and male adults were dissected in 0.9% physiological saline and immediately frozen in the liquid nitrogen until used. The total RNA was extracted as described above. The samples were treated with DNAse I (New England Biolabs, NEB, Ipswich, MA, USA) to remove any genomic DNA contamination and reverse transcriptase AMV (Takara) was employed to make first strand cDNA using oligo(dT)18 primer.
Quantitative reverse transcriptase PCR (qRT-PCR) reactions were performed in triplicate on a BioRad iQ5 Real-Time PCR Detection System using 10 ng of cDNA, 0.2 μM primers and SYBR® Premix Ex Taq™ (TaKaRa). Cycling conditions and dissociation curve analyses was performed according to the manufacturer's instructions. To amplify the SexiCSP genes, the qRT-PCR primers listed in table 1 were used. S. exigua β-actin gene (GenBank accession no. AY507963) was used as reference gene to standardize the level of other transcripts. The relative amounts of SexiCSPs transcripts were first normalized to the endogenous reference gene β-actin and normalized relative to the level of gene transcripts in embryos, larvae and adults according to the 2−ΔΔCTmethod (Livak & Schmittgen, Reference Livak and Schmittgen2001).
Double-stranded RNA preparation of SexiCSPs
Primers used to amplify cDNA fragments containing a SexiCSPs partial sequence are listed in table 1. Both the sense and antisense strands of the amplified fragments contained a T7 RNA polymerase binding site. The PCR products were purified with QIA quick columns (TransGen, Beijing, China) and cloned into the pMD20-T vector (TaKaRa). After being transferred to DH5α and sequencing confirmation, the pMD20-SexiCSP plasmids were used as a template to generate corresponding dsRNA by MEGAscript RNAi Kit (Ambion, Austin, TX, USA). All the reagents and enzymes used were from Ambion MEGAscript RNAi kit and reactions were carried out following the manufacturer's instructions. The dsRNA was suspended in 50 ml diethypyrocarbonate (DEPC)-treated water, subjected to 1% gel electrophoresis, quantified using a spectrophotometer (UV-2550; SHIMADZU) and stored at −20°C until used.
Injection of dsRNA into S. exigua female adults
Female adult of S. exigua (within one day of eclosion) was injected to investigate the function of each CSPs in female reproduction by RNAi. Three micrograms of dsRNA (about 0.22 μg μl−1) dissolved in DEPC water was injected into the abdomen of female S. exigua using 10 μl microliter syringes (Hamilton). In order to restrict insect movement, it was cooled for two minutes before the injection. Forty individuals were used for oviposition rate assay of each CSP, that was performed after 12 h of injection with four pairs (four females+four males in one box). The assay was repeated three times. Females were treated with dsRNA, whereas the males were untreated. Control groups were either injected with equivalent volume of DEPC water or uninjected. SexiCSPs mRNA levels were determined by qRT-PCR using RNA isolated from control individuals and dsSexiCSPs injected whole adults at 12 h after injection. The procedures were performed as above.
Insect oviposition rate and data analysis
Insect oviposition rate was recorded every 48 h, and the number of eggs were observed at 0–48 h, 48–96 h and 96–144 h after the mating followed by the observation of hatchability. The insect mortality was observed after 12 h of the injection. Oviposition inhibition rate (%) was calculated by following formula, X=(N 1–N 2/N 1)×100, N 1 is the cumulative number of oviposition of treated individuals, N 2 is the cumulative number of oviposition of control treatments (DEPC water injection or uninjection). Statistical analysis was performed with SAS 8.1 program (SAS Institute Inc., 2000). Differences among treatments were evaluated by analysis of variance (ANOVA) using Tukey's test at significance level of P<0.05.
Results
Cloning and sequence analysis of SexiCSPs
The SexiCSPs cDNA was obtained by 5′ and 3′ RACE. SexiCSP1 cDNA (GenBank accession no. EF186793) has the size of 809 bp with an open reading frame of 387 nucleotides (fig. 1A) and encodes a protein of 128 amino acids with a predicted mass of approximately 12.91 kDa and pI of 8.72. SexiCSP2 cDNA (GenBank accession no. EF186794) has the size of 594 bp with an open reading frame of 387 nucleotides (fig. 1B) and encodes a protein of 128 amino acids with a predicted mass of approximately 13.17 kDa and pI of 5.56. SexiCSP3 cDNA (GenBank accession no. EF186795) has the size of 502 bp with an open reading frame of 381 nucleotides (fig. 1C) and encodes a protein of 126 amino acids with a predicted mass of approximately 12.89 kDa and pI of 6.59.

Fig. 1. cDNA and predicted amino acid sequence of (A) SexiCSP1, (B) SexiCSP2 and (C) SexiCSP3. Conserved Cys sites are boxed. The signal peptide is underlined. The locations of intron are shown by arrows. The stop codons are indicated by dark arrowheads. The start codon and AATAAA-box are shown in boldface. The locations of the initial degenerate primers for 3′ RACE and the primers for 5′ RACE are represented by red and green background, respectively. The primers used for genomic DNA amplification, dsRNA preparation and real-time PCR are indicated by bullets, double underline and dashed line, respectively.
Protein sequence and phylogenetic analysis
Amino acid sequence alignment revealed that SexiCSP1, SexiCSP2 and SexiCSP3 share 49%, 50% and 46% identity, to selected Mamestra brassicae CSP1 (GenBank no. AAF71289) and MbraCSP1 having conserved Cys spacing pattern of SexiCSPs as CX6CX18CX2C (fig. 2), respectively. SexiCSP1, SexiCSP2 and SexiCSP3 have one intron of 610 bp, 135 bp and 305 bp, respectively, that conform to typical GT-AG structure (showed as supplementary data). The intron splice sites of SexiCSP1, SexiCSP2 and SexiCSP3 were after the nucleotide acids AAA (Lys) T, AAA(Lys)G and AAA (Lys)T, respectively, and all are located after 47 amino acids of SexiCSPs' mature peptide. Hydrophobic analysis revealed that the whole SexiCSPs molecule is hydrophilic except between 20–30 amino acids having the lipophilic character.

Fig. 2. Deduced amino acid alignment of SexiCSPs and M. brassicae CSPA6. Signal peptides are predicted from signal sequence cleavage sites (not shown) using Signal P 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/), and conserved cysteines residues are shaded grey. Alpha helical domains (α1–α6) are identified according to Mamestra brassicae chemosensory protein CSPA6 (GenBank no. 1KX8_A).
A neighbour-joining tree with 1000 bootstrap replicates was constructed based on 15 CSPs from Bombyx mori, 11 CSPs from Papilio xuthus and three CSPs cloned in this study (fig. 3). The tree was subdivided into several groups and provided an informative and global view on the evolution of the CSPs in insect. PxutCSP10 constitute a monophyletic clade in the outer part of the tree, indicating its role of evolution in their origin. Trees with many groups suggest that they may have diversified by gene duplication from a common ancestral gene, though numbers of clades in this tree have little bootstrap support. However, some small branch including two CSPs from Bombyx mori and Papilio xuthus, respectively, were well supported by bootstrap, for instance 99% bootstrap support for PxutCSP3 and BmorCSP7 and also 99% bootstrap support for PxutCSP7 and BmorCSP13. It may suggest a functional convergent evolution of CSPs in B. mori and P. xuthus. However, both BmorCSP3 and BmorCSP5 may be equivalent genes in B. mori with 100% bootstrap supporting. The evolutionary relationship of SexiCSPs showed in the tree suggested that SexiCSP3 is at a single branch with a more distant relationship between SexiCSP1 and SexiCSP2.

Fig. 3. Phylogeny of the CSP proteins in three lepidopteran insects. An unrooted tree was constructed with aligned protein sequences from the B. mori, the P. xuthus and S. exigua using neighbour-joining and reconstructed with 1000-replicate bootstrap analysis. The tree was condensed by a 50% cut-off value.
Developmental expression of SexiCSPs
The expression of SexiCSPs in eggs, larvae and pupae were determined by real-time PCR. The results showed that SexiCSPs expressed in all stages with different mRNA expression levels (fig. 4). SexiCSPs expression levels in seven-day-old pupae were the highest. For example, SexiCSP1, SexiCS2 and SexiCSP3 in seven-day-old pupae have 10.0, 6.4 and 1.7 times higher expression levels than eggs. If compared to first instar larvae, they were 25.6, 14.9 and 17.8 times higher, respectively. In the larval stages, the highest expression levels were expressed at the fourth instar. For example, SexiCS2 and SexiCS3 expression level in fourth instar have 31.1 and 18.5 times increase than third instar larvae, but expression of SexiCSP1 was not significantly different in third and fourth instar larvae (P<0.05).

Fig. 4. Developmental profiles of SexiCSPs mRNA expression during the egg, larva and pupa stages. The levels of SexiCSPs mRNAs relative to the β-actin mRNA levels measured with real-time PCR, and then all expression fold changes are related to egg stages. Bars with the same letter are not significantly different from each other at P<0.05 based on Tukey's test. Each point represents the mean ±SEM from three independent experiments.
Tissue distribution of CSPs in S. exigua adults
Real-time PCR was performed to compare the CSPs mRNA levels in the antennae, head, legs, wings, thoraxes, abdomens, testes and ovaries from males and female adults, respectively (fig. 5). We observed a significantly higher (58.9-fold, 35.1-fold and 42.2-fold of SexiCSP1, SexiCSP2 and SexiCSP3, respectively) expression in female antennae compared to that of male antennae (fig. 5C). In the head, the expression of three SexiCSPs was significantly higher, 2.6-fold, 2.7-fold and 5.0-fold than that of SexiCSPs in female adult (fig. 5A). However, there was significantly different expression (3.3-fold and 3.2-fold, respectively) of SexiCSP2 and SexiCSP3 in male and female wings (fig. 5D); whereas, in the case of SexiCSP3, significantly different expression (33.3-fold) between female and male was found only in the leg (fig. 5B). All the three SexiCSPs were expressed in testes and ovaries but SexiCSP2 levels remained higher than the other two SexiCSPs, thus reaching 1.9-fold higher in testes than ovaries, whereas the expression of SexiCSP1 and SexiCS3 was found to be approximately1.6-fold and 4.3-fold higher in ovaries than testes, respectively (fig. 5E). There was no significant difference found in the expression of three SexiCSPs between female and male thoraxes (fig. 5F), but their expression in abdomens was diversified, with 1.6-fold higher expression level of SexiCSP2 in male than female and 8.2-fold higher expression of SexiCSP3 in female and male (fig. 5G).

Fig. 5. Relative expression of SexiCSPs mRNA in different tissues of male and female inclued (A) head, (B) leg, (C) antennae, (D) wing, (E) ovary and testisc, (F) thorax and (G) abdomen. The levels of SexiCSPs mRNAs relative to the β-actin mRNA levels were measured with real-time PCR, and all expression fold changes are related to female head tissue. Bars with same letters are not significantly different from each other at P<0.05 based on Tukey's test. Each point represents the mean ±SEM from three independent experiments.
Survival rates and SexiCSPs transcript analysis after injection of dsRNA
dsSexiCSPs were injected into the female adults of S. exigua. SexiCSPs expression in ovaries depicts the possible role of SexiCSPs on development of female adults. After the down-regulation of SexiCSPs, the survival rates of the insects injected with dsSexiCSPs were obtained by accounting the percentage of living insects. It showed that only the dsSexiCSP3 caused 42.5% insect mortality.
To test the efficiency of RNAi, qRT-PCR was performed to detect the transcript of SexiCSPs. The results showed that SexiCSP1, 2 and 3mRNA was decreased 1.96-fold, 1.16-fold and 2.44-fold, respectively, at 12 h after the injection, compared to the negative controls (DEPC injection) (fig. 6).

Fig. 6. The efficiency of RNAi for dsSexiCSP1, dsSexiCSP2 and dsSexiCSP3. Bars with same letter are not significantly different from each other at P<0.05 based on Tukey's test. Each point represents the mean ±SEM from three independent experiments.
Function of SexiCSPs in female reproduction
To determine the function of SexiCSPs transcription in female reproduction, dsSexiCSP were injected into the female adults within 12 h of emergence, and dsRNA injected female of S. exigua were mated with uninjected male S. exigua. The numbers of eggs were recorded after three days of mating (fig. 7). Knocking down the expression of SexiCSP3 genes has affected female reproduction. As a result, the female S. exigua injected with dsRNA of dsSexiCSP3 caused 68.3% and 71.4% reduction in the number of eggs laid by the S. exigua as compared to the eggs laid by DEPC water injected and unjected control S.exigua, respectively. The eggs laid by dsSexiCSP3 injected S. exigua resulted in a hatching rate of 6.2%, whereas the DEPC water-injected or uninjected group reached 89.6% and 81.3%, respectively (table 2). These data suggests that SexiCSP3 transcription directly or indirectly regulates female reproduction.

Fig. 7. Effect of SexiCSP1, SexiCSP2 and SexiCSP3 genes silencing on the reproduction of female S. exigua. (A) 0–48 h after injection; (B) 48–96 h after injection; and (C) 96–144 h after injection. Bars with same letter are not significantly different from each other at P<0.05 based on Tukey's test.
Table 2. Effect of SexiCSPs silencing on the survival and reproduction of female S. exigua.
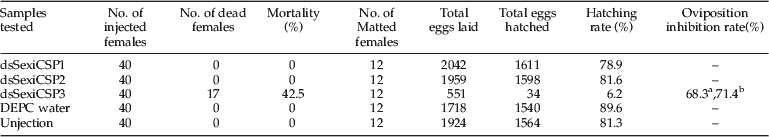
a Oviposition inhibition rate as compared to control treatment.
b Oviposition inhibition rate compared to uninjected control.
Discussion
Insect chemosensory proteins (CSPs) have been implicated in transporting chemical stimuli from air to olfactory receptors. However, CSPs are expressed broadly in ovaries or embryos of insects, suggesting that these proteins are also critical for other physiological processes. The data showed that the majority of silkworm CSP genes were expressed broadly in tissues, such as the antennae, head, thorax, legs, wings, epithelium, testes, ovaries, pheromone glands, wing disks and compound eyes, suggesting that silkworm CSPs may be involved in development (Gong et al., Reference Gong, Zhang, Zhao, Lin, Xia and Xiang2007). In this study, we report the identification and characterization of three CSP genes (SexiCSP1, SexiCSP2 and SexiCSP3) from the S. exigua. Qualitative real-time PCR analysis of these three SexiCSPs showed predominant expression in the female antennae, which have the same expressional profiling with some OBPs, like Anopheles stephensi odorant-binding protein 1 (Sengul et al., Reference Sengul and Tu2010). It may be because the antennae are the primary olfactory tissue in insect adults, and these three SexiCSPs may play important roles especially in female adults used for perceiving pheromone information materials from environments. Our analysis of SexiCSPs expression in larval and pupal stages by real-time PCR analysis depicted relatively strong expression of SexiCSPs in pupae stages, which either suggests a possible chemosensory role of this gene in pupae or a simple correlation with the onset of development of chemosensory tissues during pupation. Further study is needed to differentiate between these two possibilities. On the other hand, SexiCSPs transcripts were also detected in legs and wings revealing their possible gustatory role. Alternatively, these gustatory tissues might have olfactory capabilities that are yet to be understood in S. exigua. We observed significant differences of SexiCSP3 expressed in testes and ovaries, such as ∼4.3-fold expression of SexiCSP3 transcript level in ovaries higher than testes. This is very interesting as it indicates the possible involvement of SexiCSP3 in ovaries development.
When the expression of SexiCSP3 in females was down-regulated, a drastic reduction in the number of laid eggs was found from the treated females mating with untreated male. We hypothesized two reasons for oviposition reduction. First, expression disruption of SexiCSP3 might have seriously affected oogenesis and embryogenesis. Insect CSPs participate in the insect reproduction initiating its expression in specific tissues, such as phk-2 is related to OS-D/A10 (Phk-1), which was expressed in ejaculatory ducts of Drosophila melanogaster (Sabatier et al., Reference Sabatier, Jouanguy, Dostert, Zachary, Dimarcq, Bulet and Imler2003), whereas BmorCSP12 and BmorCSP7 were expressed in ovaries of Bombyx mori (Gong et al., Reference Gong, Zhang, Zhao, Lin, Xia and Xiang2007). However, a direct proof came from the study of CSP5 in Apis mellifera, which resembles the maternal-zygotic pattern and involved in embryonic integument formation (Forêt et al., Reference Forêt, Kevin and Wanner2007). Secondly, silencing the CSP mRNA might have confused the choice of oviposition in insects that mediates the number of oviposition. Ozaki et al. (Reference Ozaki, Utoguchi, Yamada and Yoshikawa2008) assumes some proteins of the CSP family involved in chemoreception as transporter of ligands in oviposition behavior of Papilio xuthus. This is due to the expression of a large number of CSPs in the tarsi/sensilla tissue of this species. It is interesting that disruption of SexiCSP3 expression in females can result in significant mortality rates (table 2), thus suggesting that CSPs may play key role in the regulation of vital functions in adults.
CSP have been proposed as a carrier to transport chemical stimuli through the sensillar lymph and delivery to olfactory receptors. In some cases, the CSP had very high expression in female adult antennae and could be involved in olfaction. For example, the CSP2 of Glossina morsitans morsitans with a sharp increase expression in ten-week-old female adults 48 h after a blood meal showed a clear relationship to female host-seeking behaviours (Liu et al., Reference Liu, He, Lehane, Lehane, Hertz-Fowler, Berriman, Field and Zhou2011). In the present study, the transcriptions of three SexiCSPs are higher in female antennae than in male antennae. This is very interesting as it indicates the possible physiological role of SexiCSPs associated with female moths only. Furthermore, the RNA reference technology was performed to uncover this physiological function. As shown in table 2, disruption in the expression of SexiCSP3 in a female adult would have significantly effect on its reproduction and survival. These results highlighted that the SexiCSP3 can be used as a potential insecticide target. Like PsOr1, the data suggested that adult beetles were unable to find the attractant or repellent odour stimulus after microinjection of dsRNA against PsOr1 (Zhao et al., Reference Zhao, Liu, Yang and You2011). So, this study provides the foundation for the further research in insect behaviour control, combined with RNAi-based insect control approach.
Acknowledgements
We gratefully acknowledge the role National Nature Science Foundation, People's Republic of China for funding this work under grant no. 31171870. The authors also thank the Guangdong Province guided program of university-industry cooperation (2010B090400138).
Supplementary material
The online figure can be viewed at http://journals.cambridge.org/ber.











