Introduction
Thermal stress tolerance is essential for the completion of insect life cycles, successful overwintering, and habitat exploration (McDonald et al., Reference Mcdonald, Bale, Walters, Bale, Block and Sømme1999; Hoffmann et al., Reference Hoffmann, Sørensen and Loeschcke2003; Chidawanyika & Terblanche, Reference Chidawanyika and Terblanche2011). Insects exposed to thermal stress may exhibit alterations in behavior, morphology, life history, and physiological characteristics (Taylor, Reference Taylor1981; Duman, Reference Duman2003; Kelly et al., Reference Kelly, Panhuis and Stoehr2012; Lu et al., Reference Lu, Hua, Cui and Du2014). Among these strategies, the production of heat shock proteins (HSPs) has been widely studied and is one of the best predictors of insect tolerance to temperature stress (Feder & Hofmann, Reference Feder and Hofmann1999). Furthermore, HSPs participate in diverse physiological processes (Haass et al., Reference Haass, Klein and Kloetzel1990; Johnston et al., Reference Johnston, Ward and Kopito1998; Lu et al., Reference Lu, Hua, Cui and Du2014; Lu et al., Reference Lu, Li, Zheng, Shi and Du2016) and are presumably involved in the development of some insects (Denlinger, Reference Denlinger2002; Rinehart et al., Reference Rinehart, Li, Yocum, Robich, Hayward and Denlinger2007; MacRae, Reference Macrae2010; Cheng et al., Reference Cheng, Li, Wang, Liu and Zhu-Salzman2016).
Insect forms of HSPs are divided into several families based on molecular weight and homology; these include HSP90, HSP70, HSP60, HSP40, and small heat shock proteins (sHSPs) (Lindquist & Craig, Reference Lindquist and Craig1988; Moseley, Reference Moseley1997; Feder & Hofmann, Reference Feder and Hofmann1999; Sørensen et al., Reference Sørensen, Kristensen and Loeschcke2003). HSPs function as molecular chaperones in response to a variety of stress factors, promote proper protein folding, and prevent the aggregation of denatured proteins (Gehring & Wehner, Reference Gehring and Wehner1995; Johnston et al., Reference Johnston, Ward and Kopito1998). Among HSPs, the HSP70 family is highly conserved (Boorstein et al., Reference Boorstein, Ziegelhoffer and Craig1994; Mayer & Bukau, Reference Mayer and Bukau2005) and can be subdivided into two groups (Karlin & Brocchieri, Reference Karlin and Brocchieri1998). One group is quickly and abundantly induced in response to various stress conditions and returns to a basal level of expression in the absence of stress. The other HSP70 group is not stress-inducible; it is generally constitutively expressed and is referred to as heat shock cognate protein 70 (HSC70) (Kiang & Tsokos, Reference Kiang and Tsokos1998; Tang et al., Reference Tang, Wu, Li, Ren, Huang and Liu2012; Sun et al., Reference Sun, Zhao, Sheng, Xiao, Zhang, Bai, Tan, Xiao and Xu2016; Zhang et al., Reference Zhang, Wang, Jing, Zhuang and Wu2015; Shen et al., Reference Shen, Zhao, Xie, Wei, Yang, Wang, Zhang and Tang2015).
The genus Liriomyza is an important insect pest worldwide (Spencer, Reference Spencer, Schimitschek and Göttingen1973), and L. trifolii is a highly invasive insect that has caused great losses in agricultural and horticultural crops (Johnson et al., Reference Johnson, Welter, Toscano, Tingi and Trumble1983; Parrella et al., Reference Parrella, Jones, Youngman and Lebeck1985; Reitz et al., Reference Reitz, Kund, Carson, Phillips and Trumble1999). L. trifolii, L. sativae, and L. huidobrensis are serious pests of vegetable crops in some regions of China (Kang et al., Reference Kang, Chen, Wei and Liu2009; Xiang et al., Reference Xiang, Lei and Wang2012). L. sativae is the dominant leafminer in mainland China, whereas L. huidobrensis has occurred primarily in cooler climates since its initial outbreak in the 1990s (Wen et al., Reference Wen, Wang and Lei1996, Reference Wen, Lei and Wang1998). L. trifolii, which was initially discovered in Guangzhou, has since been reported in more than ten provinces (Wang et al., Reference Wang, Guan and Chen2007; Lei et al., Reference Lei, Yao, Zhu and Wang2007; Gao et al., Reference Gao, Reitz, Xing, Ferguson and Lei2017). Several species displacement events have been reported between these Liriomyza spp. (Abe & Kawahara, Reference Abe and Kawahara2001; Reitz & Trumble, Reference Reitz and Trumble2002a, Reference Reitz and Trumbleb; Abe & Tokumaru, Reference Abe and Tokumaru2008; Gao & Reitz, Reference Gao and Reitz2016). Generally, the distribution and abundance of insect species are delineated by their adaptability to climatic stress (Bale, Reference Bale2002; Bale et al., Reference Bale, Masters, Hodkinson, Awmack, Bezemer, Brown, Butterfield, Buse, Coulson, Farrar, Good, Harrington, Hartley, Jones, Lindroth, Press, Symrnioudis, Watt and Whittaker2002); furthermore, low-temperature tolerance is a critical factor impacting the distribution and spread of Liriomyza spp. in temperate regions.
Since the initial discovery of HSPs in Drosophila melanogaster (Tissières et al., Reference Tissières, Mitchell and Tracy1974), research on insect forms of HSP70 has primarily focused on the structural characteristics and expression during different experimental conditions (Velazquez & Lindquist, Reference Velazquez and Lindquist1984; Flaherty et al., Reference Flaherty, Deluca-Flaherty and Mckay1990; Flaherty et al., Reference Flaherty, Wilbanks, Deluca-Flaherty and Mckay1994; Fung et al., Reference Fung, Hilgenberg, Wang and Chirico1996; Rinehart et al., Reference Rinehart, Yocum and Denlinger2000; Shim et al., Reference Shim, Jung, Park, Kim, Ha and Lee2006; Sonoda et al., Reference Sonoda, Ashfaq and Tsumuki2006; Morano et al., Reference Morano, Sistonen and Mezger2014; Qiao et al., Reference Qiao, Wu, Qin, Liu, Lu, Lv, Pan, Chen and Li2015; Wang et al., Reference Wang, Rreitz, Wang, Wang, Xue and Lei2014). In Liriomyza spp., an earlier study involving L. sativae and L. huidobrensis demonstrated that hsp90, hsp70, hsp40, and shsp could be induced by temperature stress; however, hsp60 was only slightly induced during heat shock and did not respond to cold stress (Huang & Kang, Reference Huang and Kang2007). Moreover, the expression of HSP genes can result in negative effects on feeding and fecundity in L. huidobrensis, which is further evidence that HSPs function in the physiology of Liriomyza spp. (Huang et al., Reference Huang, Chen and Kang2007). In L. sativae, the expression of hsps during development was investigated; the transcription of small hsps peaked during the pupal stage, whereas the expression of tcp1 (two genes), hsp60, and hsp90 gradually increased during development (Huang et al., Reference Huang, Wang and Kang2009). Compared with congener species, the study of HSPs in L. trifolii has been relatively limited (Zheng et al., Reference Zheng, Cui, Li, Cai, Gao and Shang2010; Ji et al., Reference Ji, Wang, Lei, Zhang, Wang and Zhang2013). Recently, five Lthsps were studied and showed expression profiles similar to homologous genes in L. sativae and L. huidobrensis; although L. trifolii occurs primarily in southern China, the results indicated that this pest has the potential to survive in more northern provinces (Chang et al., Reference Chang, Chen, Lu, Gao, Tian, Gong, Dong and Du2017a). In another study, Lthsp21.7 expression was induced by temperature stress and varied with different insect developmental stages (Chang et al., Reference Chang, Chen, Lu, Gao, Tian, Gong, Zhu and Du2017b). In the current study, we identify three genes encoding HSP70s in L. trifolii and describe their genomic structure. The expression of these three genes was monitored during thermal stress and during insect development with the aim of understanding molecular mechanisms of environmental tolerance and development in Liriomyza spp.
Materials and methods
Insects
L. trifolii were reared in the laboratory at 26 ± 1°C with a 16:8 h (L:D) photoperiod as described previously (Chen & Kang, Reference Chen and Kang2002). Beans (Vigna unguiculata) were seeded in plastic pots (12 cm in diameter) and transferred to screened cages (40 × 40 × 65 cm) when 5–6 true leaves were present. Bean leaves exhibiting tunnels caused by larval feeding were collected for pupation; the resulting pupae were transferred into glass tubes for experimental treatments.
Temperature treatments
Two-day-old pupae (n = 30) were placed in glass tubes, and exposed to hot (30, 32.5, 35, 37.5, 40, 42.5, 45°C) or cold stress (−5, −7.5, −10, −12.5, −15, −17.5, −20°C) for 1 h in a constant temperature controller (DC-3010, Ningbo, China). A set of pupae maintained at 25°C was regarded as a control group. After temperature stress, pupae were allowed to recover at 25°C for 1 h, frozen in liquid nitrogen, and stored at −70°C. Each treatment was repeated four times.
Developmental stage and sex
Developmental stages included third instar larvae, prepupae, 2-day-old pupae, 10-day-old pupae, male adults, and female adults (n = 30). Each experimental treatment was comprised of four independent biological replicates, except for prepupae (three repetitions).
RNA isolation and cloning experiments
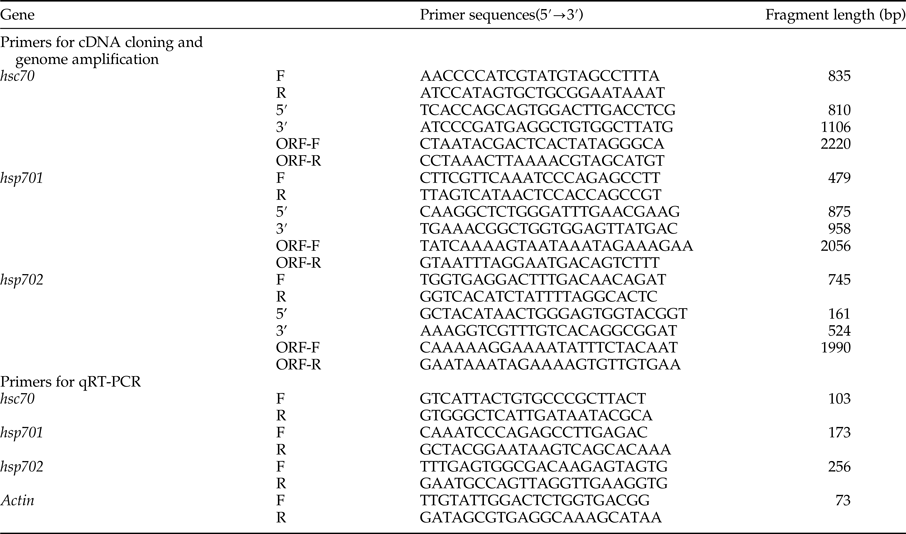
Total RNA was extracted from L. trifolii using the SV Total RNA isolation system (Promega, Fitchburg, WI, USA). The integrity and purity of RNA was determined by agarose gel electrophoresis and spectrophotometry (Eppendorf Bio Photometer plus, Hamburg, Germany). Three HSP70 genes were selected based on the analysis of our transcriptome data (unpublished). The partial segments of the three HSP70 genes were amplified using the corresponding pair of specific primers (table 1), and 5′- and 3′-RACE were utilized to obtain the full-length cDNAs (SMART RACE cDNA Amplification Kit, Clontech, CA, USA).
Table 1. Primers used in the cDNA cloning and real-time quantitative PCR.

F, forward; R, reverse; 5′, 5′-RACE primer; 3′, 3′-RACE primer; ORF-F, genome amplification forward primer; ORF-R, genome amplification reverse primer.
Characterization of genomic DNA
Genomic DNA of L. trifolii was extracted using the AxyPrep™ Multisource Genomic DNA Kit (Axygen, New York, NY, USA). The sequences of the full-length cDNAs were used to design pairs of specific primers (table 1) for amplifying Lthsp70 genomic fragments. The complete open reading frame (ORF) sequences of three genes were confirmed by 5′-RACE and genomic DNA. Amplified products were purified using a gel extraction kit (Axygen, New York, NY, USA), cloned into PGEM-T Easy vector (Promega, Fitchburg, WI, USA), and transformed into competent Escherichia coli DH5α cells. After confirmation by PCR, positive clones containing target genes were isolated and sequenced.
Quantitative real-time reverse transcriptase PCR
Total RNA was extracted as described above and 0.5 µg was reverse-transcribed into cDNA using the Bio-Rad iScript™ cDNA Synthesis Kit (Bio-Rad, CA, USA). Quantitative real-time reverse transcriptase PCR (qRT-PCR) was performed in 20 µl reaction volumes using conditions described previously (Chang et al., Reference Chang, Chen, Lu, Gao, Tian, Gong, Dong and Du2017a) and the gene-specific primers in table 1. Reactions were carried out using a CFX-96 real-time PCR system (Bio-Rad Laboratories, Berkeley, USA). Treatments included four replicates, and each reaction was performed in triplicate. The quantity of the three Lthsp70 mRNAs was calculated using the 2−ΔΔCt method (Livak & Schmittgen, Reference Livak and Schmittgen2001), and ACTIN was used as a reference gene (Chang et al., Reference Chang, Chen, Lu, Gao, Tian, Gong, Zhu and Du2017b).
Sequence alignment and data analysis
Full-length cDNAs sequences of the three Lthsp70 genes were used as queries to search for other insect hsps using the BLAST programs available at the NCBI website (http://www.ncbi.nlm.gov/BLAST/). Sequence alignments were performed using Clustal X software (Thompson et al., Reference Thompson, Gibson, Plewniak, Jeanmougin and Higgins1997), and ORFs were identified using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/). Sequence analysis tools of the ExPASy Molecular Biology Server (Swiss Institute of Bioinformatics, Switzerland) were used to analyze deduced Lthsp70 sequences. Phylogenetic trees of HSPs were generated with MEGA 6.0 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013) using the neighbor-joining method with the following parameters: Poisson correction model, pairwise deletion, and 1000 bootstrap replicates (random seed).
One-way analysis of variance (ANOVA) was used to detect the significant differences in mRNA levels among treatments, followed by Tukey's multiple comparison (P < 0.05) and analysis with SPSS v. 16.0 (SPSS, Chicago, IL, USA). For the ANOVA, data were transformed for homogeneity of variances test.
Results
Cloning and characterization of three hsp70s from L. trifolii
Three heat shock genes were cloned from L. trifolii and designated Lthsc70, Lthsp701, and Lthsp702 (GenBank accession nos. KY933450, KY933451, and KY933452, respectively). The full-length cDNAs of Lthsc70, Lthsp701, and Lthsp702 were 2332, 2261, and 2078 bp and encoded predicted proteins containing 651, 645, and 632 amino acids, respectively. The predicted protein products of Lthsc70, Lthsp701, and Lths702 had molecular weights of 71.20, 70.81, and 69.24 kDa and isoelectric points of 5.31, 5.70, and 5.70, respectively.
Three signature sequences of the HSP70 family (Gupta & Singh, Reference Gupta and Singh1994) were identified in the deduced amino acid sequences of the three Lthsc/Lthsp70 genes; these were IDLGTTYS, IFDLGGGTFDVSIL, and IVLVGGSTRIPKVQR/N/S (see sequences bound by rectangles, fig. S1). The three L. trifolii HSP70 proteins also contained the conserved EEVD motif (Pockley et al., Reference Pockley, Muthanal and Calderwood2008) at their C-termini (fig. S1). Furthermore, three other motifs typical of HSP70 were identified: an ATP/GTP binding site (AEAY/FLGK/TT) (Saraste et al., Reference Saraste, Sibbald and Wittinghofer1990), two bipartite nuclear localization signals: [(KKDLTTNKRALRR and KRALRRLRTACERAKRT) in LtHsc70 and (KKDLRSNPRA/SLRR and PRA/SLRRLRTA/EAER/KAKRT) in LtHSP701 and LtHSP702] (Knowlton & Salfity, Reference Knowlton and Salfity1996) and a non-organellar consensus motif RARFEEL (Zhang & Denlinger, Reference Zhang and Denlinger2010) (fig. S1).
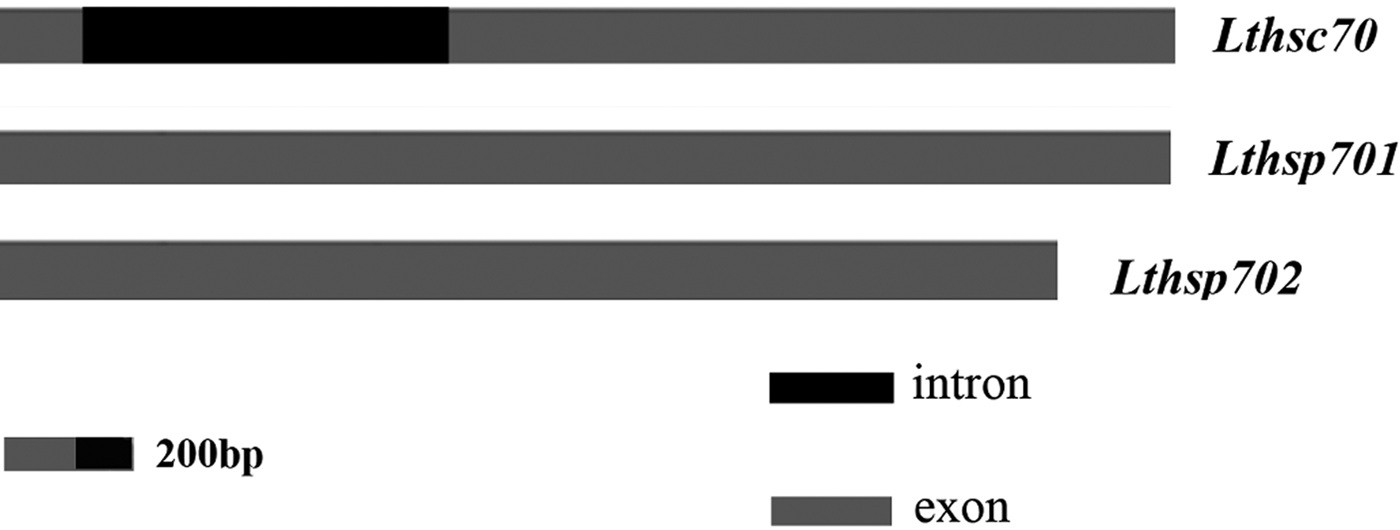
The genomic DNA sequence of Lthsc70 was identified and deposited in GenBank as accession no. KY933449. We found that Lthsp701 and Lthsp702 had no introns, whereas Lthsc70 had a 1273-bp intron, which mapped close to the N-terminus (fig. 1). The nucleotide sequences at the splice junctions in Lthsc70 are consistent with the canonical GT-AG rule (Mount, Reference Mount1982).
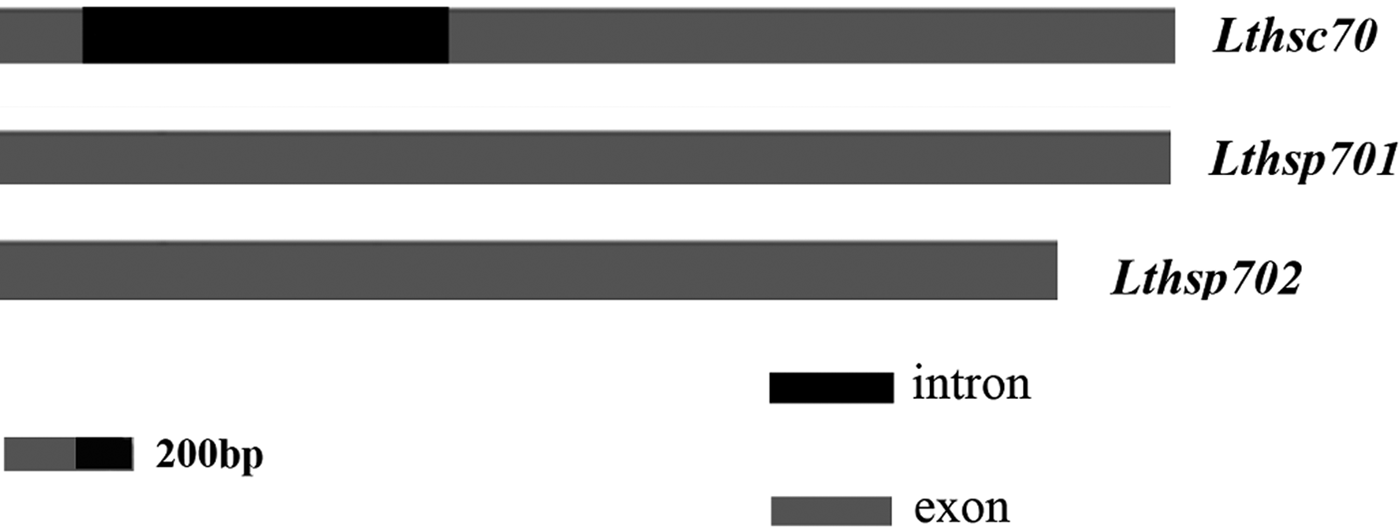
Fig. 1. Schematic representation of three Lthsp70 genomes. Light gray and black rectangles are used to highlight the exons and introns, respectively.
A TATA-box-like regulatory element was identified in the 5′ UTR of hsp701, but was absent in the 5′ UTRs of hsc70 and hsp702 (fig. 2).
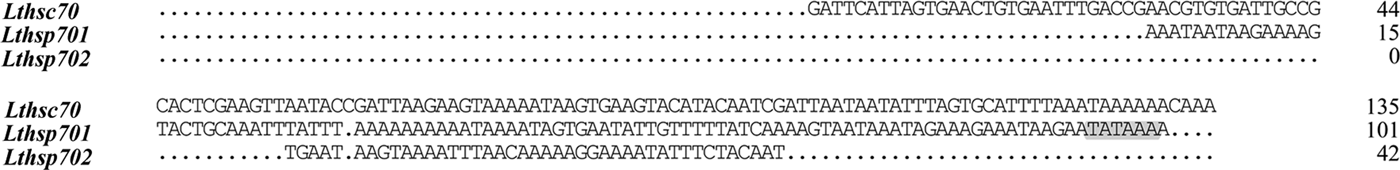
Fig. 2. Alignment of 5'UTRs of three Lthsp70s. The TATA-box-like elements are indicated by shading and the dots indicate alignment.
Phylogenetic analysis
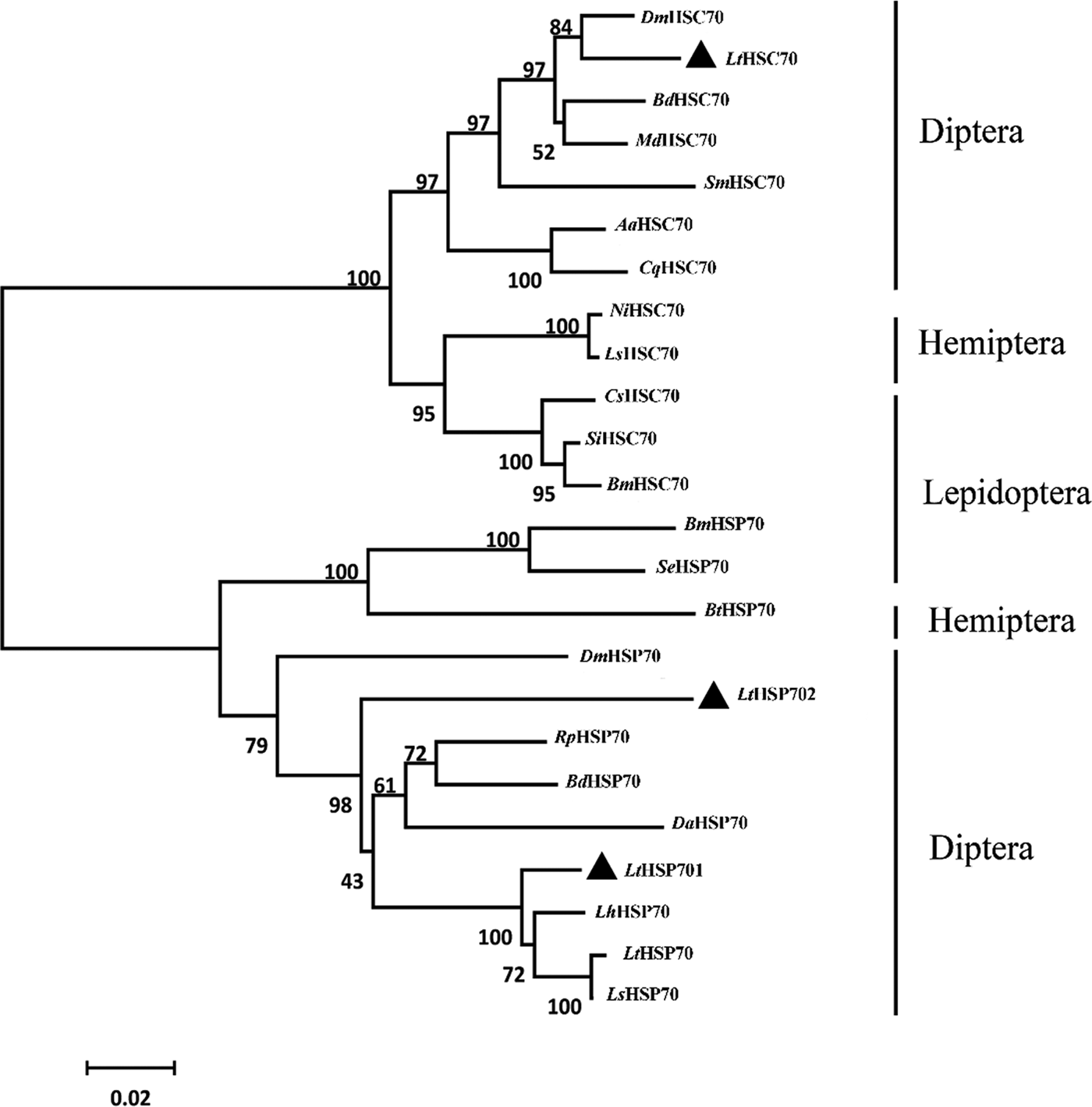
The deduced amino acid sequences of the three Lthsp70s displayed a high degree of identity with published LtHSP70 (Chang et al., Reference Chang, Chen, Lu, Gao, Tian, Gong, Dong and Du2017a) and orthologs in congener leafminers (fig. S1). To further examine the relationships between insect HSP70s, a phylogenetic tree was generated using the amino acid sequences of 20 HSP70 family members in orders Diptera, Lepidoptera, and Hemiptera (table S1; fig. 3). The resulting phylogenetic tree was divided into two distinct clusters containing HSC70 and HSP70. LtHSP701 and LtHSP702 grouped in the HSP70 cluster along with the previously published LtHSP70, LhHSP70, and LsHSP70 (fig. 3). Interestingly, LtHSP701 and LtHSP702 were located on different phylogenetic branches (fig. 3). LtHSC70 grouped with HSC70s in orders Diptera, Hemiptera, and Lepidoptera.
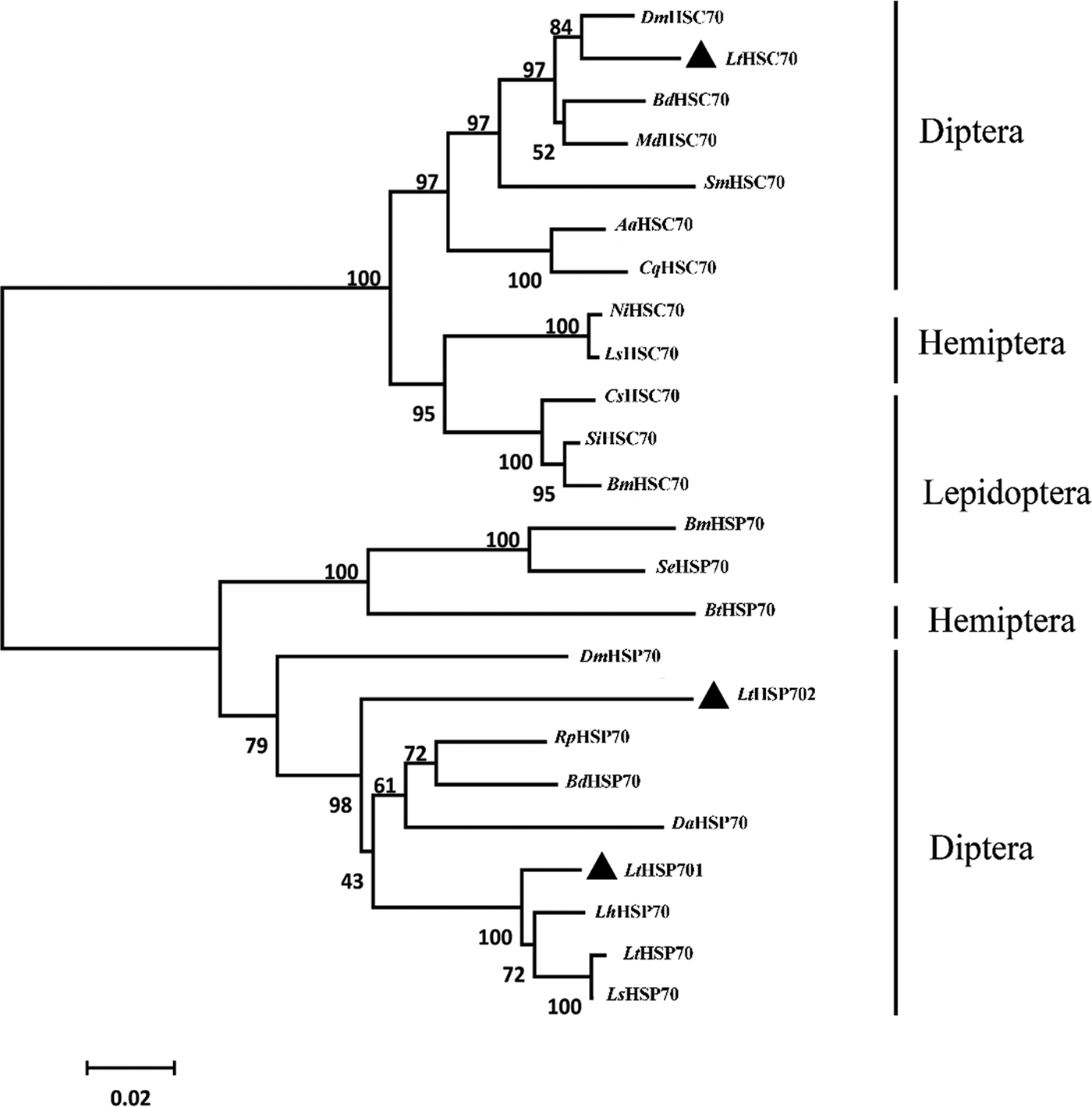
Fig. 3. Neighbor-joining phylogenetic tree of insect HSP70s. The Liriomyza trifolii HSP70s are labeled with triangles. Numbers on the branches are the bootstrap values obtained from 1000 replicates (only bootstrap values >50 are shown). The accession numbers and abbreviation for the species names are listed in Table S1.
Expression of the three Lthsp70s in response to temperature
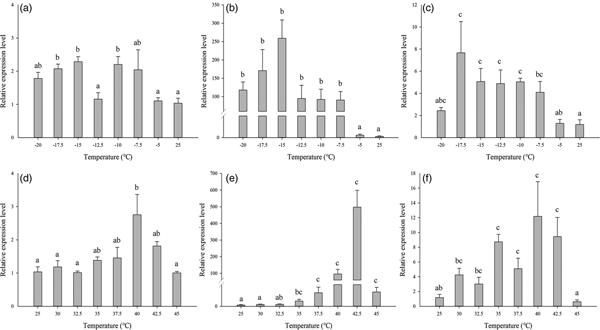
The expression of the three Lthsp70s was evaluated in response to temperature stress by qRT-PCR. The expression levels of all three Lthsp70s were significantly increased after exposure to cold stress relative to the control group at 25°C (hsc70:F 7,24 = 7.669, P < 0.001;hsp701:F 7,24 = 37.055, P < 0.001;hsp702:F 7,24 = 7.446, P < 0.001). Expression of Lthsp701 was highest at −15°C (T max), which was 258.84-fold greater than the control (fig. 4b). Lthsc70 and Lthsp702 were expressed at much lower levels than Lthsp701 during cold stress. At T max temperatures of −15 and −17.5°C, Lthsc70 and Lthsp702 were expressed at levels 2.29- and 7.66-fold higher than the control group, respectively (fig. 4a, c).

Fig. 4. Relative expression levels of three HSP70 genes in Liriomyza trifolii under low- and high-temperature treatments. The relative level of hsp expression represented the fold increase as compared with the expression in controls. Relative expression levels of hsc70 under cold stress (A), hsp701 under cold stress (B), hsp702 under cold stress (C), hsc70 under heat stress (D), hsp701 under heat stress (E), hsp702 under heat stress (F). The data were denoted as mean ± SE.
Compared with the control group (25°C), expression of the three Lthsp70s was significantly increased after exposure to heat stress (hsc70:F 7,24 = 3.602, P < 0.05;hsp701:F 7,24 = 14.348, P < 0.001;hsp702:F 7,24 = 12.590, P < 0.001). Expression of LtHsp701 was highest at 42.5°C (T max), which was 497.21-fold greater than the control (fig. 4e). Expression levels of Lthsc70 and Lthsp702 were lower than Lthsp701 in response to heat stress; both genes showed the highest expression levels (T max) at 40°C and were 2.76- and 12.18-fold greater than the control (fig. 4d, f).
Expression of Lthsp70s during L. trifolii development
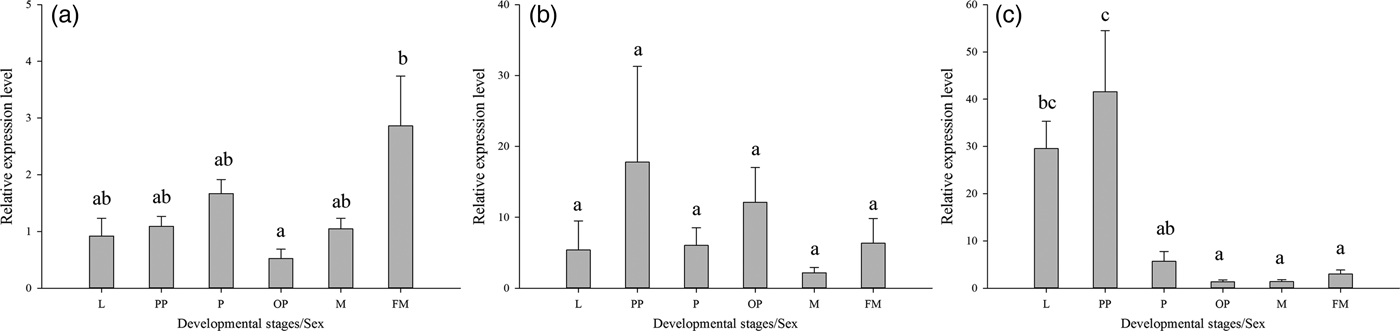
qRT-PCR was also used to monitor the expression of the three Lthsp70s in different developmental stages of L. trifolii, including third instar larvae, prepupae, 2-day-old pupae, 10-day-old pupae, male adults, and female adults. Lthsc70 and Lthsp702 exhibited significant differences in expression levels during development (Lthsc70:F 5,17 = 3.345, P < 0.05; Lthsp702:F 5,17 = 12.151, P < 0.001), whereas no significant differences were observed for Lthsp701 (F 5,17 = 0.504, P = 0.769). Expression of Lthsc70 was highest in female adults, which was 2.86-fold higher than the control group (male adults). Moreover, the expression of Lthsc70 has significant difference between female adults and 10-day-old pupae (fig. 5a). The expression level of Lthsp702 was significantly upregulated in larvae and prepupae, which were 29.54- and 41.59-fold higher than the control group (fig. 5c). Although Lthsp701 showed a slight increase in expression in pupal stages (fig. 5b), the difference was not significant.
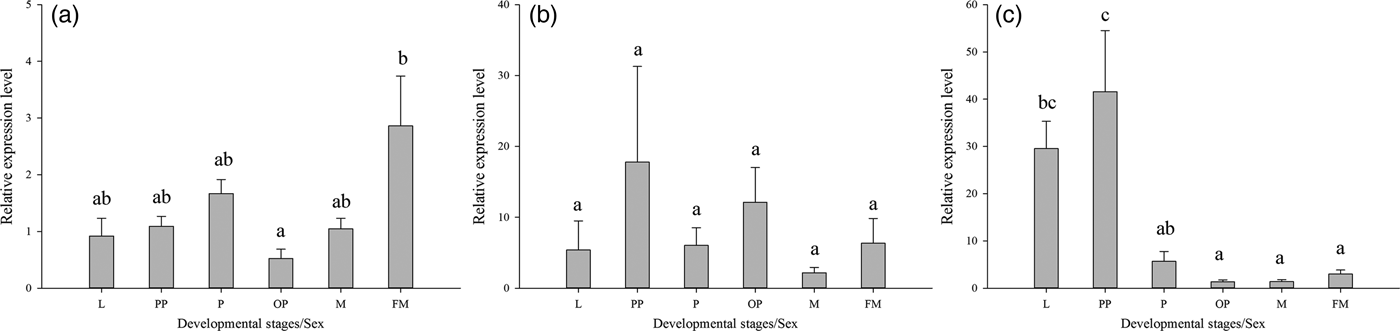
Fig. 5. Relative expression levels of three HSP70 genes in different developmental stages of Liriomyza trifolii. The relative level of hsp expression represented the fold increase as compared with the expression in controls. Relative expression levels of hsc70 (A), hsp701 (B), hsp702 (C). The data were denoted as mean ± SE. Abbreviations: FM, females adult; M, males adult; L, third instar larvae; PP, prepupae; P, 2-day-old pupae; OP, 10-day-old pupae.
Discussion
HSPs participate in a variety of physiological processes and are known to increase the thermal tolerance of diverse organisms (Burton et al., Reference Burton, Mitchell, Young and Petersen1988; Parsell & Lindquist, Reference Parsell and Lindquist1993; Parsell et al., Reference Parsell, Taulien and Lindquist1993). In insects, HSPs are produced during normal developmental stages and diapause (Joplin & Denlinger, Reference Joplin and Denlinger1990; Li et al., Reference Li, Popovabutler, Dean and Denlinger2007; Xiao et al., Reference Xiao, Wei and Xue2011). HSP70 is one of the most conserved and intensively studied members of the HSP family (Cui et al., Reference Cui, Lu and Du2010).
In this study, three genes encoding HSP70s were identified in L. trifolii, namely, Lthsc70, Lthsp701, and Lthsp702. The deduced protein products of these three genes contained signature sequences and motifs typical of the HSP70 family. The C-termini of the three LtHSP70s contain the conserved EEVD motif, which enables HSC70/HSP70 to interact with chaperones in the cytoplasm (Daugaard et al., Reference Daugaard, Rohde and Jäättelä2007). The N-termini of the LtHSP70s contained a conserved sequence with an ATP–GTP binding site, which is associated with conformational switching and ATPase activity (Siligardi et al., Reference Siligardi, Hu, Panaretou, Piper, Pearl and Prodromou2005; McLaughlin et al., Reference Mclaughlin, Sobott, Yao, Zhang, Nielsen, Grossmann, Laue, Robinson and Jackson2006).
With respect to genomic structure, a 1273-bp intron was discovered in Lthsc70; in contrast, Lthsp701 and Lthsp702 lacked introns. A negative correlation between intron size and gene expression level was suggested previously, and genes lacking introns or containing smaller introns were more highly expressed than genes with large introns (Castillo-Davis et al., Reference Castillo-Davis, Mekhedov, Hartl, Koonin and Kondrashov2002; Comeron, Reference Comeron2004). It also remains plausible that HSP-encoding genes that lack introns (e.g. LtHsp701 and LtHsp702) might be more sensitive to environmental stress. Another notable difference in genomic structure was the presence of a TATA-box-like motif in the 5′UTR of Lthsp701, which was absent in Lthsc70 and Lthsp702. We previously identified five TATA-box-like elements in Lthsp70, whereas orthologs in L. huidobrensis (Lhhsp70) and L. sativae (Lshsp70), contained one and two TATA-box elements, respectively (Huang & Kang, Reference Huang and Kang2007; Chang et al., Reference Chang, Chen, Lu, Gao, Tian, Gong, Dong and Du2017a). The position, spacing, and number of TATA elements can influence hsp expression patterns (Hunt & Morimoto, Reference Hunt and Morimoto1985; Wu et al., Reference Wu, Madabusi, Nishioka, Emanuel, Sypes, Arkhipova and Gilmour2001; Grace et al., Reference Grace, Chandrasekharan, Hall and Crowe2004).
The predicted amino acid sequences of Lthsc70, Lthsp701, and Lthsp702 shared considerable sequence similarity to HSP70 in other insect species. Interestingly, the dendrogram generated by the NJ method placed LtHSC70, LtHSP701, and LtHSP702 in different branches. This contrasts with the findings reported for Macrocentrus cingulum and Plutella xylostella (Xu et al., Reference Xu, Xiao, Li, Tong and Huang2010; Zhang et al., Reference Zhang, Wang, Jing, Zhuang and Wu2015), where intraspecific HSPs clustered together. Thus, in addition to cellular localization and expression patterns (Mahroof et al., Reference Mahroof, Yan, Neven, Subramanyam and Bai2005; Renner & Waters, Reference Renner and Waters2006; Daugaard et al., Reference Daugaard, Rohde and Jäättelä2007), there may be additional ways to classify HSP70. It is interesting to speculate that HSP genes in insects have originated from an ancestral gene but have since diverged after a long evolutionary period. In this context, HSPs can be regarded as useful markers for phylogenetic analysis in insects (Lu et al., Reference Lu, Li, Zheng, Shi and Du2016).
Multiple studies have shown that genes encoding HSPs are induced by temperature stress; furthermore, hsp70 and hsp20 are generally more susceptible to thermal stress than other hsps (Huang & Kang, Reference Huang and Kang2007). In general, hsp70 can be induced by high and low temperatures, whereas hsc70 is insensitive to thermal stress. Interestingly, our results indicate that Lthsc70 was induced by thermal stress, which is consistent with hsc70 in M. cingulum (Xu et al., Reference Xu, Xiao, Li, Tong and Huang2010), Pteromalus puparum (Wang et al., Reference Wang, Dong, Li, Hu and Ye2008), and P. xylostella (Sonoda et al., Reference Sonoda, Ashfaq and Tsumuki2006). However, our results contrasted with other studies (Rinehart et al., Reference Rinehart, Yocum and Denlinger2000; Bettencourt et al., Reference Bettencourt, Hogan, Nimali and Drohan2008; Zhang & Denlinger, Reference Zhang and Denlinger2010). Therefore, insect forms of hsc70 display species-specific transcriptional changes in response to heat stress and can be induced or constitutively expressed. The three Lthsp70s varied in terms of temperature sensitivity (fig. 4) and suggest a synergistic effect of different HSP70 family members with respect to thermal tolerance.
The role of HSPs in the regulation of insect growth and development has become an important area of inquiry. In the current study, the three Lthsp70s showed some level of expression in all developmental stages and in both sexes. The expression of Lthsp702 was highest in larvae and pre-pupae, whereas expression of Lthsc70 was highest in female adults. The latter result is similar to the findings reported for L. sativae where Lshsp expression was highest in adults; however, sexes were not discriminated in the former study (Huang et al., Reference Huang, Wang and Kang2009). In this study, female adults had a slightly larger body size vs. male adults, which could possibly explain the results with Lthsc70 expression. It remains possible that evolution may favor the expression of HSPs in female adults to preserve fecundity and reproduction; however, the tradeoffs between thermal tolerance and fitness costs should not be ignored (Huang et al., Reference Huang, Chen and Kang2007; Zhou et al., Reference Zhou, Guo, Chen and Wan2010; Lü et al., Reference Lü, Wang, Zhu, Yu, Guo and Wan2014). Functional connections between hormones and heat shock regulatory systems have been previously suggested (Lezzi, Reference Lezzi, Gilbert, Tata and Atkinson1996). The expression of selected HSPs was induced by ecdysone in Drosophila and steroid hormones in mammals (Ryan & Hightower, Reference Ryan, Hightower, Puga and Wallale1998). Therefore, the expression of HSP genes during L. trifolii development may influence the secretion of hormones.
The expression of Lthsp702 in larvae and prepupae was significantly higher than the control group (fig. 5), which consisted of male adults. The larvae of L. trifolii cause damage by feeding inside foliar tunnels, which form a micro-environment that provides some protection from temperature fluctuations. In the thermal stress experiments, we removed larvae from leaves, and the removal process may have elevated HSP gene expression. In the case of prepupae, the larvae simply exit the leaves, and the soft puparium may be more sensitive to temperature fluctuations. We observed a no negligible increase in Lthsp701 and Lthsp702 expression during the transition from larvae to prepupae, though there were no significant difference between those two developmental stages (fig. 5). Thus, in addition to diapause, metamorphosis may also induce HSP gene expression. Many tissues and organs are degraded and re-constructed during insect metamorphosis. It is quite possible that HSPs, which function as chaperones (Feder & Hofmann, Reference Feder and Hofmann1999), facilitate the reconstruction of new tissues and organs during the pupal stage. The metamorphosis period is characterized by large physical changes, and the change in protein structure was previously shown to result in high expression levels of hsps (Huang et al., Reference Huang, Wang and Kang2009). Interestingly, Lthsp701 was also expressed at different developmental stages; however, there was no significant difference in Lthsp701 expression between stages, which indicates that Lthsp701 may not function in the leafminer growth and development.
In conclusion, the genes encoding LtHSP70 in L. trifolii showed different expression profiles in response to temperature stress and during different leafminer development. This study provides new insights into the potential role of Hsp70s in insect behavior and development. Future studies are underway to further investigate the function of HSPs in the physiology of L. trifolii.
Acknowledgements
The authors express their deep gratitude to the Testing Center of Yangzhou University. This research was funded by the Jiangsu Science & Technology Support Program (BE2014410), the Basic Research Program of Agricultural Application of Suzhou (SNG201602), the Science and Technology Program of Yangzhou (YZ2014171), the Modern Agriculture Industrial Technology System Program of Jiangsu (SXGC [2017]218) and the Priority Academic Program Development of Jiangsu Higher Education, the National Science and Technology Support Program (2012BAD19B06).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485318000354.