Introduction
The eastern spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae), is the most destructive insect pest in the maritime and boreal forests of North America. Populations of this forest defoliator have reached outbreak densities over extensive forested areas on a fairly regular basis for the past three centuries, at the very least (Blais, Reference Blais1965). Losses to the forest products industry that could be attributed to spruce budworm during its last outbreak amounted to 44 million m3 of wood per year (Sterner & Davidson, Reference Sterner and Davidson1982). Budworm feeds on several conifer hosts, most preferably on balsam fir (Abies balsamea (L.) Mill.), followed by white spruce (Picea glauca (Moench) Voss), red spruce (P. rubens Sarg.) and black spruce (P. mariana (Mill.) BSP) (Henningar et al., Reference Henningar, MacLean, Quiring and Kershaw2008).
This univoltine insect emerges as an adult from its pupa in mid-summer to mate and lay its eggs. Immediately after egg hatch, first-instar larvae spin hibernacula, moult to the second-instar and enter into an obligatory diapause until the following spring (Han & Bauce, Reference Han and Bauce2000). During this long overwintering period, the larvae do not feed. Their survival depends exclusively on the reserves provided by the female parent (Han & Bauce, Reference Han and Bauce2000). While the quality of these reserves may be affected by the quality of food which had been consumed by the mother, the quality and quantity of food that is subsequently consumed by a larva influences its overall fitness and performance, thereby affecting growth rate, developmental time, final body mass, dispersal ability and probability of survival (Slansky & Scriber, Reference Slansky, Scriber, Kerkut and Gilbert1985). Further, larval food quality is important to the adult insect because its effects can carry over to affect larval performance in the succeeding generation (Slansky & Scriber, Reference Slansky, Scriber, Kerkut and Gilbert1985).
Thus, the nutritional state of parents can influence the performance of their progeny (Rossiter, Reference Rossiter1991a,Reference Rossiterb; Fox et al., Reference Fox, Wadell and Mousseau1995; Carisey & Bauce, Reference Carisey and Bauce2002). For example, the progeny of gypsy moth (Lymantria dispar L.) females that had fed on leaves from defoliated trees dispersed less successfully than those of females that had not suffered such nutritional stress (Diss et al., Reference Diss, Kunkel, Montgomery and Leonard1996). At a more fundamental level, as Rossiter (Reference Rossiter1991b) found, the nutritional experience of gypsy moth parents can influence the length of the pre-feeding larval period, together with the developmental time and pupal mass of the offspring.
Henningar et al. (Reference Henningar, MacLean, Quiring and Kershaw2008) found that balsam fir is more prone to being defoliated than is white spruce, red spruce or black spruce. Although balsam fir is known to be the preferred host of the budworm, field observations (Blais, Reference Blais1957; Craighead, 1924, cited by Lavallé & Hardy, Reference Lavallé and Hardy1988) and laboratory studies (Koller & Leonard, Reference Koller and Leonard1981; Mattson et al., Reference Mattson, Haack, Lawrence and Slocum1991) have found that larvae reared on white spruce develop more rapidly and are larger than those reared on balsam fir. The difference between balsam fir and white spruce may be due to faster growth, greater development and more foliage per unit area in the shoots of spruce compared to those of balsam fir (Greenbank, Reference Greenbank1963). In contrast, naturally occurring budbreak and shoot elongation occurs later in black spruce than in balsam fir, making the latter more susceptible to defoliation than the former (Blais, Reference Blais1957). Thus, susceptibility to spruce budworm attack varies among host species, depending upon differences in phenology and shoot performance.
It has been proposed that balsam fir, white spruce and black spruce are equally susceptible targets for spruce budworm oviposition and that all are suitable for completion of its life cycle (Nealis & Régnière, Reference Nealis and Régnière2004). However, differences in synchrony between spruce budworm and host tree phenology are responsible for differences in host susceptibility and insect performance (Blais, Reference Blais1957; Mattson et al., Reference Mattson, Haack, Lawrence and Slocum1991; Nealis & Régnière, Reference Nealis and Régnière2004). Nevertheless, differences in host nutritional quality may also play an important role. For example, Thomas (Reference Thomas1989) found that black spruce was less suitable than white spruce and red spruce as a source of food because of the presence of allelochemicals which reduce budworm performance. Differences in food quality provided by host species can thus affect population dynamics and adaptions of the budworm (Carisey & Bauce, Reference Carisey and Bauce2002). Understanding the effects of food quality on the performance of spruce budworm progeny may help us in developing methods that reduce the damage caused by this insect. The objectives of this study were to determine (i) if differences in nutritional quality existed among balsam fir, white spruce and black spruce, and (ii) if differences in host nutritional quality affected spruce budworm progeny development and survival. We reared budworms in the field to elucidate the importance of host trees on insect performance. Also, a laboratory insect rearing study that used an artificial diet was used to determine whether or not the effect of host tree is passed on to the progeny.
Materials and methods
Field insect rearing
Our research was conducted in the Montmorency experimental forest (47°19′N, 79°09′W), which is located 60 km north of Quebec City, Canada. This forest is typical of the Laurentide-Onatcheway region (Rowe, Reference Rowe1972), and most of the stands fit Grandtner's (Reference Grandtner1966) description of the balsam fir-white birch association. We evaluated the effect of food quality on spruce budworm by using foliage of three different host trees: balsam fir, white spruce and black spruce. Balsam fir and white spruce trees were selected from sites with good quality drainage (Class 3, mesic with seepage), whereas black spruce trees were selected only on sites with poor drainage (Class 5, hydric) because this species only grows on the hydric drainage class in the Laurentide-Onatcheway region (Rowe, Reference Rowe1972). Bélanger et al. (Reference Bélanger, Lemay and Cardinal2004) should be consulted for further details regarding drainage class. Site selection avoided using stressed trees, which could have altered results of the study. Also, the study site was free of spruce budworm. Six dominant or codominant individuals were randomly selected for each tree species. Trees were at least one km apart, which allowed us to treat them as independent experimental units. This gave three ‘treatments’ (species), each with six replicates, which yielded 18 individuals that included subsamples (pseudoreplication). Two 75-cm-long branches, from the north-northwest aspect of the tree canopy, were selected from the mid-crown of each tree. Each branch was enclosed with a fine-mesh cloth sleeve cage, which served as an enclosure for 20 post-diapausing second-instar larvae (parental generation) (n=360 larvae). Larvae came from a colony in the Laboratory of Forest Entomology, Laval University. The colony has been maintained with regular introductions (every three years) of wild populations for the last seven years. To simulate normal field emergence following winter diapause, insects were placed in the field cages when 150 degree-days had been accumulated, which is two to three weeks prior to vegetative budbreak in the study area. Branches were cut and brought to the laboratory when 90% of the larvae had turned into pupae. During travel from the forest to the laboratory, pupae were kept under ambient conditions. The dry mass of frass produced in each rearing sleeve cage was recorded, larval mortality was determined, and pupae were sexed and weighed using an electronic balance with 10 μg accuracy (MC 1 Analytic AC 210 S, Sartorius Canada, Mississauga, ON, Canada). Results from an earlier study on spruce budworm food utilisation that combined laboratory and field rearing experiments indicated that the rearing technique used in the present study gives an accurate estimate of frass production (Bauce et al., Reference Bauce, Crepin and Carisey1994).
Laboratory insect rearing
Spruce budworm second-instar larvae were obtained from the parental generation which had been reared in the Montmorency forest. At emergence, moths were sexed and placed in a rearing room (23°C, 60% RH, L:D 16:8). To increase mating success, two males were placed with one female in a clear plastic vial (9.5 cm high×4.5 cm in diameter), covered on top with a piece of cheesecloth and on the bottom with a plastic cap. Throughout the oviposition period, the eggs laid by each female were collected every two days using a fine brush and weighed to evaluate total and individual egg mass. Eggs were incubated (23°C, 65% RH and L:D 16:8) for one week and were enclosed in clear plastic boxes (4×2.5×1.5 cm), the lids of which were lined with cheesecloth that could be used by first-instar larvae for building hibernacula.
Twenty-five post-diapausing second-instar larvae from each selected tree (n=450 larvae) were reared to pupation in a growth chamber (23°C, 60% RH, L:D 16:8). Male and female post-diapause second-instar larvae emerged in rearing containers which contained an artificial diet (McMorran, Reference McMorran1965). Larvae were monitored twice each day to record mortality and instar. These data were then used to establish the time (hours) from second-instar to the adult stage. Pupae were weighed 8 h after pupation and sexed. After emergence, moths were mated and the eggs that were laid were collected each day. The number of eggs laid by each female during its lifetime, the number of fertile eggs and the subsequent number of second-instar larvae that entered diapause were recorded. Larvae were placed in an outdoor insectarium near Laval University (46°47′N, 71°18′W) for the duration of their winter diapause and were exposed to low ambient temperatures. Winter survival was evaluated the following spring.
For each host tree, 50 sixth-instar larvae were randomly chosen to determine relative growth rates and relative consumption rates, which were estimated on a dry-mass basis (gravimetric experiment). Newly-moulted sixth-instar larvae were weighed and placed in individual rearing containers (4×2.5×1.5 cm). Larval developmental time was monitored twice daily. Ingested food and excreted dry faeces that had been produced during larval development were quantified, as described by Bauce et al. (Reference Bauce, Crepin and Carisey1994).
Relative growth rate (RGR) and relative consumption rate (RCR) were determined from the following formulae:
where:
G=gained mass=(final mass−initial mass)
MW=mean larval mass=G/log (final mass/initial mass)
I=ingested food
Linear or non-linear regression was used to predict the numerator as a function of the denominator for each ratio so that an adjusted ratio could be calculated on the basis of a common denominator, thereby enabling indices to be compared (Bauce et al., Reference Bauce, Crepin and Carisey1994).
Chemical analysis of the foliage
For chemical analysis of the foliage, we collected mid-crown branches from each tree that had been selected for field rearing. Foliar chemical content was determined on each sample tree using north-northwest facing mid-crown branches which had been not infested by budworm. Foliar chemistry was determined twice during each growing season: 15 days after insect installation and when budworm infested-branches were collected (at pupal stage). For chemical determinations, 3 g of fresh current-year foliage were collected from each sample tree (n=18 samples per collection date). The samples were returned to the laboratory on dry ice, flash-frozen in liquid nitrogen, freeze-dried, ground in a Wiley mill (maintained below −30°C to avoid deterioration of polyphenolics), and maintained at −20°C until they were analysed for protein, mineral nutrients (P, K, Ca, Mg), total soluble sugars, total tannins, hydrolysable tannins, condensed tannins and total phenolic content using the methodologies described in Bauce (Reference Bauce1996) and Bauce et al. (Reference Bauce, Kumbaşlı, van Frankenhuyzen and Carisey2006). Two subsamples of 15 current-year twigs were collected from each sample tree to determine moisture content. Two additional subsamples of fresh current-year twigs were collected on each tree, placed in crimped sealed vials and kept at −20°C until the needles could be analysed for monoterpenes using gas chromatographic techniques described in Bauce et al. (Reference Bauce, Crepin and Carisey1994). Extracts were analysed with a Varian GC3900 gas chromatograph equipped with a flame ionisation detector and a 30 m×0.25 mm fused silica capillary column (supelco SPB-5), controlled by a Varian Workstation running Galaxie software. Three μl aliquots were injected into the column and carried by hydrogen (split 1:20). Column temperature was programmed to increase at 2°C min−1 from 60°C to a final temperature of 110°C, which was maintained for 3 min. Monoterpenes were identified by comparing retention times with authentic standards (Aldrich Chemical Co. Inc., Milwaukee, WI, USA) under identical conditions. Our identifications were confirmed at the LASEVE laboratory (Université du Québec à Chicoutimi) using data obtained from a gas chromatography/mass spectrometry system. Quantification of monoterpenes was based on injecting known amounts of authentic compounds under identical conditions and determining response factors for each monoterpene relative to known amounts of the internal standard, tetradecane. The results are expressed as percent dry weight.
Statistical analyses
Multiple responses for each property were averaged for each tree to avoid pseudoreplication (i.e. the tree was the experimental unit). Normality and homogeneity of variance tests were performed before data were subjected to multivariate analysis of variance (MANOVA), with individual trees as experimental units. In the case of chemical analysis, the data were analysed using a split-plot complete randomised factorial design with six replicates, with block (tree species) as the error term. The main plots corresponded to tree species (3), and the sub-plots to collection date (2). MANOVA was performed on each of the following groups of variables: parental generation performance, progeny performance, gravimetric experiment (males and females were analysed separately), host tree monoterpenes, host tree tannins and phenols, and nutrient elements. Furthermore, monoterpenes were divided into two groups because there were not sufficient degrees of freedom to perform MANOVA on a single group. The first group corresponded to monoterpenes reported as oviposition stimulants to the budworm in the scientific literature (group 1: α-pinene, β-pinene, limonene, myrcene, thujone) (Städler, Reference Städler1974; Grant et al., Reference Grant, Guo, MacDonald and Coppens2007), whereas the second group corresponded to monoterpenes reported as feeding deterrents to the insect (group 2: camphene, terpinolene, δ-3-carene, bornyl acetate, borneol) (Mattson et al., Reference Mattson, Haack, Lawrence and Slocum1991; Bauce et al., Reference Bauce, Crepin and Carisey1994). If MANOVA found a significant effect in a group of variables, data from that group were analysed using Analysis of Variance (ANOVA) to determine which variables were affected by the factors that were studied. Duncan's test was used for comparison of means. When data did not meet assumptions of normality and homogeneity of variance, the tests were performed on ranked data (PROC GLM). Mortality was analysed in a generalised linear model for binary response data, assuming that the random component in the model has a binomial distribution, followed by two-by-two contrasts for comparing differences between treatments (PROC GENMOD: SAS Institute, 2003).
Canonical correlation analysis (CCA) examined the degree to which components of each group of insect performance variables were correlated with each foliar chemistry group of variables (PROC CANCORR: SAS Institute, 2003). For each group, we used only the variables that were statistically different among host tree species because the large number of variables in these groups compared to the relatively small number of repetitions did not permit the use of CCA on the entire data set. Finally, the variables developmental time (DT), development time of the sixth-instar (DT6) and total development time (TDT) were transformed into developmental rate (DR), development rate of the sixth-instar (DR6) and total development rate (TDR) by using the following formulae: DR=1/DT; DR6=1/DT6 and TDR=1/TDT.
Results
Field insect rearing
Results of MANOVA on field insect rearing measurements indicated that host species had a significant effect on spruce budworm performance (Wilks' Lambda: F(14,18)=5.34, P=<0.01). For both sexes, the larval developmental period was approximately four days shorter on white spruce than on black spruce and balsam fir (table 1). Male larvae fed on white spruce were about 12% heavier than larvae fed on balsam fir and 19% heavier than those fed on black spruce. Males also had a shorter developmental time. Further, male spruce budworm fed on black spruce had a lower pupal mass than males fed on the other hosts. Larvae fed on white spruce exhibited higher realised fecundity but lower fertility than larvae fed on the other hosts. Female pupal mass (table 1), egg mass (F(2,15)=1.25, P=0.31) and total mortality (χ2(2)=3.08, P=0.21) did not differ among the three host trees species.
Table 1. Spruce budworm performance tabulated by host species (mean±SEM).

Values in each row followed by the same letter do not differ significantly at P<0.05 according Duncan's multiple range test.
1 Total number of eggs laid by a female moth.
2 Total number of viable larvae produced by a female moth.
* Number of larvae in parentheses.
Laboratory insect rearing
Host tree species affected spruce budworm performance in laboratory reared larvae (Wilks' Lambda: F(12,18)=3.22, P=<0.02). Budworm progeny from parents reared on balsam fir exhibited the greatest female pupal mass, while budworms from parents reared on white spruce showed shorter developmental time for both sexes (table 2). Also, larvae with parents reared on black spruce had a longer developmental time, greater mortality and lower pupal mass than those reared on the other hosts. Finally, fertility (F(2,15)=2.56, P=0.110), fecundity (F(2,15)=1.12, P=0.352) and winter larvae survival (F(2,15)=0.24, P=0.792) were not significantly affected by the parental host tree.
Table 2. Spruce budworm performance in laboratory tabulated by host species (mean±SEM).
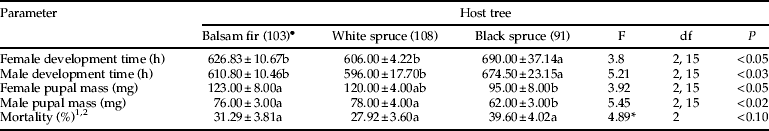
Values in each row followed by the same letter do not differ significantly at P<0.05 according Duncan's multiple range test.
1 Mortality was analysed in a generalised linear model for binary response data, followed by two-by-two contrasts (PROC GENMOD).
2 Test was considered statistically significant at P<0.1.
* Chi-square value.
• Number of larvae in parentheses.
Host tree species also had a significant effect on performance of female (Wilks' Lambda: F(14,8)=3.83, P=<0.04) and male (Wilks' Lambda: F(14,14)=3.86, P=<0.01) larvae used in the gravimetric experiment. Females that were obtained from parents reared on balsam fir had shorter total developmental time and reached sixth-instar 10 h and 86 h earlier than those with parents fed on white and black spruce, respectively (table 3). Females obtained from parents reared on black spruce had 53% and 65% higher RGR than those whose parents had fed on balsam fir and white spruce, respectively. No differences were detected among parental host species in terms of female sixth-instar initial (F(2,15)=0.74, P=0.503) and final masses (F(2,15)=0.38, P=0.694), RCR (F(2,15)=3.52, P=0.069) and pupal developmental time (F(2,15)=1.20, P=0.341).
Table 3. Female spruce budworm performance and indices in laboratory tabulated by host species (mean±SEM).

Values in each row followed by the same letter do not differ significantly at P<0.05 according Duncan's multiple range test.
1 Larval mass at the beginning of the sixth-instar.
2 Developmental time from second to sixth-instar.
3 Pupal mass.
4 Developmental time from second-instar to pupal stage.
* Number of larvae in parentheses.
Males obtained from parents reared on balsam fir had 10% higher sixth-instar initial mass than those with parents reared on white spruce and 46% higher than those with parents reared on black spruce (table 4). They also reached sixth-instar stage 14 h and 84 h earlier, and had a shorter total developmental time than those with parents fed on white and black spruce, respectively. In contrast, males obtained from parents reared on black spruce had 87% and 59% higher RGR than those with parents fed on balsam fir and white spruce, respectively. No differences in male sixth-instar final mass (F(2,15)=1.15, P=0.346), RCR (F(2,15)=0.17, P=0.846) and pupal developmental time (F(2,15)=1.47, P=0.267) were detected among parental host species. Finally, larvae of parents reared on black spruce exhibited greater mortality than those whose parents were reared on balsam fir and white spruce.
Table 4. Male spruce budworm performance and indices in laboratory tabulated by host species (mean±SEM).

Values in each row followed by the same letter do not differ significantly at P<0.05 according Duncan's multiple range test.
1 Larval mass at the beginning of the sixth-instar.
2 Developmental time from second to sixth-instar.
3 Pupal mass.
4 Developmental time from second-instar to pupal stage.
5 Mortality (males and females included) was analysed in a generalised linear model for binary response data, followed by two-by-two contrasts (PROC GENMOD).
* Chi-square value.
• Number of larvae in parentheses.
Foliar chemical analysis
Host tree species were significantly different in terms of their foliar chemistry (tables 5 and 6). Balsam fir foliage contained higher concentrations of N, Ca, total tannins and total hydrolysable tannins than the other hosts, whereas black spruce foliage had higher concentrations of P, Mg and total phenolics (table 5). Potassium (F(2,21)=0.33, P=>0.05), and sugars (F(2,21)=3.54, P=>0.05) did not differ among host trees. As for monoterpenes, black spruce had high concentrations of camphene, bornyl acetate and myrcene (table 6). Balsam fir also had high concentrations of certain monoterpenes, including α- and β-pinene.
Table 5. Nutrient, tannins and phenolic contents according to host species (balsam fir, white spruce and black spruce) (mean±SEM).

Foliar chemistry was determined twice during each growing season: 15 days after insect installation and when budworm infested-branches were collected (at pupal stage). A total of six trees were chosen at random from each host species (n=18 samples per collection date). Data were analyzed using multivariate analysis of variance (MANOVA). If MANOVA found a significant effect in these groups of variables, data from that group were analysed using analysis of variance (ANOVA) to determine which variables were affected by the factors that were studied (host species and collection date).
Values in each row followed by the same letter do not differ significantly at P<0.05 according Duncan's multiple range test.
1 P-value is for univariate analysis of variance.
2 Results of MANOVA for group of nutrients.
3 Results of MANOVA for group of tannins and phenolics.
Table 6. Monoterpene contents according to host species (balsam fir, white spruce and black spruce) (mean±SEM).

Foliar chemistry was determined twice during each growing season: 15 days after insect installation, and when budworm infested-branches were collected (at pupal stage). A total of six trees were chosen at random from each host species (n=18 samples per collection date). Data were analyzed using multivariate analysis of variance (MANOVA). If MANOVA found a significant effect in these groups of variables, data from that group were analysed using analysis of variance (ANOVA) to determine which variables were affected by the factors that were studied (host species and collection date).
Values in each row followed by the same letter do not differ significantly at P<0.05 according Duncan's multiple range test.
1 P-value is for univariate analysis of variance.
2 Results of MANOVA for group of monoterpenes stimulants.
3 Results of MANOVA for group of monoterpenes deterrents.
There were marked shifts in host foliar chemistry over the growing season, but temporal variation differed among host tree species (table 5). Variation in foliar chemistry over the sampling dates was detected for N, P, Mg, Ca, total tannins, total hydrolysable tannins and total phenolics (table 5). The interactions between date and host species were also significant for those compounds, further indicating that seasonal trends differed among the hosts. Seasonal declines were observed in all three hosts for N (47%), P (51%) and Mg (17%), with the foliage of black spruce being the most affected. Seasonal elevation in concentrations were observed for Ca (121%), total tannins (2477%), total hydrolysable tannins (2415%) and total phenolics (1395%), with the foliage of balsam fir showing the greatest increases.
With respect to monoterpenes, date of collection affected deterrent monoterpenes, whereas the date by host species interaction affected oviposition-stimulating monoterpenes (table 6). Variations in monoterpenes over sampling dates were detected for myrcene, camphene and bornyl acetate, whereas a date by host species interaction was detected for α-pinene, β-pinene, limonene and myrcene (table 6). Black spruce foliage retained the highest concentrations of deterrent monoterpenes, such as camphene and bornyl acetate, throughout the growing season (table 6).
Canonical correlation analysis
The canonical correlation analysis (CCA) showed a significant correlation between parental generation performance and nutrient data set only (Rc2=0.96, Wilks' Lambda: F(40,24)=1.89, P=<0.05). The first canonical variate for parental performance was positively associated with female (r=0.78) and male (r=0.68) developmental rate, and fecundity (r=0.93), whereas the first canonical variate for nutrients was negatively associated with Ca on the first (r=−0.51) and second (r=−0.56) collection dates. Scores on the first canonical variate of parental performance for Ca for the first (r=−0.49) and second (r=−0.55) collection dates suggest that Ca has a negative effect on female and male developmental rate, and fecundity. Canonical redundancy analyses (CRA) indicated that the nutrient data set extracted 44.12% of the variance in the parental performance data set.
As for progeny, their performance was significantly correlated with deterrent monoterpenes only (Rc2=0.91, Wilks' Lambda: F(16,22)=2.91, P=<0.02). The first canonical variate for progeny performance was negatively associated with female developmental rate (r=−0.16) and male pupal mass (r=−0.11), and positively associated with female pupal mass (r=0.95), whereas the first canonical variate for deterrent monoterpenes was positively associated with camphene (r=0.99) and bornyl acetate (r=0.54) at the second collection. The analysis of correlations between the first canonical variate of progeny performance and camphene (r=0.94) and bornyl acetate (r=0.52) suggests that these monoterpenes have a negative effect on female developmental rate and male pupal mass, while they have a positive effect, surprisingly, on female pupal mass. CRA indicated that the proportion of variance in progeny performance explained by the nutrient data set was 21.79%.
Finally, CCA indicated that only female performance in the gravimetric experiment data set had a significant correlation with deterrent monoterpenes (Rc2=0.86, Wilks' Lambda: F(12,16)=4.44, P=<0.01) and tannin and phenol (Rc2=0.96, Wilks' Lambda: F(18,11)=2.72, P=<0.05). The first canonical variate for female performance was positively associated with developmental rate of sixth-instar larvae (r=0.71), total developmental rate (r=0.46) and relative growth rate (r=0.47), whereas the first canonical variate for deterrent monoterpenes was negatively associated with bornyl acetate (r=−0.53) on the first collection date. Scores for bornyl acetate (r=−0.49) on the first collection date for the first canonical variate of female performance suggest that this monoterpene has a negative effect on the three variables of female performance. CRA indicated that the nutrient data set extracted 27.49% of the variance in the progeny performance date set.
With respect to correlations between female performance and tannin and phenols, the first canonical variate for female performance was positively associated with relative growth rate (r=0.86), whereas the first canonical variate for tannin and phenol was positively associated with total tannins (r=0.81) and total hydrolysable tannins (r=0.56) on the first collection date and negatively associated with total phenolics on the second collection date (r=−0.27). Scores for these elements suggest that total tannins (r=0.80) and total hydrolysable tannins (r=0.55) at the first collection have a positive effect and that total phenolics at the second collection (r=−0.26) have a negative effect on relative growth rate. CRA indicated that the proportion of variance in female performance explained by nutrient data set was 21.79%.
Discussion
Results of this experiment show that budworm larvae fed on white spruce foliage performed best in terms of fitness indicators, as expressed in the shorter larval developmental time for both sexes, and high male pupal mass, compared with larvae that fed upon black spruce and balsam fir. Shorter developmental time may be important to larval survival because the duration of larval exposure is reduced with respect to predators, pathogens and other potentially harmful agents (Slansky, Reference Slansky1990). Moreover, developmental time may alter the adult's ability to mate, and the timing and rate of reproduction, together with its fecundity and dispersal abilities (Slansky & Scriber, Reference Slansky, Scriber, Kerkut and Gilbert1985). Furthermore, male insect mass may affect fecundity because it has been discovered that male body size affects female fecundity (Fox et al., Reference Fox, Wadell and Mousseau1995; Delisle & Hardy, Reference Delisle and Hardy1997).
Foliar analyses revealed significant differences among host species. For example, balsam fir had higher concentrations of N and Ca, whereas black spruce contained greater concentrations of camphene, bornyl acetate and total phenolics than did the other hosts. Canonical correlation analysis suggests that Ca might play an important role in budworm performance by reducing developmental rate and fecundity. Mattson et al. (Reference Mattson, Haack, Lawrence and Slocum1991) hypothesised that high concentrations of calcium might interfere with the uptake of micronutrients, such as iron and zinc, which are known to be important catalysts of several enzyme reactions (Mattson & Scriber, Reference Mattson, Scriber, Slansky and Rodriguez1987). Moreover, nitrogen is by far the most important nutrient for insect growth and survival (Mattson & Scriber, Reference Mattson, Scriber, Slansky and Rodriguez1987). In fact, larvae of spruce budworm fed on a diet containing sufficient nitrogen have higher growth rates and shorter developmental times (Mattson et al., Reference Mattson, Haack, Lawrence and Slocum1991; Bidon, Reference Bidon1993; Bauce et al., Reference Bauce, Crepin and Carisey1994; Carisey & Bauce, Reference Carisey and Bauce1997a,Reference Carisey and Bauceb). Nevertheless, the lack of strong correlation between insect performance and the other nutrients suggests that a proper balance of nutrients would be more important to spruce budworm than a high concentration of a given nutrient. For example, Clancy (Reference Clancy1992) reported that western spruce budworm response to increased N in artificial diets was neither positively linear nor convex; rather, response was dependent on levels of minerals in the diets. Thus, the stronger seasonal decline of nutrients such as N, P and Mg in black spruce may affect budworm performance by making its foliage nutritionally unbalanced and, therefore, less suitable for the insect than the foliage of the other hosts.
The effects of secondary compounds on insect progeny appeared to be stronger than nutrients, as reflected in the negative correlations between monoterpenes, such as camphene and bornyl acetate, and female developmental rate and pupal mass. Surprisingly, these monoterpenes also had a positive effect on female pupal mass. This effect was not consistent with results from previous studies, which have found that bornyl acetate and camphene exert deleterious or toxic effects on budworm larvae, including higher mortality and lower growth rates (Mattson et al., Reference Mattson, Haack, Lawrence and Slocum1991; Bauce et al., Reference Bauce, Crepin and Carisey1994; Carisey & Bauce, Reference Carisey and Bauce1997a). Furthermore, some phenolics such as pungenin are feeding deterrents for spruce budworm and can cause a reduction in consumption, thereby affecting the size of females (Heron, Reference Heron1965; Strunz et al., Reference Strunz, Giguère and Thomas1986).
Mortality did not differ among the host trees, but we could observe that larvae fed on balsam fir exhibited slightly higher mortality (60.83%) than those fed on black (55%) and white (52.5%) spruce. This result is not in accordance with previous works, which have reported higher larval mortality in black spruce (Blais, Reference Blais1957; Nealis & Régnière, Reference Nealis and Régnière2004; Henningar et al., Reference Henningar, MacLean, Quiring and Kershaw2008) compared to other spruce budworm host trees. Late budbreak of black spruce has frequently been suggested as the most important factor responsible for high larval mortality and reduced defoliation on this species compared with balsam fir and white spruce because this phenological delay forces budworms to mine old foliage, which is an unsuitable food source (Blais, Reference Blais1957; Greenbank, Reference Greenbank1963).
Nevertheless, Lawrence et al. (Reference Lawrence, Mattson and Haack1997) found that late budbreak (i.e. budbreak follows larval emergence by several weeks) does not significantly affect post-diapause spruce budworm survival. By contrast, early budbreak (i.e. budbreak occurs prior to or during larval emergence) dramatically reduced budworm survival and body mass because larvae start feeding too late to take advantage of the high levels of foliar nitrogen, which diminishes rapidly during and immediately following budbreak. This same pattern has been observed by Bauce (unpublished data) in white spruce. The results reported by Lawrence et al. (Reference Lawrence, Mattson and Haack1997) thus may be explained by the ability of young budworms to mine one- and two-year-old foliage, where they obtained the required amount of nitrogen from nitrogen-rich tissues under the nitrogen-poor outer needle layer (Trier & Mattson, Reference Trier and Mattson1997). Acquisition of nitrogen is more important than sugars for young larvae (Albert & Bauce, Reference Albert and Bauce1994), thereby allowing larvae to survive up to four weeks prior to budbreak (Trier & Mattson, Reference Trier and Mattson1997). This ability of young budworms may explain larval mortality on black spruce. Likewise, the use of sleeve cages may have reduced the loss of early-stage larvae by preventing the redistribution of budworms among more suitable host trees.
An earlier study on spruce budworm (Carisey & Bauce, Reference Carisey and Bauce2002) suggests that the nutritional experience of the parents may affect the performance of the progeny. The laboratory rearing results indicate that progeny with parents fed on black spruce exhibited longer developmental times and greater mortality, and had lower pupal masses. Despite reaching the sixth-instar later, the progeny of parents that fed on black spruce also exhibited higher RGR than progeny of parents fed on balsam fir and white spruce, suggesting the existence of a nutritional-based parental effect. Canonical correlation analysis suggests that concentrations of bornyl acetate and phenolics in the foliage fed upon by the parental generation might have affected offspring performance.
The poorer performance exhibited by the offspring of parents fed on black spruce points to this species as poor quality food for spruce budworm. The nutritional quality of this host may have caused a reduction in the quantity or quality of reserves which the parents provided to their eggs. Egg provisioning is very important because it represents the sole energy source available to the progeny for embryogenesis and maintenance prior to hatching. Besides, these reserves can affect larval survival, development and behaviour (Rossiter, Reference Rossiter1991a).
The lack of significant differences between the parental generations fed on balsam fir and black spruce does not concur with previous research, which has shown that larvae fed on black spruce foliage performed more poorly than those fed on other budworm host species (Blais, Reference Blais1957; Thomas, Reference Thomas1989). However, a similar phenomenon has been reported by Carisey & Bauce (Reference Carisey and Bauce2002). They found that the parental generation did not show significant differences between the two poorest artificial diets that were used, but its progeny was affected by them. Unfortunately, the parameters measured in the current study do not allow us to explain the cause of this pattern. Examination of the nutritional composition of eggs and feeding behaviour of the progeny may help us to explain the parental effects reported in this study. These results also show the importance of studying effects of food quality on at least two generations to fully understand the effect that this variable exerts on insect performance; otherwise, we could have concluded that black spruce is an optimal source of food for spruce budworm.
In conclusion, our results demonstrate that black spruce foliage is an inferior food for budworm. This is because of the negative effects that black spruce imposed on the progeny of those insects that had been reared on it. These effects are not apparent if only one generation is studied. It is necessary to incorporate this phenomenon into budworm control, particularly by adjusting predictive models accordingly. We have shown that, for budworm and doubtless for other lepidopteran forest pests, carry-over effects cannot be ignored.
Acknowledgements
We are grateful to R. Alfaro of the Pacific Forestry Centre (Canadian Forest Service) and S. Flores for reviewing an earlier version of this paper, and W.F.J. Parsons for checking the English. Financial support was provided to the iFor Research Consortium by the Natural Sciences and Engineering Research Council of Canada (NSERC-CRSNG), the Ministère des Ressources naturelles et de la Faune du Québec (MRNFQ), the Conseil de l'Industrie Forestière du Québec (CIFQ), the Canadian Forest Service, and the Société de Protection des Forêts contre les Insectes et les Maladies du Québec (SOPFIM). This work was also supported by a CRSNG-Kruger Inc. grant to Éric Bauce.








