Introduction
Among insecticidal transgenic crops, Bt-plants are the most widely used and have generated a rich inventory of studies on environmental side effects, as reported by many reviews (Groot & Dicke, Reference Groot and Dicke2002; Lövei & Arpaia, Reference Lövei and Arpaia2005; Andow et al., Reference Andow, Löwei and Arpaia2006; Romeis et al., Reference Romeis, Meissle and Bigler2006; Lövei et al., Reference Lövei, Andow and Arpaia2009). Transgenic plants provide economically attractive pest-control methods because they can be very effective against many target pest species (Fischhoff, Reference Fischhoff and Persley1996; Carozzi & Koziel, Reference Carozzi and Koziel1997). Before commercialization, transgenic plants need to undergo a thorough risk assessment analysis to detect potential harm to the environment. The environmental side effects include many complex aspects, including gene flow, the impact on non-target organisms (i.e. pollinators, soil indicators and other beneficial organisms) and resistance management.
The potential non-target effects of genetically modified (GM) crops are some of the more debated topics within applied biotechnologies in agriculture and environmental risk assessment. As indicated by some authors (Andow et al., Reference Andow, Löwei and Arpaia2006; Romeis et al., Reference Romeis, Meissle and Bigler2006), the evaluation of the effects of GM crops is a controversial topic. Besides problems concerning the selection of the proper bioindicators and methods to use for monitoring, meta-analysis and critical review of the studies remain very complex issues (Marvier et al., Reference Marvier, McCreedy, Regetz and Kareiva2007; Lövei et al., Reference Lövei, Andow and Arpaia2009; Shelton et al., Reference Shelton, Naranjo, Romeis, Hellmich, Wolt, Federici, Albajes, Bigler, Burgess, Dively, Gatehouse, Malone, Roush, Sears and Sehnal2009). A general methodology for risk assessment, concerning GM organisms, was suggested by the Italian Ministry of Environment with the aim of reviewing the main risk sources and to propose a monitoring programme within a risk evaluation plan (Sorlini et al., Reference Sorlini, Buiatti, Burgio, Cellini, Giovannelli, Lener, Massari, Perrino, Selva, Spagnoletti and Staiano2005). A detailed proposal for an ecotoxicological testing concept for GM crops that leads to a comparable number of tests as used for the testing of pesticides was also proposed (Hilbeck et al., Reference Hilbeck, Jänsch, Meier and Römbke2008). In particular, considering that environmental risk is a combination of likelihood of exposure and adverse effect, a basic pre-requisite for the selection of testing candidate organisms for risk assessment is to evaluate their exposure pathways (Hilbeck et al., Reference Hilbeck, Jänsch, Meier and Römbke2008).
Among the potential impacts, an important aspect is whether the transgene product can be transported in the phloem sap. Because aphids and other pests are obligatory phloem sap feeders, they represent a potential way of toxin exposure for beneficial insects, such as predators and parasitoids. Concerning the Bt-toxin uptake by non-target herbivore, many studies demonstrated that Bt-protein was not ingested by aphids which feed on phloem-sap from selected Bt-cultivars (Head et al., Reference Head, Brown, Groth and Duan2001; Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001; Dutton et al., Reference Dutton, Klein, Romeis and Bigler2002; Schuler et al., Reference Schuler, Clark, Clark, Poppy, Stewart and Denholm2005; Obrist et al., Reference Obrist, Dutton, Albajes and Bigler2006; Torres et al., Reference Torres, Ruberson and Adang2006; Lawo et al., Reference Lawo, Wäckers and Romeis2009); and, only in few cases, Bt-protein was detected in aphid samples (Zhang et al., Reference Zhang, Wan, Lövei, Liu and Guo2006; Burgio et al., Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007). Concerning the few cases of Bt-toxin uptake, most of the positive samples were collected in greenhouse studies and some authors hypothesized that Bt-proteins detected in these studies could be due to contamination of the samples by other herbivores or their faeces, which contain large amounts of this protein (Lawo et al., Reference Lawo, Wäckers and Romeis2009; Romeis & Meissle, in press). However, this hypothesis did not explain the positive samples of aphids feeding on transgenic oilseed rape collected in laboratory trials (Burgio et al., Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007).
The general aim of the research was a detailed study of the potential Bt-toxin uptake by the aphid species Myzus persicae Sulzer (Hemiptera: Aphididae) feeding on a transgenic Bt-oilseed rape to improve the knowledge on the possible transfer of the Bt-protein to phloem sap feeders. A specific aim was to replicate a previous experiment (Burgio et al., Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007) concerning Bt-toxin uptake, in order to avoid or minimize the risk of contamination, leading to potential false positives. For this reason, the study was carried out only in the laboratory, thus permitting to control and standardize the experimental conditions. Additionally, the toxin expression of the Bt-oilseed rape used in the experiment was also assessed, to better clarify the physiological aspects linked to the studied system. In the experiment, vernalized (V) and not-vernalized (not-V) plants were used to determine if this aspect of plant physiology would influence toxin expression.
Materials and methods
Transgenic Bt-oilseed rape and corresponding isogenic line
Transgenic oilseed rape plants (Brassica napus L. cv. ‘Westar’ lines GT 2–4), expressing a truncated and synthetic version of the cry1Ac gene from Bacillus thuringiensis var. kurstaki, were employed for the study. Transgenic oilseed rape expresses a toxin active against Lepidoptera under the constitutive CaMV 35S promoter (Harper et al., Reference Harper, Mabon, Leffel, Halfhill, Richards, Moyer and Stewart1999). The corresponding isogenic line was employed as control. These two genotypes will be referred throughout the manuscript as (Bt+) and (Bt−).
Plant growth
One set of (Bt+) and (Bt−) oilseed rape plants were grown singularly in plastic pots (h. 20 cm, d. 12 cm) containing a 2:1 (v:v) peat:sand sterile potting mix. Plants were placed in a growth chamber with a photoperiod of 12 h at the constant temperature of 24±2°C, 50±5% relative humidity (RH). Plants were fertilized and irrigated weekly. At the BBCH 34–36 growth stage (rosette with 4–6 fully expanded leaves) (Lancashire et al., Reference Lancashire, Bleiholder, Van dem Boom, Langeluddecke, Staugs, Weer and Witzenber1991), plants were transferred in another growth chamber at 5±1°C, 50±5% RH and 8 h of light for the vernalization process. After six weeks the (Bt+) and (Bt−) V plants were transferred to a growth chamber set at 24±2°C, 50±5% RH and 12 h of light and grown until the BBCH 36–38 growth stage (rosette with 6–8 fully expanded leaves).
The not-V plants were sown in single plastic pots and grown at 24±2°C, 50±5% RH and 12 h of light until the BBCH 36–38 growth stage.
Aphids fed on oilseed rape genotypes and Bt-toxin plant expression
A colony of M. persicae, maintained in laboratory according to Lanzoni et al. (Reference Lanzoni, Accinelli, Bazzocchi and Burgio2004) and Burgio et al. (Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007), was used for the experiments. Aphid populations were transferred on (Bt+) and (Bt−), V and not-V oilseed rape plants at the BBCH 36–38 growth-stage. After two weeks from aphid inoculation, three and six herbivore samples were collected from (Bt+) V and (Bt+) not-V plants, respectively. Actually, the vernalization process in laboratory conditions negatively affected plant growth leading to fewer and smaller leaves than not-V plants. For this reason, aphid populations reared on V-plants were lower in number and in such conditions only three samples were set up. Aphid samples were also collected, as negative checks, from both (Bt−) V and (Bt−) not-V plants. Great care was taken in collecting aphid samples to prevent contamination with plant material; aphids were brushed carefully onto a white plastic tray and then individually collected. Each sample, consisting of 200–400 adult aphids, was weighed and stored at –20°C until analyses.
To investigate the Bt-toxin plant expression, leaf samples were collected at four pre-determined time intervals (0, 5, 10 and 14 days after the transfer of aphid population on the plants). Sampling was stopped when V plants reached the pre-flowering growth stage, corresponding to about 14–15 days after the start of the experiment. At each sampling time, three leaf discs (of approximately 50 mg) from each of nine selected (Bt+) V, (Bt+) not-V, (Bt−) V and (Bt−) not-V oilseed rape plants were collected, weighted and stored at –20°C until analyses.
Cry1Ac toxin in herbivore and plant samples was extracted and quantified using a double sandwich Enzyme-Linked ImmunoSorbent Assay (ELISA) QuantiPlate kit Cry1Ab/Cry1Ac (EnviroLogix Inc. Portland, Maine) following manufacturer's instructions. Spectrophotometric measurements were conducted with a microtitre plate reader (LabsystemMultiscan, Dasit, Italy) at 405 nm. Bt-toxin concentrations were expressed in μg Cry1Ac kg−1 of fresh weight (FW). Based on μg kg−1 FW, the limit of detection (LOD) was 1.2. All samples were centrifuged for 5 min at 13,000 rpm before they were introduced into the ELISA plate.
Statistical analysis
The comparison of toxin concentration between treatments was performed by Student t-test for paired data. The time trend of Bt-toxin expression in V and not-V transgenic oilseed rape plants was studied by a linear correlation analysis.
Results and discussion
In V plants, a significant positive linear trend (R=0.80, P<0.01) between Bt-toxin content and sampling time was observed (fig. 1). In contrast, the linear correlation was not significant for not-V plants (P>0.05) (fig. 1). It is likely that the lack of significance for the linear trend in not-V plants was mainly due to high levels of variability of Bt-toxin expression within each time replicate. For each time replicate, the coefficient of variation ranged between 15.7 and 24.3% for not-V plants, while for V plants it was between 1.8 and 4%. The statistical analysis on overall data (pooling all the time replicates) showed a significantly (P<0.01) higher content of the Bt-toxin in V plants than in not-V plants (table 1). No false positives were detected in (Bt−) plants and in aphids reared on (Bt−) plants. Based on these experiments, we do not have a clear explanation of the observed differences between V and not-V plants; it is, however, plausible that the different growing conditions of oilseed rape plants affected the plant expression of Bt-toxin. Other authors have also observed that the toxin expression in Bt-crops can vary considerably, according to plant age and environmental factors (Dong & Li, Reference Dong and Li2007; Lawo et al., Reference Lawo, Wäckers and Romeis2009). In our experiment, the Bt-toxin expression in not-V plants showed high variability, in comparison with V plants whose expression was significantly more stable. Previously, changes in the Cry1Ac content in transgenic cotton leaves in response to temperature were reported (Olsen et al., Reference Olsen, Daly, Finnegan and Mahon2005). The expression patterns of the Cry1Ab gene were studied in the progenies derived from different Bt-transgenic japonica rice lines under field conditions: the Cry1Ab content in different tissues of transgenic rice varied individually as a function of the environmental temperature (Wu et al., Reference Wu, Cui, Ye, Xia, Sardana, Cheng, Li, Altosaar and Shu2002).

Fig. 1. Bt-toxin expression in vernalized (V) and not-vernalized (not-V) transgenic oilseed rape plants. R=0.80, P<0.01 for V plants; P>0.05 for not-V plants (linear correlation analysis) over 14 days. V plants: at the BBCH 34–36 growth stage kept at 5±1°C, 50±5% relative humidity (RH) and 8 h of light for six weeks and then at 24±2°C, 50±5% RH and 12 h of light until the BBCH 36–38 growth stage. Not-V plants: grown at 24±2°C, 50±5% RH and 12 h of light until the BBCH 36–38 growth stage (![]() , V; ∆, not-V).
, V; ∆, not-V).
Table 1. Plant expression of the Bt-toxin (μg Cry1Ac kg−1 of fresh weight) in vernalized (V) and not-vernalized (not-V) oilseed rape plants. For each time interval, mean and median values are calculated on 27 samples (three leaf disks for each plant).
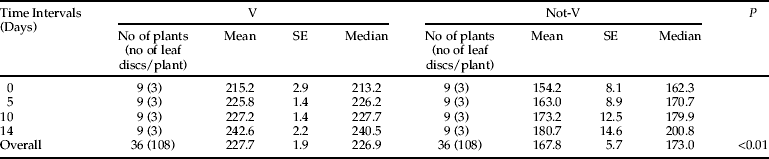
Mean concentrations of 227.7±1.9 and 167.8±5.7 μg kg−1 FW of Cry1Ac were found in (Bt+) oilseed rape leaves of V- and not-V plants, respectively (fig. 2). The Cry1Ac toxin was also detected in all three aphid samples reared on V plants and in three out of six aphid samples reared on not-V plants (fig. 3). There was a trend towards higher levels of Cry1Ac in positive aphid samples in the not-V (7.1±1.2 μg kg−1 FW) than in the V (4.8±0.6 μg kg−1 FW) plants, although no statistical differences were detected between these two treatments (P>0.05, Student t-test) (fig. 2). The mean Bt-toxin concentration of all the positive aphid samples was 5.9±1.0 μg kg−1 FW, whereas Bt-toxin of all the samples (including positive and negative samples reared on (Bt+) plants) was 4.0±1.2 μg kg−1 FW (fig. 2). As expected, none of the (Bt−) oilseed rape leaves or (Bt−) aphid samples contained any Bt-protein.

Fig. 2. Levels of Bt-toxin in transgenic oilseed rape leaf and Myzus persicae (aphid (+), positive samples; aphid (–), negative samples). V, vernalized plants; not-V, not vernalized plants.

Fig. 3. Levels of Bt-toxin based on ELISA test of the aphid samples reared on vernalized (V) and not vernalized (not-V) transgenic oilseed rape plants in laboratory conditions. The numbered codes indicate the samples.
Previous studies demonstrated that no or very little Cry1Ab toxin could be detected on Rhopalosiphum padi (Linnaeus) (Hemiptera: Aphididae) fed on Bt-maize (Head et al., Reference Head, Brown, Groth and Duan2001; Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001). In another laboratory study, ELISA tests showed that M. persicae did not ingest Cry1Ac when feeding on Bt-oilseed rape (B. napus), cv. Oscar (Schuler et al., Reference Schuler, Clark, Clark, Poppy, Stewart and Denholm2005). Concerning the Bt-toxin uptake by non-target herbivores, few studies have reported amounts of Bt-proteins in aphid samples reared on Bt-crops. Bt-toxin uptake in aphids was reported by Zhang et al. (Reference Zhang, Wan, Lövei, Liu and Guo2006) conducting ELISA analyses of aphids which previously had fed on Bt-cotton lines. The toxin was also detected in ladybirds preying on Bt-fed aphids, and its quantity increased as the predatory period extended. Burgio et al. (Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007) detected Cry1Ac protein in some of the aphid samples, reared on Bt-oilseed rape plants, in both greenhouse and climate chamber studies. In particular, the mean toxin concentration detected in positive aphid samples was 2.0±0.8 μg kg−1 FW. In addition, Burgio et al. (Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007) reported also the presence of Cry1Ac toxin in the phloem sap of Bt-oilseed rape plants, with a mean concentration of 2.7±1.5 μg kg−1 FW.
The present paper and our previous studies (Burgio et al., Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007) seem to show that a small fraction of Bt-toxin could be found in aphids. It is probable that this fraction is still significant enough, depending on how high the concentration in the plant material is, to cause chronic, long term effects in some non-target organisms, including predators that were shown to exhibit a certain sensitivity. Better investigations to support that inference ought to be carried out.
Considering that all positive samples except one of our previous study were collected in the greenhouse, some authors (Lawo et al., Reference Lawo, Wäckers and Romeis2009; Romeis & Meissle, Reference Romeis and Meisslein press) argued that the detection of Bt-proteins can be attributed to a contamination of the samples by other herbivores, such as spider mites or thrips, or their faeces, which contain large amounts of Bt-protein. Moreover, Romeis & Meissle (Reference Romeis and Meisslein press) reported that positive readings can be due to contamination with pollen that can be collected together with the aphids, stuck to their bodies. Herbivorous contamination can involve greenhouse or field samples, as reported by Lawo et al. (Reference Lawo, Wäckers and Romeis2009), but it is unlikely for laboratory experiments carried out in controlled conditions. Moreover, even though pollen contamination cannot be ruled out for aphid samples collected on V plants, this can be excluded for samples from not-V plants that did not bloom. Therefore, our conclusion was that aphids can ingest Bt-toxin from a variety of transgenic oilseed rape.
In the present research, the toxin expression in (Bt+) plants (mean concentration of toxin=227.7 μg kg−1 FW, in V plants and 167.8 μg kg−1 FW in not-V plants) was higher in comparison to that of (Bt+) plants of the previous cited study (mean concentration of toxin=64.3 μg kg−1 FW), using the same variety of Bt-oilseeed rape (Burgio et al., Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007). Also, the mean Bt-toxin concentration of positive aphids in the present experiment was higher in comparison with that found by Burgio et al. (Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007). This result may be attributed to the higher toxin expression of the plants used in the present research. Moreover, the present data seem to demonstrate that Bt-toxin expression in oilseed rape can also be affected by the vernalization process.
This study confirms that the transfer of Cry1Ac from (Bt+) plants to aphids may increase the release of this toxin in the environment. The reason for which only a part of the aphid samples were positive (six aphid samples out of nine in this study and only one out of eight samples in Burgio et al. (Reference Burgio, Lanzoni, Accinelli, Dinelli, Bonetti, Marotti and Ramilli2007)) is unknown. This dynamic could be affected by the aphid-plant interaction, plant expression or climatic factors, including laboratory conditions. Comparison of Bt-toxin expression levels is difficult because they vary with plant age and environmental factors (Dong & Li, Reference Dong and Li2007). Analogously, ELISA test results can differ among studies conducted with different methods and materials (Lawo et al., Reference Lawo, Wäckers and Romeis2009).
Some discrepancies exist on the potential effect of Bt-toxin against aphids. Field tests evidenced no short-term effects of Bt-maize on aphids, and laboratory tests showed the lack of direct toxic effects (Lozzia et al., Reference Lozzia, Furlanis, Manachini and Rigamonti1998). In contrast, four Bt-endotoxins obtained by recombinant microbial strains were found to exhibit low to moderate toxicity on the pea aphid, Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae), in terms of mortality and growth rate; in particular, Cry1Ab was essentially non-toxic except at high rates (Porcar et al., Reference Porcar, Grenier, Federici and Rahbé2009). Recently, in laboratory bioassays, it was investigated that the performance of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae), was affected by three Indian Bt-cotton varieties, expressing the Cry1Ac protein, and their corresponding non-transformed near isolines (Lawo et al., Reference Lawo, Wäckers and Romeis2009). ELISA analyses revealed that none of the aphid samples contained detectable Bt-protein and that plant transformation did not have any influence on aphid performance.
In any case, potential transfer of Bt-toxin from transgenic plants to herbivore populations should be taken into account to evaluate the possible impact of transgenic crops on non-target organisms, for example predators like coccinellids. In a laboratory study, Adalia bipunctata (L.) (Coleoptera: Coccinellidae) larvae fed with the Cry1Ab toxin exhibited significantly higher mortality than the control group (Schmidt et al., Reference Schmidt, Braun, Whitehouse and Hilbeck2009). In that experiment, Ephestia eggs sprayed with the respective treatment dilution of Cry1Ac were used as food. In another bioassay, for three predators (A. bipunctata, Atheta coriaria (Kraatz) (Coleoptera: Staphylinidae), Cryptolaemus mountrouzieri Mulsant (Coleoptera: Coccinellidae)), there was a lack of mortality associated with oral ingestion of microbial Cry1Ab and Cry3Aa using an artificial diet (Porcar et al., Reference Porcar, Grenier, Federici and Rahbé2009).
Multiple interactions among Bt-plants-herbivores-predators have been studied mostly in the maize agroecosystem. Exposure of ladybirds to Bt-toxins in Cry1Ab maize fields seems to be unlikely, because they feed mainly on aphids, which do not uptake the Bt-toxin from transgenic corn (Raps et al., Reference Raps, Kehr, Gugerli, Moar, Bigler and Hilbeck2001). Anyway, as remarked by many authors (e.g. Hodek & Honek, Reference Hodek and Honek1996; Triltsch, Reference Triltsch1999), coccinellids can consume pollen; by feeding on pollen of transgenic Bt-plants, larvae and adults are potentially exposed to transgene products expressed by the plants (Schmidt et al., Reference Schmidt, Braun, Whitehouse and Hilbeck2009). Bt-toxin can be transferred to predators, like Orius spp., Chrysoperla spp. and Stethorus sp., when Bt-maize pollen or spider mites were available, even if other predators contained no or negligible toxin levels even when pollen or spider mites were present (Obrist et al., Reference Obrist, Dutton, Albajes and Bigler2006). Moreover, consumption of alternative insect prey, which have ingested transgene products from plants, might also expose ladybirds to Bt-toxins (Schmidt et al., Reference Schmidt, Braun, Whitehouse and Hilbeck2009).
At present, few studies have focused on the potential accumulation of Bt-toxins in food webs, involving multiple and complex interaction among beneficial organisms. In a study, significant quantities of detectable Cry1Ab endotoxin were found within nontarget herbivores which feed on transgenic corn; furthermore, arthropod predators (Coccinellidae, Araneae and Nabidae) collected from these agroecosystems also contained significant quantities of Cry1Ab endotoxin, indicating its movement into higher trophic levels (Harwood et al., Reference Harwood, Wallin and Obrycki2005). In a similar approach, it was demonstrated that adults of three coccinellid species contained low, but detectable, quantities of Bt-toxin by means of ELISA analysis; in the study, the Bt-toxin in gut samples was not confined to the period around anthesis, suggesting tri-trophic linkages in their food chain (Harwood et al., Reference Harwood, Samson and Obrycki2007). By an analysis of the literature, some discrepancies have emerged, mainly due to different cultivars, trophic systems, methods and materials. For example, thrips collected from Bt-maize fields did not contain significant toxin levels either, even when collected directly from inflorescences during pollen shed (Obrist et al., Reference Obrist, Dutton, Albajes and Bigler2006). On the contrary, in another laboratory study, the toxin concentrations in larvae of a thrip species, Frankliniella tenuicornis (Uzel) (Thysanoptera: Thripidae), reared on Bt-maize plants were up to 50 times higher than those found in the field before pollen shed (Obrist et al., Reference Obrist, Klein, Dutton and Bigler2005); as remarked by the authors, this discrepancy could be due by the different cultivars used in the two studies.
For these reasons, there is the need of more standardization of experimental methods and criteria (Arpaia, Reference Arpaia2004; Romeis, Reference Romeis2004); and, in this direction, test methods and strategies have been proposed (Hilbeck et al., Reference Hilbeck, Jänsch, Meier and Römbke2008). As remarked also in other studies, future work should address more specific immuno-based bioassays (i.e. Western blot analysis) to demonstrate if the toxin remains active in the non target species.
Acknowledgements
This work was supported by the Ministero dell'Ambiente e della Tutela del Territorio (Direzione generale per la salvaguardia ambientale). The authors thank Metapontum Agrobios, Matera, Italy for kindly providing transgenic and isogenic oilseed rape seeds.






