Introduction
Larger or heavier insects usually have higher fecundity and fitness than their smaller or lighter conspecifics (Nylin & Gotthard, Reference Nylin and Gotthard1998). This is especially true for females, with larger individuals laying more eggs (Honěk, Reference Honěk1993). For males, larger individuals usually have better chances in male–male competition for females or have higher mobility and ability to find females (for review see Nylin & Gotthard, Reference Nylin and Gotthard1998). In social insects, like the honey bee (Apis mellifera), where the chance of reaching sexual maturity and survival of reproductive individuals until mating is additionally regulated by the colony members, the correlation between body mass and fitness of males might be less obvious.
In a honey bee colony, on average, thousands of drones and only a few queens are reared, creating a male-biased sex ratio (Page & Metcalf, Reference Page and Metcalf1984; Baer, Reference Baer2005). As a result, only a few drones will have a chance to mate. High mating competition between drones has been suggested to result in individuals investing in features enabling higher mating success. Natural selection should favour drones with high flight stamina, which is necessary to succeed during nuptial flight and mating. There is also some evidence that body mass and wing symmetry of drones positively correlate with mating success (Berg et al., Reference Berg, Koeniger, Koeniger and Fuchs1997; Jaffé & Moritz, Reference Jaffé and Moritz2010).
The body mass of drones changes with age (Gençer & Firatli, Reference Gençer and Firatli2005; Mazeed & Mohanny, Reference Mazeed and Mohanny2010), but the body mass of a drone at emergence depends mostly on the care and feeding received from nursing bees. Nursing bees care for drones from eclosion until emergence, adjusting the food provided relative to the age of larvae, feeding them with either royal jelly or honey enriched with pollen (Haydak, Reference Haydak1970; Hrassnigg & Crailsheim, Reference Hrassnigg and Crailsheim2005). Drones start consuming honey on their own about a week after emergence; however, they still require further feeding with a protein- and amino acid-rich diet by the nursing bees (Free, Reference Free1957; Haydak, Reference Haydak1970; Szolderits & Crailsheim, Reference Szolderits and Crailsheim1993; Schmickl & Crailsheim, Reference Schmickl and Crailsheim2004). In order to reproduce, drones need to reach sexual maturation, which occurs about 2 weeks after emergence (Rhodes et al., Reference Rhodes, Harden, Spooner-Hart, Anderson and Wheen2011). At this age, they start to perform mating flights (Witherell, Reference Witherell1971), and copulation occurs most often around their 3rd week of life (Couvillon et al., Reference Couvillon, Hughes, Perez-Sato, Martin, Roy and Ratnieks2010), therefore life span of drones after maturation and survival until successful copulation is important for the colony's reproductive output.
Honey bee colonies regulate the rearing and maintenance of drones depending on internal (colony size or queen presence) and externals conditions (season or food availability) (Free & Williams, Reference Free and Williams1975; Wharton et al., Reference Wharton, Dyer and Getty2008; Boes, Reference Boes2010). When food is scarce workers can evict drones from the hive (Page & Peng, Reference Page and Peng2001; Wharton et al., Reference Wharton, Dyer, Huang and Getty2007). In some cases, all drones are evicted, but eviction is most often selective with only a fraction of the drones being evicted. In the latter case, workers may bias acceptance and their interactions with accepted drones in a manner that could increase the colony's reproductive output and increase their inclusive fitness (Goins & Schneider, Reference Goins and Schneider2013).
To date, body size has been used as one of the most important predictors of drone mating success (Berg, Reference Berg1991; Berg et al., Reference Berg, Koeniger, Koeniger and Fuchs1997; Couvillon et al., Reference Couvillon, Hughes, Perez-Sato, Martin, Roy and Ratnieks2010). Body size is usually assessed using either the lengths of various drone body parts (Berg, Reference Berg1991; Berg et al., Reference Berg, Koeniger, Koeniger and Fuchs1997) or measured as body mass at certain life stages (Hrassnigg & Crailsheim, Reference Hrassnigg and Crailsheim2005). Larger and heavier drones are believed to be of higher quality, because they start their nuptial flights earlier (Rueppell et al., Reference Rueppell, Chandra, Pankiw, Fondrk, Beye, Hunt and Page2006a, Reference Rueppell, Page and Fondrkb) and have a greater chance of mating with queens (Koeniger et al., Reference Koeniger, Koeniger, Gries and Tingek2005; Couvillon et al., Reference Couvillon, Hughes, Perez-Sato, Martin, Roy and Ratnieks2010) and also produce more spermatozoa (Schlüns et al., Reference Schlüns, Schlüns, van Praagh and Moritz2003; Gençer & Firatli, Reference Gençer and Firatli2005; Taha & Alqarni, Reference Taha and Alqarni2013). Size and body mass of drones can change due to a number of conditions during their development. Drones that are undernourished (Czekońska et al., Reference Czekońska, Chuda-Mickiewicz and Samborski2015; Szentgyörgyi et al., Reference Szentgyörgyi, Czekońska and Tofilski2016), tended in suboptimal temperatures (Jaycox, Reference Jaycox1961), or infested with parasites (Duay et al., Reference Duay, De Jong and Engels2003; Retschnig et al., Reference Retschnig, Williams, Mehmann, Yañez, De Miranda and Neumann2014) during larval period are smaller and lighter at emergence; however, drones reared in queenless colonies are heavier than those reared in queenright colonies (Mazeed, Reference Mazeed2011). Drones reared in larger colonies (Free & Williams, Reference Free and Williams1975), colonies that more intensively hoard pollen (Rueppell et al., Reference Rueppell, Chandra, Pankiw, Fondrk, Beye, Hunt and Page2006a, Reference Rueppell, Page and Fondrkb) or have better nutritional conditions (Czekońska et al., Reference Czekońska, Chuda-Mickiewicz and Samborski2015) are also usually larger and heavier at emergence. Therefore, aside from potential genetic influences, drone body mass at emergence reflects the investment of nursing bees in rearing drone larvae (Crailsheim & Hrassnig, Reference Crailsheim and Hrassnig1998).
In addition to body size or mass, the symmetry of bilateral body parts has also been shown to vary according to conditions the drones are exposed to during development, and thus symmetry may also serve as a possible indicator of fitness (Palmer, Reference Palmer and Markow1994; Palmer & Strobeck, Reference Palmer, Strobeck and Polak2003). It is often assumed that bees developing under suboptimal conditions are more asymmetrical (Brückner, Reference Brückner1976; Abaga et al., Reference Abaga, Alibert, Dousset, Savadogo, Savadogo and Sedogo2011). Therefore, more asymmetrical individuals can be predicted to be of lower quality. Increasing deviations from perfect symmetry were also found to indicate the decreasing quality of drones (Jaffé & Moritz, Reference Jaffé and Moritz2010). Jaffé & Moritz (Reference Jaffé and Moritz2010) found that drones captured at drone congregation areas had more symmetrical wings than drones collected at their maternal colonies. They suggested that more symmetrical drones may have higher reproductive success and therefore better quality and fitness. Other studies revealed that wing asymmetry was not affected during development by malnutrition (Rueppell et al., Reference Rueppell, Fondrk and Page2006a, Reference Rueppell, Chandra, Pankiw, Fondrk, Beye, Hunt and Pageb; Szentgyörgyi et al., Reference Szentgyörgyi, Czekońska and Tofilski2016); however, in both cases, body mass was significantly smaller due to the food shortage indicating a decrease in drone quality.
Current beekeeping practices concentrate mainly on drone genetic properties. Evaluation of those properties is based on the colony from which they were obtained, not on the drones themselves. Properties of individual drones are largely neglected by beekeepers. In the case of instrumental insemination, only the age of drones is controlled and it is assumed that drones older than 12 days are mature (Rhodes et al., Reference Rhodes, Harden, Spooner-Hart, Anderson and Wheen2011). However, it is known that environmental conditions influence sexual maturation (Czekońska et al., Reference Czekońska, Chuda-Mickiewicz and Chorbiński2013, Reference Czekońska, Chuda-Mickiewicz and Samborski2015). Drones that are not mature do not provide semen or provide it in small quantities. Consequently, the number of drones required for insemination of one queen is larger (Czekońska et al., Reference Czekońska, Chuda-Mickiewicz and Chorbiński2013, Reference Czekońska, Chuda-Mickiewicz and Samborski2015; Czekońska et al., Reference Czekońska, Chuda-Mickiewicz and Samborski2015). It is sometimes suggested that drone quality should be considered in breeding programmes (Niño & Jasper, Reference Niño and Jasper2015). In particular, it was suggested that viability and motility of spermatozoa should be used as a selection criteria (Rhodes, Reference Rhodes and Barton2008), but the assessment of these parameters is relatively difficult. Drone mass at emergence is much easier to measure and can be used to select drones of higher quality.
The aim of our study was to assess drone life span after emergence in honey bee colonies. We correlated drone life span, which depends on the acceptance and maintenance of these individuals by the nursing bees, with body mass at emergence, wing size and the asymmetry of their forewings. We expected that larger and heavier drones with more symmetrical forewings would be maintained better and therefore survive longer after emergence in the colony and reach sexual maturity more often.
Materials and methods
This study was conducted between May and July in 2015 and 2016 in the experimental apiary at Garlica Murowana (lat: 50.140382, long: 19.930825) near Kraków, Poland. Fifteen unrelated colonies of A. mellifera carnica with 2-year-old queens were used. Experimental drones were obtained each year from two maternal colonies (A and B in 2015, C and D in 2016), altogether from four maternal colonies in two consecutive years. In 2015, the drones were maintained in two maternal colonies (A and B) and one foreign (host) colony (hereafter called foreign-maintaining colony 3) prepared for drone maintenance. In 2016, seven foreign colonies (foreign-maintaining colonies 4–10) were prepared for maintaining the drones originating from maternal colonies C and D. The colonies were kept in hives consisting of two hive bodies (Wielkopolski type). A queen excluder was placed between two hive bodies and each hive body contained ten combs. The level of Varroa destructor infestation was detected using standard methods (Dietemann et al., Reference Dietemann, Nazzi, Martin, Anderson, Locke, Delaplane, Wauquiez, Tannahill, Frey, Ziegelmann, Rosenkranz and Ellis2013). In the maternal colonies, the infestation level was approximately <4 mites/100 drone pupae and in the maintaining colonies, it was <2 mites/100 workers.
In maternal colonies, larvae were reared on drone combs built by the workers on beeswax drone comb foundations obtained from the same serial production of OSP, Kraków. The queens of each maternal colony were isolated on drone combs and released after 10 h. The drone combs with newly laid eggs were moved to the upper hive body above the queen excluder. One day before expected emergence, the combs with capped drone brood were placed in cages made of queen excluder and moved to incubators with a constant temperature set at 34.5°C. After the emergence of the first drone, the combs were inspected regularly every 2 h.
Drones were weighed after emergence using an analytical balance (RADWAG PS 210/C/2) with readability to 1 mg and individually marked using coloured and numbered tags. After marking within one day, these drones were collected in small wooden bee cages (8 × 10 × 5 cm) for transport to maintaining colonies from the laboratory to the apiary.
In 2015, drones that emerged from maternal colonies A and B were either returned back to their maternal colonies, or were introduced to the foreign-maternal colony (from colony A to colonies B and 3, or from colony B to colonies A and 3) for further fostering and maintenance (table 1) to assess if drone maintenance by a foreign colony affects their life span. With this arrangement, the drones from a maternal colony were maintained both in their own colony and in two different foreign colonies. Based on the 2015 results, in 2016, drones from maternal colonies C and D were introduced to the seven maintaining colonies for further fostering and maintenance until sexual maturity (table 1). Only in one foreign-maintaining colony, drones were originating from two maternal lines (C and D) while in the remaining six, drones were either coming from maternal colony C or D (table 1). No marked drones were maintained in their own maternal colonies C and D in 2016.
Table 1. Drone maintenance after emergence during two seasons.

Drones originated from four colonies (two in 2015 and two in 2016) and after weighing and marking were maintained in either their own or in a foreign-fostering colony after emergence, where life span was noted for all recovered drones in 2015 and until the 30th day of the drones’ lives in 2016. Survival rate to full maturation (more than 15 days of life) was also assessed.
The drones that were weighed and marked were maintained in cages (37 × 27 × 7 cm) made of plywood and queen excluder. Inside each cage, there was one comb partially filled with honey. The cage had a removable bottom made of plywood allowing quick removal of dead drones. The cages with marked drones were installed in the centre of the upper hive body (three combs were removed from the upper hive body to get empty space for the cage). In 2015, the cages were inspected every day until the last drone died to determine the maximum and the mean drone life span. In 2016, the cages were inspected every day for up to 30 days. During each inspection, all alive and dead drones were recorded. Drone survival and life span for the two sampling years were analysed separately, because different methodologies were used.
Drone life span was compared statistically in each maintaining colony from 2015 using a Mann–Whitney U test to check for possible differences between the maintenance of natal and foreign individuals in these colonies (colonies A and B) and the maintenance of two foreign groups of drones in the same colony (colony 3). In 2016, the maintenance of two foreign groups of drones (C and D) in the same colony was possible to be compared in one case (colony 8).
We also compared life span of drones originating from the two maternal colonies regardless of fostering conditions in both years using a Mann–Whitney U test. Life span depending on fostering conditions was compared using a Kruskal–Wallis test for multiple comparisons.
Differences in the body mass of drones originating from maternal colonies were analysed using one-way analysis of variance (ANOVA), followed by Tukey's test for uneven sample sizes. Body mass of all drones was correlated with their life span in each colony using Spearman correlation with a significance set at α < 0.005, based on Bonferroni's correction for ten repetitions.
The body mass of drones surviving or not until expected maturation (15 days) in both years was compared using one-way ANOVA for each maintaining colony with significance set at α < 0.005, based on a Bonferroni correction. We assumed 15 days as the time necessary for full sexual maturation of drones based on the study of Rhodes et al. (Reference Rhodes, Harden, Spooner-Hart, Anderson and Wheen2011).
In 2015, all dead drones were collected and whenever possible both their right and left forewings were collected and mounted on slide frames for morphometric measurements. Wing veins are fully developed at the time of bee emergence and do not change their structure during the bees life. Therefore, the age at wing collection does not affect their asymmetry measures. In some cases, drone wings were destroyed by workers attempting to remove their remains after death, so there is less data for wing size and shape than for life span. Altogether, 424 drones that had intact pair of wings from the three maintaining colonies (colony A: 126, colony B: 110, colony 3: 188 drones) were analysed. Wing images were obtained using a CMOS camera (UCMOS09000KPB, ToupTek Photonics) equipped with a 25 mm lens (FL-CC2514-2 M, Ricoh). The resolution of the images was 2400 pixel per inch (945 pixels per cm). The forewings were automatically measured three times using DrawWing software (Tofilski, Reference Tofilski2004). The three measurements were independent of each other and were used to assess measurement error (Palmer, Reference Palmer and Markow1994; Graham et al., Reference Graham, Raz, Hel-Or and Nevo2010). Nineteen landmarks were determined on each forewing as described in earlier studies (Szentgyörgyi et al., Reference Szentgyörgyi, Czekońska and Tofilski2016). The configurations of landmarks were aligned using Procrustes superimposition (Dryden & Mardia, Reference Dryden and Mardia1998) in MorphoJ software (Klingenberg, Reference Klingenberg2011). Centroid size (Dryden & Mardia, Reference Dryden and Mardia1998) was used as a measure of wing size. Shape was described by Procrustes coordinates of the landmarks scaled to the same size. Wing size asymmetry was measured as the absolute difference between the centroid sizes of the right and left forewings divided by the mean centroid size for each individual. Wing shape asymmetry was measured as the Procrustes distance (Procrustes FA score) between the shapes of the right and left wings. Centroid sizes and Procrustes FA scores were calculated using MorphoJ software (Klingenberg, Reference Klingenberg2011). Wing size and asymmetry of wing size and shape were compared between maternal lines using a one-way ANOVA. Next, all three parameters were correlated to body mass regardless of drone origin using Spearman correlation. Life span was correlated with all three wing measurements for each maintaining colony separately using Spearman correlation. All statistical analyses were performed using Statistica 12 software (StatSoft, 2014).
Results
Not all drones introduced to colonies could be assessed, because some lost their tags or their remains were removed from the cage by workers before inspection. In 2015, of 820 emerged drones, only 677 had their life span assessed, and in 2016, only 547 of 657 marked drones were assessed. Out of these, 404 died before terminating the experiment and 143 were still alive.
Life span did not differ between drones reared in their own colony and drones reared in an unrelated colony (colony A: U = 2228.5, P = 0.685; colony B: U = 3093.5, P = 0.836). In colony 3, which had drones from two maternal colonies (A and B), drone life span also did not differ between colonies from which they originated (U = 15,302.5, P = 0.078). The maximum life spans in the maternal colonies A, B and foreign-maintaining colony 3 were 15, 91 and 85 days, and the mean life spans (±SE) were 13.4 ± 1.5, 43.2 ± 22.9 and 19.3 ± 16.4 days, respectively (fig. 1a). In 2016, only one maintaining colony (colony 8) contained drones from two various queens (C and D) and also in this colony life span of drones did not differ between the two maternal lines (U = 888.0, P = 0.756).

Fig. 1. The survival of drone honey bees maintained after emergence in ten different colonies. In 2015 (a), drone survival was followed until the death of the last individual, and in 2016 (b), until the 30th day of the drone's life.
Drone life span differed slightly between maternal colonies A and B in 2015 (U = 51,271.0, P = 0.024), regardless of maintaining colony, but not between maternal colonies C and D in 2016 (U = 34,756.0, P = 0.192), but they differed markedly between maintaining colonies in both 2015 (H (2, 667) = 144.60, P < 0.001) and 2016 (H (6, 547) = 68.73, P < 0.001) (fig. 1a, b).
The mean body mass of drones originating from colonies A, B, C and D differed significantly (F (3, 1471) = 244.4, P < 0.001). In 2015, drones from both maternal colonies A and B were significantly heavier than drones from maternal colonies C and D in 2016. In addition, drones from colony A were significantly lighter than those from colony B, but the drones from colonies C and D did not differ in 2016 (table 2). Because drone life span varied markedly between foreign-maintaining colonies, the relationship between body mass and life span was analysed in each colony separately, regardless of drone origin. There was a positive correlation between life span and body mass of drones in all ten colonies (table 3), with five colonies retaining statistically significant correlations after the application of Bonferroni's correction (α < 0.005) (table 3).
Table 2. Mean (±SE), minimum and maximum body mass (mg) at emergence of drones originating from four different maternal colonies in two different years (A and B in 2015, C and D in 2016).

Statistically significant differences in body masses among maternal lines are indicated by different letters (a, b and c).
Table 3. Correlation of drones’ body mass at emergence with life span in ten honey bee colonies tested in 2015 and 2016.

Data are shown for drones with full life spans assessed before the termination of the experiment (max. 91 days in 2015, living not more than 30 days in 2016). The significance level after Bonferroni's correction was set at α < 0.005.
* Indicates statistically significant difference after applying Bonferroni's correction.
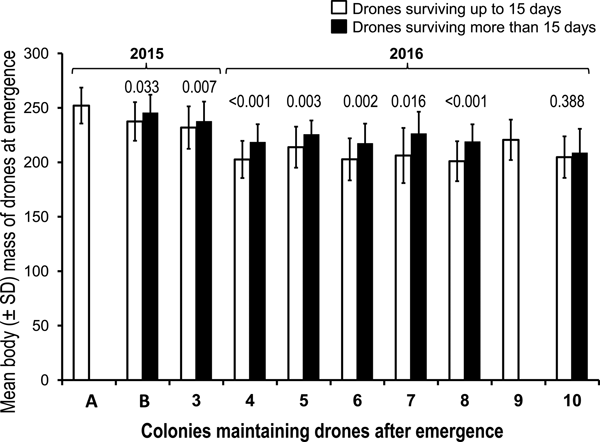
The comparison of body mass of drones reaching full maturity (surviving more than 15 days) or not reaching maturity showed that drones reaching maturity were heavier in all colonies (fig. 2). In four out of eight maintaining colonies, where drones survived more than 15 days, this difference was statistically significant after applying Bonferroni's correction (α < 0.005).

Fig. 2. Body mass at emergence of drones reaching maturity (surviving 15 days) or not in ten different maintaining colonies in 2015 and 2016. The values above each pair of columns are the P values of the one-way ANOVA comparison for each maintaining colony. After applying Bonferroni's correction, the significance level was set at α < 0.005.
Wing size (F (1, 404) = 0.058, P = 0.809), wing size asymmetry (F (1, 404) = 2.309, P = 0.129) and wing shape asymmetry (F (1, 404) = 1.166, P = 0.281) of drones originating from colonies A and B were similar. Drone wing size was positively correlated with body mass (r s = 0.369, P < 0.001), but there was no significant correlation between body mass and wing size asymmetry (r s = −0.045, P = 0.365) or wing shape asymmetry (r s = 0.027, P = 0.591). We did not find any correlation between life span and wing size in the maintaining colonies (colony A: r s = 0.146, P = 0.116; colony B: r s = −0.087, P = 0.408; colony 3: r s = 0.079, P = 0.287) or between life span and wing size asymmetry (colony A: r s = −0.048, P = 0.601; colony B: r s = 0.137, P = 0.194; maintaining colony 3: r s = 0.041, P = 0.577). In two colonies, there was also no significant correlation between life span and wing shape asymmetry (colony A: r s = 0.111, P = 0.233; maintaining colony 3: r s = 0.035, P = 0.635). However, in colony B, drones with greater wing shape asymmetry lived significantly longer (r s = 0.317, P = 0.002).
Discussion
Our results clearly demonstrated that drones that were heavier at emergence had a longer life span in the colony and also survived until maturation more often. There were marked differences in drone life span between the colonies, but within all of the colonies survival was positively correlated with body mass. Even in colonies where drone eviction resulted in losing all drones before expected full maturation (15th day of life), heavier individuals were still surviving longer. The results presented here suggest that a colony, when adjusting the number of maintained drones, first eliminates the drones that have a lower body mass at emergence and probably a lower quality and predicted fitness.
Variation in body mass of drones at emergence is the result of the interplay of genetic and environmental factors. One of the most important environmental factors affecting drone size and body mass is cell size. Most drones are reared in drone cells of 6.2–6.9 mm in diameter; however, in some situations, drones can also develop in smaller, worker cells of 5.2–5.7 mm in diameter (Berg, Reference Berg1991; Boes, Reference Boes2010). The size of the cell in which a drone larva develops markedly affects body mass at emergence (Berg et al., Reference Berg, Koeniger, Koeniger and Fuchs1997; Schlüns et al., Reference Schlüns, Schlüns, van Praagh and Moritz2003). Larger drones, reared in standard drone cells, usually weigh between 201 and 275 mg (Hrassnigg & Crailsheim, Reference Hrassnigg and Crailsheim2005; Mazeed & Mohanny, Reference Mazeed and Mohanny2010; Szentgyörgyi et al., Reference Szentgyörgyi, Czekońska and Tofilski2016), which is consistent with our results. Smaller drones, reared in worker cells, can weigh between 147 and 175 mg (Gençer & Firatli, Reference Gençer and Firatli2005; Couvillon et al., Reference Couvillon, Hughes, Perez-Sato, Martin, Roy and Ratnieks2010; Gençer & Kahya, Reference Gençer and Kahya2011). Here, the same standard drone comb foundation was used in all colonies, so the results were not affected by cell size. Differences in drone mass can also be the result of differences in rearing conditions including colony size, colony health, the number of larvae being reared by the colony (Boes, Reference Boes2010; Brodschneider & Crailsheim, Reference Brodschneider and Crailsheim2010; Czekońska et al., Reference Czekońska, Chuda-Mickiewicz and Samborski2015), mean temperature in the nest (Czekońska et al., Reference Czekońska, Chuda-Mickiewicz and Chorbiński2013), or available provisions (Hrassnigg & Crailsheim, Reference Hrassnigg and Crailsheim2005; Wharton et al., Reference Wharton, Dyer, Huang and Getty2007; Boes, Reference Boes2010; Brodschneider & Crailsheim, Reference Brodschneider and Crailsheim2010; Czekońska et al., Reference Czekońska, Chuda-Mickiewicz and Samborski2015; Szentgyörgyi et al., Reference Szentgyörgyi, Czekońska and Tofilski2016). These conditions are difficult to assess and could cause the observed statistically significant differences in body mass between seasons and also during 2015 in our study, although we made an effort to choose colonies under similar conditions. There can be also some genetic differences among colonies that might affect drone body mass e.g. via the amount of stored pollen and feeding regime (Rueppell et al., Reference Rueppell, Page and Fondrk2006a, Reference Rueppell, Chandra, Pankiw, Fondrk, Beye, Hunt and Pageb; Page et al., Reference Page, Fondrk and Rueppell2012).
Honey bee workers can evict drones when the expected cost of maintaining them outweighs the expected fitness benefits (Free, Reference Free1957; Wharton et al., Reference Wharton, Dyer and Getty2008; Boes, Reference Boes2010). As shown by our results, there is a considerable variation among colonies in acceptance and maintenance of drones. In some colonies, all drones were evicted before their sexual maturation, whereas other colonies allowed drones to mature and survive for up to 3 months after emergence. These results are in agreement with other studies conducted under more natural conditions without confinement (Fukuda & Ohtani, Reference Fukuda and Ohtani1977; Rueppell et al., Reference Rueppell, Fondrk and Page2005). It is also known that maintaining drones in a large laboratory cage did not affect their semen quality (Abdelkader et al., Reference Abdelkader, Kairo, Tchamitchian, Cousin, Senechal, Crauser, Le Conte, Belzunces, Barbouche and Brunet2014).
The observed differences in drone life span and survival were not dependent on the relatedness of drones to the bees maintaining them in fostering colonies. This lack of difference in maintenance of related and unrelated drones shows that nursing bees might not recognize their relatedness with freshly emerged drones, and the workers’ decisions on maintaining them in the colony are based on other factors. We suggest that body mass can be one of the factors determining drone acceptance and maintenance.
Although body mass was correlated with life span contrary to our expectations, wing size and asymmetry measures did not. Drones originating from the two different colonies did not differ in their wing size or wing size asymmetry and wing shape asymmetry measures, despite the significant difference in their body mass. This was a surprise, because here and in another study (Es'kov & Es'kova, Reference Es'kov and Es'kova2013), there were positive correlations between body mass and wing size. Possibly, the lower number of wing pairs collected during the experiment could affect the statistical significance of these comparisons.
Life span was mostly independent of wing size asymmetry and shape asymmetry measures, except in colony 2, where wing shape asymmetry was positively correlated with life span. This result was unexpected, and it is difficult to explain. The lack of negative correlation between symmetry measures and life span is contrary to prior work that showed mature drones with more symmetrical forewings seems to have a better chance of mating and probably higher fitness (Jaffé & Moritz, Reference Jaffé and Moritz2010). However, based on our results, more symmetric drones do not survive better until maturity, even if later in life more symmetrical drones reach drone congregation areas more often.
It is well known that the body size of mature drones determines their reproductive success and competitiveness during nuptial flights (Couvillon et al., Reference Couvillon, Hughes, Perez-Sato, Martin, Roy and Ratnieks2010). Our study showed that the body mass of drones at emergence can also be a good predictor of their future quality. In honey bee management practices, measuring body mass at emergence could be used as a non-invasive method predicting the quality of drones used later for both natural and artificial insemination of honey bee queens. Heavier drones will not only survive longer, but also produce more semen; therefore, they will compete more successfully with foreign drones.
Acknowledgements
The authors thank Bernadeta Rzeźnicka for her technical assistance. This study was supported by the Polish National Science Centre (NCN) grant number DEC-2013/10/E/NZ9/00682 and Polish Ministry of Science and Higher Education (MSHE) grant number DS-3506.