Introduction
Biting insects impose a number of costs on ungulate hosts, including blood loss, decreased feeding and resting time caused by disturbance and disease transmission (Mooring et al., Reference Mooring, Blumstein, Reisig, Osborne and Niemeyer2007).
Tabanids are free-living adult haematophagous flies that are major livestock pests due to their painful and persistent biting behaviour (Foil & Hogsette, Reference Foil and Hogsette1994; Mullen & Durden, Reference Mullen and Durden2002). One study in southern Louisiana (USA) recorded landing rates on horses of up to 1000 h−1 (Foil & Foil, Reference Foil and Foil1988). Moreover, tabanids are mechanical vectors of agents of animal disease, such as bovine besnoitiosis and equine infectious anaemia (Bigalke, Reference Bigalke1968; Foil et al., Reference Foil, Meek, Adams and Issel1983; Foil & Issel, Reference Foil and Issel1991), which are considered emerging diseases in Europe (Hans et al., Reference Hans, Poncon and Zientara2012; Alvarez-García et al., Reference Alvarez-García, Frey, Mora and Schares2013).
Cattle are highly exposed to tabanids in summer pastures, where larval habitats are independent of domestic livestock because wild animal blood sources are also available to maintain annual tabanid populations (Foil & Hogsette, Reference Foil and Hogsette1994). Mountains, where cattle pasturing in valleys may have been at the origin of the spread of the bovine besnoitiosis, an enzootic disease in the Pyrenees, to neighbouring regions (Jacquiet et al., Reference Jacquiet, Lienard and Franc2010). However, the biting behaviour of tabanids on cattle is poorly known in such mountainous areas. A better understanding of the alighting and feeding behaviour of tabanids on vertebrate hosts could lead to better intervention strategies and improved disease management (Muzari et al., Reference Muzari, Skerratt, Jones and Duran2010). For example, documenting alighting sites allows a more economical use of residual insecticides on hosts (Mohamed-Ahmed & Mihok, Reference Mohamed-Ahmed and Mihok2009). Although many observations have been conducted on various tabanid hosts, especially ungulates, in different parts of the world, most have been in plains (Mullens & Gerhardt, Reference Mullens and Gerhardt1979; Hollander & Wright, Reference Hollander and Wright1980; Lewis & Leprince, Reference Lewis and Leprince1981; Raymond & Rousseau, Reference Raymond and Rousseau1987; Phelps & Holloway, Reference Phelps and Holloway1990; Barros & Foil, Reference Barros and Foil2007; Mohamed-Ahmed & Mihok, Reference Mohamed-Ahmed and Mihok2009; Muzari et al., Reference Muzari, Skerratt, Jones and Duran2010). Previous studies have not been made on tabanid species in high-altitude summer pastures.
To reduce the pain, blood loss and other negative impacts of insect bites, ungulates use a varied arsenal of behaviours to repel or dislodge biting insects (Mooring et al., Reference Mooring, Blumstein, Reisig, Osborne and Niemeyer2007). Defensive reactions against biting flies include insect-repelling behaviour (e.g., tail flicking and leg stamping), evasive behaviour (e.g., moving to an area such as a forest or windy hilltop with less insects activity) and herding behaviour (gathering into groups to protect each other and to lessen insect's intensity per animal) (Raymond & Rousseau, Reference Raymond and Rousseau1987; Hart, Reference Hart1992; Mullens et al., Reference Mullens, Lii, Mao, Meyer, Peterson and Szijj2006). Defensive reactions are effective in removing flies and reducing the impact of insect bites (Mooring et al., Reference Mooring, Blumstein, Reisig, Osborne and Niemeyer2007); indeed, the rate of insect-repelling behaviour is negatively correlated with the feeding success of biting flies (Baylis, Reference Baylis1996; Torr & Mangwiro, Reference Torr and Mangwiro2000). Defensive behaviour to avoid tabanids has been observed in horses, moving together to an area usually exposed to the prevailing wind (Hughes et al., Reference Hughes, Duncan and Dawson1981), as well as in individual cattle responses, including head throwing, leg stamping and tail flicking (Raymond & Rousseau, Reference Raymond and Rousseau1987; Ralley et al., Reference Ralley, Galloway and Crow1993). These defensive reactions may play a major role in protecting cattle from tabanid bites, particularly in summer pastures, and the frequency of such reactions could perhaps be used as an index of tabanid abundance for herders.
As with other biting insects, the annoyance level of tabanids to livestock is closely related to the flies’ abundance in the field; large numbers of bites can directly reduce weight gain, milk yield and feeding efficiency in cattle, and can hide potential injury caused by the bites (Perich et al., Reference Perich, Wright and Lusby1986; Mullen & Durden, Reference Mullen and Durden2002). Moreover, the intensity of contact between hosts and vectors is key to the mechanical transmission of pathogens, as highlighted by a mathematical model developed by Desquesnes et al. (Reference Desquesnes, Biteau-Coroller, Bouyer, Dia and Foil2009). In a previous study of Pyrenean summer pastures, tabanid abundance appeared to be influenced by altitude and landscape structure (Baldacchino et al., Reference Baldacchino, Porciani, Bernard and Jay-Robert2013d ). Maximum abundance was observed between the end of June and the beginning of August, and the main species showed a peak of activity at midday. Several other studies have also shown the abundance and daily activity of tabanids to be related to landscape parameters (altitude, vegetation and presence of water bodies) or climatic conditions (temperature, humidity and wind) (Chvála et al., Reference Chvála, Lyneborg and Moucha1972; Sheppard & Wilson, Reference Sheppard and Wilson1977; Hackenberger et al., Reference Hackenberger, Jarić and Krčmar2009; Van Hennekeler et al., Reference Van Hennekeler, Jones, Skerratt, Muzari and Fitzpatrick2011). However, these findings were all based on field experiments using trapping. Although in most population surveys efficient traps are assumed to adequately reflect the actual abundance of flies, this approach generally requires comparisons with other sampling methods, such as direct counts of tabanids on cows (Thomas et al., Reference Thomas, Berry, Berkebile and Skoda1989; Gilles et al., Reference Gilles, David, Duvallet, De La Rocque and Tillard2007). Therefore, our study aimed to estimate the annoyance level of tabanids on cattle through entomological observations, and to test the influence of environmental variables on tabanids’ biting behaviour.
To identify tabanid annoyance of cattle in summer pastures, a herd of cows was followed for 6 days in the French eastern Pyrenees. Entomological and behavioural observations were conducted in parallel with insect trapping. Our objectives were (i) to observe the selection of feeding sites by tabanids depending on tribe, (ii) to study the cattle host's defensive reactions, (iii) to determine if tabanids collected in traps correspond to direct counts of landings on cows during the day and (iv) to assess the influence of meteorological conditions, altitude and landscape structure on tabanids alighting on cattle.
Materials and methods
Study site and cattle
The summer pasture where the study took place is located in a valley of the Mantet Nature Reserve (French eastern Pyrenees) on the northwest slope of the Costabona massif (2°18′E 42°28′N), at an altitude ranging from 1450 to 2700 m a.s.l. The climate is influenced both by moderate Atlantic conditions and orogenic continental conditions. It has a mean annual temperature of 9.5 °C and an annual rainfall of 855 mm (mean for Mantet village, 1545 m a.s.l.) (Mantet Nature Reserve data, unpublished). The geology of the area includes primarily metamorphic rocks from the Mantet-Fillos rift. The landscape is a complex mosaic of woodlands (primarily Pinus uncinata and Betula spp.), moorland (primarily Cytisus oromediterraneus), rocks, pastures and grasslands. Wetlands and aquatic habitats are poorly represented.
A total of 180 cows were present in the valley during the study period (in the summer of 2012). The study was conducted on a mixed-age and mixed-breed (Limousine and Aubrac) herd of 68 individuals (fig. 1). This herd was followed for 6 days (17, 18, 19, 20, 25 and 26 July) between 10:00 and 16:00. Its position was georeferenced every hour using a GPS TwoNav Sportiva (CompGPS Team SL, Barcelona, Spain). In addition, the temperature, relative humidity and wind speed were measured using a Kestrel 4500 pocket weather tracker (KestrelMeters.com, Birmingham, MI, USA). This meteorological data were recorded every 30 min (table 1).

Fig. 1. A Limousine cow and a Nzi trap.
Table 1. Ranges of environmental variables with their mean values over the period of the study (6 days).
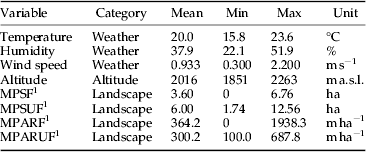
1 Mean patch size for forested patches (MPSF), mean patch size for unforested patches (MPSUF), mean perimeter-area ratio for forested patches (MPARF) and mean perimeter-area ratio for unforested patches (MPARUF).
Mapping
For the three periods of each study day (morning, noon and afternoon), a circle with a 200 m radius was overlaid on the herd position. Within each circle, polygon-shaped patches of homogeneous habitat were delineated according to the vegetation cover using aerial orthophotography dating from 2009 and classified into two land-cover classes, forested and unforested, using ArcMap 9.3 (ESRI Inc., California, USA) as in Baldacchino et al. (Reference Baldacchino, Porciani, Bernard and Jay-Robert2013d ). Forested patches (F) corresponded to conifer forests, and unforested (UF) patches corresponded to open areas such as grasslands, pastures, moors, bushes, rocky areas, water or mixed vegetation. Landscape metrics were calculated in each circle using the patch analyst extension for ArcGis 9.x. Forested and unforested patches were characterized by mean patch size (MPS, ha) and mean perimeter – area ratio (MPAR, m ha−1) as an indicator of the patch-shape complexity (table 1).
Tabanid landings and host defensive behaviour
The study was conducted over 6 days, and counts were carried out three times a day: morning (10:00–11:00), noon (12:30–13:30) and afternoon (15:00–16:00). For each time period, ten adult cows were chosen randomly in the herd and tabanid landings and host defensive reactions were counted on one side of the cow during 1 min.
Tabanid landings were estimated by counting each tabanid that landed on the visible side of the cow's body. To report the alighting sites on the cow, its body was divided into seven parts (head and neck, back, flank, belly, udder, forelegs and hind legs). Observations were made from a distance of about 3–5 m, using binoculars as needed. In these circumstances, female tabanids could not be easily identified to genus, and even less to species, level. As a consequence, they were allocated to tribe (Tabanini, Diachlorini or Haematopini) to avoid identification mistakes. One observer did all the counting over the 6 days.
In parallel, cow behaviour was recorded using a DCR–SR21E Digital Video Camera Recorder (Sony, Tokyo, Japan). Three insect-repelling behaviours were selected as in Mullens et al. (Reference Mullens, Lii, Mao, Meyer, Peterson and Szijj2006): tail flicking, leg stamping and head throwing.
Trapping
On the study days, two Nzi traps (Mihok, Reference Mihok2002) were set near the cattle between 10:00 and 16:00 (fig. 1). The traps were made from blue and black components (SuperMaine 300 g cotton/polyester 65/35, TDV industries, France) and polyester mosquito netting. Aged cow urine (50 ml) was used as an attractant and placed under each trap in plastic vials with a 2.5-cm aperture. The traps were collected every hour and moved as necessary according to the movements of the cattle. The tabanids were identified using Chvála's key (Reference Chvála, Lyneborg and Moucha1972).
Statistical analysis
For each tribe, the correlation between the daily catches of tabanids per trap and the daily mean landings per cow and per minute was tested using the Spearman's rank-order correlation coefficient.
The Kruskal–Wallis test and Bonferroni-corrected Mann–Whitney pairwise comparisons were used to test the effect of the period (morning, noon and afternoon) on tabanid landings as well as to compare landings on the different parts of a cow's body.
To describe the relationships between the host's defensive reactions (head throwing, leg stamping and tail flicking) and tabanid landings, linear regressions were conducted.
The effects of meteorological parameters (wind, temperature and humidity), altitude and landscape parameters (MPSF, MPSUF, MPARF and MPARUF: see acronym definitions in table 1) on tabanid landings were assessed using generalized linear mixed models (GLMM). GLMM were developed for tabanid landings according to the ‘best-practice’ data analysis of Bolker et al. (Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009). Because the response variable (landings per cow per minute) was count data, we used a Poisson model (log link). The mean number of landings per cow within a day or a period of the day was sometimes <5, so we fitted the GLMM using Laplace approximation. The position of the cattle at each observation period was considered a random effect. A day of observations represented pseudoreplication for each cow and was set as a continuous random effect in the model (Crawley, Reference Crawley2007; Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009).
Meteorological data, altitude, landscape variables and period of the day were fixed effects. All environmental variables were standardized before modelling, and correlations between the variables were tested using Pearson's correlation test. Altitude was highly correlated with MPSUF and MPARUF; MPSUF was highly correlated with MPSF and MPARUF (Pearson's coefficient r>0.7). The function lmer in the package lme4 from R was used to compute the GLMM (Bates et al., Reference Bates, Maechler and Bolker2012). Different models were built with the variable response defined as landings of all tabanids, landings of Tabanini, landings of Diachlorini or landings of Haematopini: (i) models with the interaction of meteorological data and the period of the day, (ii) models with altitude in addition to the fitted models with the meteorological data, (iii) and models with landscape parameters (except MPSUF) in addition to the fitted models with the meteorological data. The overdispersion of the models was estimated using the function overdisp_fun in R. The models were compared using the Akaike Information Criterion (AIC). All statistical analyses were performed using R (Team, 2013).
Results
Tabanid trapping and landings
The field experiments (trapping and observations) were carried out during sunny days. A total of 3921 tabanids (all females) belonging to 15 species in 6 genera (Tabanus, Hybomitra, Atylotus, Philipomyia, Dasyrhamphis and Haematopota) were collected over the study period (table 2). Tabanini was the most abundant tribe caught (65.3%) and the richest in species (11). The four main species were Tabanus bromius and Hybomitra spp. (H. montana, H. auripila and H. caucasica), representing 84% of the Tabanini caught. Diachlorini represented 28.2% of the catches, whereas Haematopini represented only 6.5% of the catches. Diachlorini and Haematopini were both dominated by one species, Philipomyia aprica and Haematopota pluvialis, respectively. Therefore, for these two tribes, tribe results can be interpreted as species results.
Table 2. Collected Tabanidae species per tribe and the number of individuals per species.

The mean (±SE) landing count was 4.3±0.3 per cow per minute, 49.2% of which were Tabanini, 28.1% Diachlorini and 22.7% Haematopini. For Diachlorini (P. aprica), the catches per trap were highly correlated with the landings (rs=0.88, P<0.05), unlike Tabanini (rs=0.20, P=0.44) and Haematopini (rs=0.30, P=0.54). Landing counts and trap catches for each day are illustrated for each tribe in fig. 2. Tabanids were active from 10:00 to 16:00. There were no significant differences in landings according to the period of the day except for Haematopini (Hc=17.95, P<0.001).

Fig. 2. Tabanid landings per cow per minute (mean±SE) and tabanid catches per trap (mean) recorded per period of the day for each tribe (Tabanini, Diachlorini and Haematopini). Differences between landings and period were tested using the Kruskal–Wallis test and Bonferroni-corrected Mann–Whitney pairwise comparisons (N=60). Significant differences (P≤0.01) are represented by different letters (a, b).
Alighting sites and host defensive reactions
Observations showed that Tabanini and Diachlorini landed preferentially on the forelegs (55.8 and 53.9%, respectively), the hind legs (19.2 and 8.6%, respectively) and the udder (16.8 and 31.7%, respectively) (fig. 3). In contrast, Haematopini were observed frequently on the hind legs (48.4%) and the flank (24.7%). Haematopota spp. were the only species that fed on the head (including the neck), and Tabanus sudeticus, the largest species observed (distinctly identified because of its size), was the only species that landed on the back. Overall, observations indicated that a cow is most frequently bitten by tabanids on the legs (67%) and the udder (20%).

Fig. 3. Percentages of landings of Tabanini (N=129), Diachlorini (N=97) and Haematopini (N=80) on the different body parts of a cow (cows without any landings were excluded from the count for N).
In parallel with tabanid landings, host defensive reactions were counted on one side of a cow during 1 min (fig. 4). Linear regressions were conducted to describe the relationships between defensive reactions and tabanid landings. The r 2 values were highly significant (P<0.001) for leg stamping (r 2=0.181) and tail flicking (r 2=0.115), but not significant for head throwing (r 2=0.009, P=0.113).

Fig. 4. Plots of the number of host defensive reactions (head throwing, leg stamping and tail flicking) vs. the number of tabanid landings and the linear regression with a 95% confidence interval for each plot. The r 2 values were highly significant (P<0.001) for leg stamping (r 2=0.181) and tail flicking (r 2=0.115), but not significant for head throwing (r 2=0.009, P=0.113).
Modelling tabanid landings in relation to environment variables
First, we modelled the landing of all tabanids (without distinguishing between the tribes) depending on meteorological data, including altitude or landscape parameters as variables or not. The model that included the interaction of meteorological data and the period of the day showed that increased tabanid landings were significantly associated with higher temperatures at noon and in the afternoon, lower wind speed at noon and lower humidity during the day. The model that included altitude showed that higher altitude caused a significant decrease in tabanid landings, as did higher temperatures in the morning. These two models were slightly overdispersed with a ratio equal to 1.20 and 1.25, respectively, and their AIC values were extremely close (415.5 and 413.8, respectively). The model that included landscape parameters showed no significant effect of size or shape complexity of forested and/or unforested patches on tabanid landings.
Next, we modelled the landings of each tribe; we observed different results according to the tribe. For Tabanini, landings were negatively associated with wind speed at noon, relative humidity, morning temperature and two periods of the day: noon and afternoon. For Diachlorini, landings were negatively associated with humidity at noon and wind speed in the morning and at noon. For Haematopini, landings were negatively associated with wind speed, and positively correlated with temperature and the period of the day at noon. Models that differentiated landings by tribe did not show any association with altitude or landscape parameters.
Discussion
Trapping efficiency and landings
In a 2011 study, a total of 1289 tabanids, belonging to 13 species, were collected at the same study site, using nine Nzi traps and nine Vavoua traps set along an elevation gradient during eight 48-h sessions (Baldacchino et al., Reference Baldacchino, Porciani, Bernard and Jay-Robert2013d ). In the 2012 study discussed in this paper, we collected three times as many tabanids, belonging to 15 species, with only two Nzi traps set close to a herd of cattle during 6 days. P. aprica (43%) and T. bromius (39%) were in similar proportions in 2012 whereas P. aprica (51%) was three times more abundant than T. bromius (16%) in 2011. Identifications of blood meals using PCR-assays conducted on specimens collected in 2011 showed that T. bromius fed mainly on cattle and that P. aprica fed mainly on red deer (Baldacchino et al., Reference Baldacchino, Gardes, De Stordeur, Jay-Robert and Garros2013b ). Therefore, it is consistent to catch more T. bromius in the vicinity of cattle. In the vast mountainous summer pastures, a herd of cattle appeared to be very attractive for horse flies, which use visual and olfactory cues to locate a host (Gibson & Torr, Reference Gibson and Torr1999; Horváth et al., Reference Horváth, Majer, Horváth, Szivak and Kriska2008, Reference Horváth, Blahó, Kriska, Hegedüs, Gerics, Farkas and Akesson2010). Our experiment's trapping design showed that visually attractive, baited traps can be very effective at catching tabanids in the vicinity of a herd. Setting more than two traps might even help to decrease tabanid populations. In Louisiana, horse fly populations on cattle were reduced by setting sticky traps baited with dry ice around a pasture where the animals were confined (Wilson, Reference Wilson1968). Yet no such practical experiment has been conducted since the 1970s despite the fact that trap attractiveness for tabanids has been improved by coloured fabrics and odorant baits (Mihok, Reference Mihok2002; Mihok et al., Reference Mihok, Carlson, Krafsur and Foil2006; Mihok & Mulye, Reference Mihok and Mulye2010; Mihok & Lange, Reference Mihok and Lange2012). New traps have also been designed based on the polarotaxis in tabanids (Blahó et al., Reference Blahó, Egri, Barta, Antoni, Kriska and Horváth2012; Egri et al., Reference Egri, Blahó, Szaz, Barta, Kriska, Antoni and Horváth2013a , Reference Egri, Blahó, Szaz, Kriska, Majer, Herczeg, Gyurkovszky, Farkas and Horváth b )
Comparisons between trapping and landings show that for Tabanini and Diachlorini, catches adequately correlate to the intensity of landings, but catches of Haematopini underestimated the number of landings (22.7% of the total landings vs. 6.5% of the total catches). This result is not surprising, as in 2011, Nzi traps appeared to be less effective for Haematopota sp. (Baldacchino et al., Reference Baldacchino, Porciani, Bernard and Jay-Robert2013d ). Nonetheless, Nzi traps can be a useful means of reducing tabanid biting of cattle because the majority of the annoyance is caused by Tabanini and Diachlorini.
Influence of meteorological parameters on landings
Meteorological parameters appeared to have a great influence on tabanid biting behaviour, especially in conjunction with the period of the day. In our models, wind speed and the temperature at noon were closely associated with the number of landings. This is consistent with other studies that show the peak activity of tabanids at midday in the Pyrenees (Baldacchino et al., Reference Baldacchino, Porciani, Bernard and Jay-Robert2013d ). Tabanids are haematophagous Diptera that are mostly diurnal, and their daily activity patterns have been observed to be related to meteorological conditions, such as wind velocity, temperature, relative humidity, evaporation, atmospheric pressure and sky radiation or cloud cover (Burnett & Hays, Reference Burnett and Hays1974; Dale & Axtell, Reference Dale and Axtell1975; Alverson & Noblet, Reference Alverson and Noblet1977; Van Hennekeler et al., Reference Van Hennekeler, Jones, Skerratt, Muzari and Fitzpatrick2011). In a study in Southeastern France, daily catches of T. bromius and Atylotus quadrifarius appeared to be positively correlated with temperature and/or negatively correlated with wind speed (Baldacchino et al., Reference Baldacchino, Cadier, Porciani, Buatois, Dormont and Jay-Robert2013a , Reference Baldacchino, Manon, Puech, Buatois, Dormont and Jay-Robert c ), as in our observations in the Pyrenees. More generally, temperature and wind speed have been shown through GLMM analysis to drive variation in the daytime aerial density and displacement speed of insects (Bell et al., Reference Bell, Aralimarad, Lim and Chapman2013). The relationship was positive for temperature and negative for wind speed. Low temperatures seem to limit the initiation of flight activity (Amano, Reference Amano1985), while high wind velocity inhibits flight activity, particularly when the wind exceeds the insect's maximum air speed. It also affects the airborne olfactory cues available to insects (Gibson & Torr, Reference Gibson and Torr1999).
Surprisingly, when altitude was included as an explicative variable in the model, tabanid landings were negatively associated with an increase of temperatures in the morning. This is likely to be explained by diurnal mountain winds, also known as thermally driven winds (Zardi & Whiteman, Reference Zardi, Whiteman and Chow2012). In the morning, winds flow upwards as the atmosphere warms up after sunrise. So the negative effect of temperature on tabanid landings in some circumstances may be explained by the influence of thermal mountain winds. Chvála (Reference Chvála1979) suggested that these winds were a decisive factor alongside temperature and relative humidity in explaining the daily activity of Tabanus sp. and P. aprica in the Caucasus.
Van Hennekeler et al. (Reference Van Hennekeler, Jones, Skerratt, Muzari and Fitzpatrick2011) also found that different species of horse fly respond differently to weather variables throughout the day. For instance, Tabanus pallipennis was most active at high temperatures in the late of the day where as Pseudotabanus silvester was most active when barometric pressure and humidity were low. In our study, we observed that tabanids of different tribes respond differently to weather variables. Medium-sized and large species (Tabanini and Diachlorini) were most active when wind speed and relative humidity were low, whereas smaller species (Haematopini) were most active when temperatures were high. The period of the day was also associated with landing frequency. For Haematopini, landings increased at noon; this finding is consistent with the daily activity of H. pluvialis, mostly active from midday to afternoon (Chvála et al., Reference Chvála, Lyneborg and Moucha1972). For Tabanini, landings at noon and in the afternoon were lower in comparison with landings in the morning, corresponding to a decrease in Tabanus sp. and Hybomitra sp. captures during the day.
Feeding site selection and host defensive reactions
In Pyrenean summer pastures, cows’ legs and udders appeared to be tabanids’ preferred feeding sites. Similar observations have been made on ungulates in other parts of the world. In French Guiana, Tabanus spp. landed most often on the legs of a cow (>90%) (Raymond & Rousseau, Reference Raymond and Rousseau1987). In Queensland (Australia), Tabanus spp. most often preferred landing on the legs of a horse (70–100%) (Muzari et al., Reference Muzari, Skerratt, Jones and Duran2010). In Louisiana (USA), tabanids fed preferentially on the anterior of a cow, and the number of tabanids on the front legs was a reflection of the overall annoyance for the cow (Hribar et al., Reference Hribar, Leprince and Foil1992). In comparison, the lower legs of a human were the preferred feeding sites for Tabanus spp. and Philipomyia graeca, whereas H. pluvialis preferred the head and neck (Krčmar & Marić, Reference Krčmar and Marić2006). In our study, Haematopota sp. were the only species that fed on the head and neck of cows, whereas T. sudeticus was the only species that fed on a cow's back. Studies of horse flies in the USA show that the smallest Tabanus spp. most often land on the legs, flanks or lower body, whereas large Tabanus spp. land almost exclusively on the back (Mohamed-Ahmed & Mihok, Reference Mohamed-Ahmed and Mihok2009). As tabanids appear to favour certain alighting sites, chemical treatments topically applied to the animal must be effective on these body regions.
Of the three host defensive movements observed, leg stamping is the most common response to tabanid landings. Several authors have made similar observations concerning defensive reactions of cattle against horse flies. In Sudan, a significant correlation was found between a cow's leg stamping and alighting frequency of tabanids (Mohamed-Ahmed & Mihok, Reference Mohamed-Ahmed and Mihok2009). In Canada, horse flies increased individual avoidance responses of cattle, and the amount of tail switching and leg stamping was significantly higher in a control herd compared to a herd treated with insecticide (cypermethrin) (Ralley et al., Reference Ralley, Galloway and Crow1993). In French Guiana, tail flicking seemed to be proportionate to the logarithm of the number of horse flies on a cow, but this relation was significantly dependent on the day of observation and on the cow (Raymond & Rousseau, Reference Raymond and Rousseau1987). However, although tail flicking and leg stamping are well correlated with tabanid landings, the relationship is not as close as might be expected. Indeed, host defensive reactions also depend on other flies, the excitability of the host and its habituation to pain (Raymond & Rousseau, Reference Raymond and Rousseau1987; Warnes & Finlayson, Reference Warnes and Finlayson1987; Mullens et al., Reference Mullens, Lii, Mao, Meyer, Peterson and Szijj2006). Excitability and habituation effects have been demonstrated in studies of behavioural responses of cattle to stable flies. Individuals that respond vigorously suffer less from insect bites than more placid individuals (Warnes & Finlayson, Reference Warnes and Finlayson1987). In contrast, habituation to the pain associated with fly biting results in decreased defensive reactions over time (Mullens et al., Reference Mullens, Lii, Mao, Meyer, Peterson and Szijj2006). Nonetheless, practically speaking, monitoring defensive reactions may be easier than monitoring tabanid landings, so in this way counting leg stamps could prove a useful tool for assessing the intensity of tabanid biting of cattle. This method could help in evaluating the annoyance they cause as well as testing the effectiveness of treatments.
Conclusion
The results of our study indicate that in high-altitude summer pastures, landscape structure is not associated with the biting activity of tabanids, although their distribution has been observed to be influenced by landscape variables in relation to breeding sites, host-seeking areas and resting sites (Baldacchino et al., Reference Baldacchino, Porciani, Bernard and Jay-Robert2013d ). This is likely to be explained by the high attractiveness of the herd and the great dispersal capacity of tabanids. On the other hand, their biting activity was strongly associated with weather variables, and altitude was also a factor in terms of how it affects the climate. It seems that it is difficult for animals to avoid the biting of tabanids during summer days, although host defensive reactions are quite effective at dislodging females and limiting their blood-feeding success. Our results suggest that an integrated pest management strategy should implement protective measures for livestock during the peak of horse fly abundance, in July and August, when the days are very hot and dry and there is little wind. Nzi traps set close to livestock were very effective in catching tabanids. Further investigation of the practical use of visually attractive traps such as these is warranted for reducing tabanid bites on cattle in pastures.
The supplementary material for this article can be found at http://www.journals.cambridge.org/BER
Acknowledgements
We would like to thank Claude Guisset of the Mantet Nature Reserve for his invaluable help in the field. We are also grateful to Aurélien Besnard (CEFE), Sophie Padie (CEFE), Sophie Monsarrat (CEFE) and Thomas Balenghien (CIRAD) for their useful contributions to the manuscript.








