Introduction
Climate changes have affected environmental conditions, acting predominantly on mean global temperatures, and may cause many socioeconomic impacts, such as worldwide production losses of important crops (Estay et al., Reference Estay, Lima and Labra2009). The temperature increase has a direct effect on plant fitness, besides favoring or preventing the occurrence of pest outbreaks. The geographical distribution, occurrence, development and fecundity of insects may be positively or negatively affected, depending on the temperature variations. In addition, temperature can indirectly affect insect performance by altering the availability of food resources or affecting the presence or lack of natural enemies (Bowler & Terblanche, Reference Bowler and Terblanche2008). Studies in the state of Rio Grande do Sul, Brazil show that a 1°C increase in the current mean temperature may shorten the life cycle of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae), leading to up to eight generations annually, which might increase its importance as a key-pest for corn crops (Afonso et al., Reference Afonso, Wrege, Martins and Nava2009). Milano et al. (Reference Milano, Berti-Filho, Parra and Cônsoli2008), studying the copulation activity of insects (A. gemmatalis and S. frugiperda), observed a negative effect on the frequency of copulation at the extreme temperatures of 15°C and 35°C, leading to a drastic reduction in reproductive activity. In spite of these findings, there is still a large demand for studies of temperature effects on many economically important pests and crops.
The velvetbean caterpillar (VBC) (Anticarsia gemmatalis Hübner, 1818 (Lepidoptera: Noctuidae)) is widely distributed in tropical and subtropical regions. In the larval stage, it has five to six instars, and this number can be influenced by variations in temperature and food quality. Mild temperatures can increase the number of instars in the VBC (Fugi et al., Reference Fugi, Lourenção and Parra2005). Generally, the entire biology of this insect (development of larval, pupal and adult stages, reproduction and oviposition) as well as feeding behavior are highly influenced by temperature (Magrini et al., Reference Magrini, Silveira Neto, Parra and Botelho1996; Fugi et al., Reference Fugi, Lourenção and Parra2005; Milano et al., Reference Milano, Berti-Filho, Parra and Cônsoli2008). Therefore, the objective of the present study was to evaluate the effect of different temperatures on biological traits (duration of larval, pupal and adult stages, longevity, viability of eggs and sex ratio) and growth (weight of pupa×time) of A. gemmatalis, for three consecutive generations, and, thus, produce useful information to analyze possible impacts of global warming on its occurrence in the future.
Material and methods
The experiments were carried out at the Entomology Laboratory of Embrapa Soybean, in Londrina, State of Paraná, Brazil, under controlled environmental conditions (fixed temperature, 80±10% RH, 14:10 h photoperiod (L:D)), using a fully randomized design, with the treatments arranged in a 5×3 factorial (five temperatures×three generations). The studied temperatures were 25°C, 28°C, 31°C, 34°C and 37±1°C.
Caterpillars were obtained from the lepidopteran mass rearing facility at Embrapa Soybean, where this colony had been kept for two years under controlled conditions (25±2°C, 80±10% RH, 14:10 h photoperiod (L:D)). One thousand newly emerged larvae from the same laboratory generation of this colony were split into the five studied temperatures, forming the generation F0 of the trial. Four replicates of 50 individual caterpillars were used for each temperature (totaling 200 caterpillars per temperature). These caterpillars were individually kept in 50-ml plastic cups sealed with cardboard caps, containing an artificial diet developed by Greene et al. (Reference Greene, Leppla and Dickerson1976) and modified by Hoffmann-Campo et al. (Reference Hoffmann-Campo, Oliveira and Moscardi1985). Daily observations were carried out to evaluate the changing and duration of each instar, as well as larval mortality. The diet was replaced three times per week. No-break batteries were provided to each chamber to avoid any lack of energy or equipment malfunctioning.
When caterpillars reached the pupal stage, they were separated by sex; and, soon after emergence, the adults were placed in 34 cm wide×34 cm deep×47 cm high acrylic cages (Magrini et al., Reference Magrini, Silveira Neto, Parra and Botelho1996) to allow wing expansion for flying and mating. These cages were lined with paper towels and contained a plastic container with adult food (10% honey solution). To maintain the minimum luminosity necessary for the insects to mate during the scotophase period, 15-watt light bulbs were kept on (Hoffmann-Campo et al., Reference Hoffmann-Campo, Oliveira and Moscardi1985; Magrini et al., Reference Magrini, Silveira Neto, Parra and Botelho1996). After 72 h, 18 pairs of the insects were taken randomly from the acrylic cages and placed in couples in 20 cm tall×10 cm diameter cages made from PVC tubes (Milano et al., Reference Milano, Berti-Filho, Parra and Cônsoli2008). These cages were sealed with plastic film at the top and placed in a petri dish lined with filter paper, containing a cotton ball soaked with food (10% honey solution). To allow oviposition and egg collection, the cages were lined internally with white paper.
For estimating egg viability and longevity of adults leading to the next generations, the individual couples were maintained at the same temperatures in which they had been reared during the three generations. The cages were inspected daily, to replace the food and record the mortality in each replicate. For assessment of egg viability, 100 eggs were collected for three consecutive days from the onset of egg-laying. The eggs were placed in plastic cups containing artificial diet and sealed with a plastic lid, where they remained until larval eclosion. The first-instar caterpillars were counted and used to compose the next generations (F1 and F2). Since the development time to complete the biological cycle was different at each temperature, the second (F1) as well as the third generation (F2) started at different dates in each temperature; however, it was standardized that the following generation always started with eggs of the second day of oviposition of the adults from the previous generation. Also, the same number of replicates per temperature (four replicates of 50 individualized caterpillars each) was kept at all three studied generations except when the generation was not evaluated.
The sex ratio was computed by dividing the number of females by the total number of individuals, and the rate of survival for each instar was obtained by dividing the number of surviving pupae by the total number of individuals of the previous instar. For growth assessment, 100 48-h pupae, also maintained in the same environmental conditions, were dried at 60°C for 72 h and then individually weighed (Piubelli et al., Reference Piubelli, Hoffmann-Campo, Moscardi, Miyakubo and Oliveira2005).
Statistical analysis of all variables was carried out in three steps. First, statistical tests of the residuals were used to confirm if the data showed all the assumptions for the analysis of variance (ANOVA), followed by Tukey and Tukey-Kramer tests for comparison of treatment means, at a 5% probability level. Last, the analysis of covariance (ANCOVA) was carried out to estimate the insects' growth, as proposed by Raubenheimer & Simpson (Reference Raubenheimer and Simpson1992) (SAS Institute, 2001). For the pupa weight analyses, development time was used as covariate since the time required to complete the development was different at each temperature which may have influenced the recorded pupa weight. Throughout the covariate analysis, it was possible to check if the differences in pupa weight were only due to temperature or if development time had any influence on this parameter.
Results
Significant statistical effects of generation and temperature were indicated by the ANOVA (P<0.001), as well as the interactive effect of generation versus temperature for the duration of instars and survival (table 1).
Table 1. Summary of the ANOVA (df, degrees of freedom; and F-values) for the duration of instars and survival rate of Anticarsia gemmatalis reared on artificial diet and subjected to different temperatures for three generations.

*** P<0.001.
At the F0 generation, the survival from the first to the fourth instars remained above 95% and no differences among the temperatures were observed (table 2). At the fifth larval instar and prepupal stages, this rate remained above 90% at temperatures up to 34°C, and decreased to 88.12% and 24.62%, respectively, at the highest tested temperature (37°C). At the pupal stage, the percentage survival gradually decreased from 94.87% at 25°C to 76.68% at 34°C. At 37°C, no insects reached full development.
Table 2. Survival (%) (mean±SE) of A. gemmatalis reared on artificial diet under different temperatures for three consecutive generations.

* Means followed by the same lowercase letter in the columns and by the same capital letter on the lines are not statistically different by the Tukey test at 5% probability;
** no survivors.
The percentage survival of the F1 generation was higher at 25°C, compared to 28°C, at the second and third instars, and development of the insects was only observed in these two temperatures. At the F2 generation, insect survival was higher than 95% and did not vary significantly at the two lowest temperatures (25°C and 28°C) (table 2).
When generations are compared within each temperature, a difference in survival percentage of caterpillars reared at 25°C occurred only at the fifth larval instar and the pupal stages. At these development stages, the highest survival percentage was observed in the F2 generation. At 28°C, until the fourth instar, the survival of caterpillars was lower in the F1 than in the subsequent generations. From the fifth instar on, no difference in survival of caterpillars was observed in all generations. In temperatures of 31°C and above, there were no survivors in the F1 and F2 generations (table 2).
The length of A. gemmatalis developmental phases also varied according to the temperatures as well as the generations (table 3). The time spent in the first instar was significantly higher in the F0 generation, at 25°C; at the F1 and F2 generations, there were no statistically significant differences between 25°C and 28°C, the temperatures in which survivors were observed. Comparing generations, the shortest time taken to complete the first larval instar was at 28°C, in the F0 generation.
Table 3. Time (in days) spent (mean±SE) in each larval instar, prepupal and pupal stage by A. gemmatalis reared on artificial diet under different temperatures for three consecutive generations.

* Means followed by the same lowercase letter in the columns and by the same capital letters on the lines are not statistically different by the Tukey test at 5% probability;
** no survivors.
At the second larval instar, in the F0 and F1 generations, there was no difference in the duration of instars at 25°C and 28°C (table 3). However, comparing F0 and F2, a reduction of the instar duration started at 31°C and 28°C, respectively. In the F2 generation, a longer development time was observed for larvae reared at 25°C.
At the F0 generation, the temperature of 25°C increased the time spent in all except the fourth instar (table 3). The shortest periods in the third instar occurred at 28°C (1.10 days), 31°C (1.14 days) and 34°C (1.17 days). Among the generations, there was a difference between 25°C and 28°C; the shortest third-instar periods were also observed in the F1 and F2 generations.
The fourth-instar durations in the F0 and F1 generations were similar in the temperature range and at 25°C and 28°C, respectively (table 3). Nonetheless, comparing within the same temperature, in the three generations, a longer fourth instar was observed in the F1 generation, at 28°C, which was 2.27 days. Comparing temperatures at the F0, an extended fifth instar occurred at 25°C (2.71 days), followed by 37°C (2.25 days) (table 3). However, when generations were compared, the slowest development time was observed in the F0 for caterpillars reared at 25°C and 28°C. At the prepupal phase of F0, there was no difference in the time necessary to complete this stage for those insects reared at 25°C and 28°C (table 3). These values, however, were higher than those observed for all other tested temperatures. When generations were compared at the two temperatures that allowed insect survival (25°C and 28°C), the differences were not statistically significant.
The mean time (days) for the pupal stage, within the F0 generation was inversely proportional to the increase in temperature, and the longest and shortest times were observed at 25°C (11.34) and 34°C (6.98), respectively (table 3). Larvae of A. gemmatalis maintained at 37°C did not complete the pupal stage. At 25°C, the pupal stage was shorter in the F1 and F2 than in the F0 generation, while at 28°C the duration of this phase remained practically constant in all generations.
The sex ratio was not affected significantly by increases in temperature in the three different generations. The sex ratio oscillated between the maximum of 0.56 at F0, 37°C and the minimum of 0.43 at F1, 25°C (table 4). Egg viability did not differ among generations at 25°C, although at 28°C these values increased along the generations. Statistically significant differences were observed in longevity of males among and within generations at 25°C and 28°C. In the F0 generation, the longevity of males was lower than in the remaining generations at 25°C. At 28°C, however, the inverse occurred, i.e. the F0 males lived longer and a gradual decrease in longevity in the F1 and F2 was observed (table 4).
Table 4. Sex ratio, adult longevity (days), and egg viability (%) (mean±SE*) of A. gemmatalis reared on artificial diet under different temperatures for three consecutive generations.

* Means followed by the same lowercase letters in the columns and by the same capital letters on the lines do not differ statistically by the Tukey test at 5% probability; ns, differences statistically non-significant;
** no survivors.
Differences in longevity of females were recorded within and among generations, and also between 25°C and 28°C. At 25°C, the longevity of females was similar in F0 and F1, but was lower in F2. However, comparing this parameter at the tested temperatures, differences between 25°C and 28°C were observed, with the shortest times observed for the F0 and F2 generations, respectively (table 4). At 28°C, a gradual reduction in female longevity occurred between the F0 and the F2 generations (table 4).
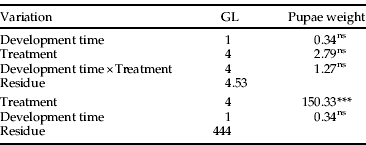
The ANCOVA indicated that the interactions among the covariates time of development and temperature were not significant regarding pupal weight (table 5). This relationship can be represented by the parallel-lines model, considering only the effects of temperature and, thus, validating the results obtained by the ANOVA. Increases in temperature negatively affected the weight of pupae in some of the treatments (fig. 1). At 25°C, 28°C and 31°C, no differences in pupal weight were observed. Nevertheless, at 34°C and 37°C, the mean weight of pupae decreased sharply, reaching 49.13 mg and 33.15 mg, respectively. The last value is approximately half the weight of pupae originated from caterpillars maintained at 25°C.

Fig. 1. Effect of five different temperatures on the weight (mg) of A. gemmatalis pupae (mean±SE).
Table 5. Covariance analyses (ANCOVA) used to study the effects of different temperatures in the pupa weight adjustment of A. gemmatalis using the development time (days) as covariable.
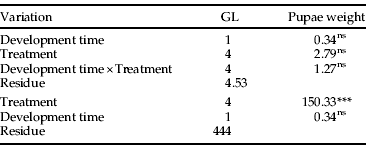
*** P<0.001; ns, non-significant.
Discussion
Through three successive generations and during the entire developmental cycle, the biological parameters of A. gemmatalis were negatively affected by temperature increases. In most cases, the thermal increase and developmental time were inversely correlated, mainly concerning survival and duration of the development stages of the insect, i.e. the higher the temperature, the lower the survival and duration of the larval instars.
Many researchers emphasize that the survival rate can be altered as a function of insect feeding on natural or artificial diet, or also on different hosts, at an ideal temperature, i.e. 25°C (Bortoli et al., Reference Bortoli, Takao, Silva and Brito2005; Santos et al., Reference Santos, Meneguim and Neves2005; Sá et al., Reference Sá, Fonseca, Boregas and Waquil2009) or in different temperatures, but have studied only a single generation (Magrini et al., Reference Magrini, Silveira Neto, Parra and Botelho1996; Milano et al., Reference Milano, Berti-Filho, Parra and Cônsoli2008). Several reports refer to different temperature effects in more than one generation. Here, this effect was evaluated in three successive generations, thus making it possible to observe that, even though the insects reached the pupal stage, or even adulthood, at temperatures above 31°C, the females did not oviposit or produced only infertile eggs.
During our study, the time taken in each instar and insect survival did not vary widely at the temperatures of 25°C and 28°C, which are considered appropriate for insect development. In the first generation (F0), at the temperature of 31°C, the insects were fertile and oviposited normally, but produced a large amount of infertile eggs and, thus, had few offspring. Furthermore, a drastic increase in temperature (above 31°C) impaired the development of A. gemmatalis at the immature stages. This phenomenon is, in fact, common for other insect species. Stenoma catenifer Walsingham (Lepidoptera: Elachistidae) also did not complete its larval development in temperatures above 32°C (Nava et al., Reference Nava, Haddad and Parra2005); and, at 30°C, the larval viability decreased, with only 43% emergence.
The percentage survival until the fifth instar was higher than 90% in the range of 25°C to 34°C. However, mortality increased in the prepupal and pupal stages, likely because of the high moisture content that larvae and pupae require in these stages. Several morphological and behavioral alterations, including cocoon secretion, lack of larvae feeding and movement, were also observed. Insects react to thermal changes by inducing alterations in the metabolism, which vary from species to species. A peculiar behavior was observed in A. gemmatalis at the uppermost temperatures (31°C and 34°C). The larvae remained most of the time on the food, without moving, possibly because of changes in their metabolism and/or as an attempt to save energy. Subsequently, generally, dehydration and death of individuals occurred.
The larval period of A. gemmatalis was reduced by approximately ten days, when the temperature increased from 25°C to 34°C. Magrini et al. (Reference Magrini, Silveira Neto, Parra and Botelho1996), in studies with one generation of the same species, reported that reductions in the larva-adult period occurred in the range from 24°C to 33°C. Hochachka & Sommero (Reference Hochachka, Sommero, Hochachka and Sommero1984) found that changes in temperature affect the enzyme substrate as compensation and, as a consequence, speed up the metabolism, which also affects the development of insects. Food consumption by the insects was not evaluated in this experiment; but likely, with the shortening of the cycle, the insects were not able to supply their nutritional needs. This suggestion is supported by the much lower weight of pupae in the highest temperatures.
Although characterization of the prepupal stage is difficult for some insects (Specht et al., Reference Specht, Formentini and Corseuil2006), in this experiment it was easy to detect when A. gemmatalis at the prepupal stage stops feeding, digs a cavity in the artificial diet, where it remains immobile, closing this space with silk threads mixed with feces, forming a cocoon. Thus, it was observed that the duration of prepupal stage was longest at 25°C and shortest at 37°C. For another lepidopteran species, Spodoptera cosmioides Walk., shortening of the prepupal phase was also reported when the temperature increased from 25°C to 32°C (Bavaresco et al., Reference Bavaresco, Garcia, Grützmacher, Foresti and Ringenberg2002).
At the highest temperature studied (37°C), the weight of pupae was lower than at the remaining temperatures tested, and adults did not emerge. The time (days) spent in the pupal stage, without distinction between males and females, was inversely proportional to the increase in the temperature. When A. gemmatalis caterpillars were maintained under different temperatures, Magrini et al. (Reference Magrini, Silveira Neto, Parra and Botelho1996) also observed a decrease in the length of the pupa stage of approximately six and seven days for females and males, respectively, when the temperature rose from 24°C to 33°C. In our experiments, the pupae were sexed and maintained individually in the same conditions until emergence of adults, and thus it was possible to observe the ‘protogeny’ (Crocomo & Parra, Reference Crocomo and Parra1985), i.e. the emergence of the females before the males. This phenomenon is presumed to be a mechanism to reduce the probability of mating between individuals from the same egg mass, allowing the females to emerge before the males and fly to other sites or, if they remain at the same site, they can only mate with males born from a different egg mass.
The longevity of A. gemmatalis adults varied as a function of temperature, but may also vary with type of feeding and mating behavior. This is an important aspect and it may impact adult mating. According to Milano et al. (Reference Milano, Berti-Filho, Parra and Cônsoli2008), the lower the female longevity, the higher are the number of copulations. Feeding on an artificial diet and in five different temperatures, the longevity of A. gemmatalis varied from 11.2 days (32.2°C) to 24.8 days (21.1°C) (Moscardi et al., Reference Moscardi, Barfield and Allen1981). In the present study, the longevity of adults in the first generation (F0) was higher at 28°C, for both males and females, but decreased in the subsequent generations (F1 and F2), in agreement with the results obtained by Moscardi et al. (Reference Moscardi, Barfield and Allen1981) and Magrini et al. (Reference Magrini, Silveira Neto, Parra and Botelho1996). At an ideal temperature, i.e. 25°C, both males and females in the F1 and F2 generations showed higher longevity and a trend for females to live longer.
The temperature of 25°C is the most appropriate for A. gemmatalis oviposition (Milano et al., Reference Milano, Berti-Filho, Parra and Cônsoli2008). Here, we recorded a reduction in the viability of A. gemmatalis eggs with increasing temperature, from approximately 11% at 25°C to 32% at 28°C. This reduction is possibly related to the lower copulation activity under high temperatures (Milano et al., Reference Milano, Berti-Filho, Parra and Cônsoli2008), which may impair egg fertilization. At 28°C, the viability of eggs was low during the first generation (F0), but increased in the next generations (F1 and F2), indicating that the species possibly experienced a process of adaptation to this small increase in temperature. Busato et al. (Reference Busato, Grützmacher, Garcia, Giolo, Zotti and Stefanello Júnior2005) observed that the highest viability of eggs of S. frugiperda also occurred at 25°C.
The sex ratio of pupae (table 4) was not affected by the increase in temperature. On the other hand, negative effects of temperatures lower than those studied here were observed by other authors for different insect species. According to Pereira & Berti-Filho (Reference Pereira and Berti-Filho2009), a higher number of females at 18°C and males at 30°C was observed, for example, in populations of Cerconota anonella Sepp (Lepidoptera: Oecophordae).
The weight of pupae (fig. 1) varied according to the treatments. Again, the highest dry weight of pupae was recorded at the ideal temperature for A. gemmatalis development, i.e. 25°C. The smallest pupal weight occurred at 37°C. Up to 31°C, however, there were no differences from the lower temperatures. If this result were analyzed for only one generation, 31°C would appear to be the limiting temperature for A. gemmatalis development. However, it is necessary to consider that no fertile eggs were produced at this temperature. In general, the variation in pupal weight is a non-adaptive response, due to unfavorable environmental conditions; however, if we consider the adverse environmental conditions to which insects may be exposed, this reduction in weight and body size may be advantageous in certain areas where the thermal regime is more rigorous (Slansky, Reference Slansky, Stamp and Casey1993; Scriber, Reference Scriber1996; Nylin & Gotthard, Reference Nylin and Gotthard1998).
The data obtained in our study allow us to conclude that global warming may cause negative impacts on A. gemmatalis populations, reducing their occurrence and consequently diminishing the problems with this species on soybeans. However, mild climate changes, such as from 25°C to 28°C, allow the insects to adapt to regions with small environmental alterations, making possible a shortening of their developmental cycle and, thus, increasing the incidence of pests, such as A. gemmatalis, in soybean crops. Moreover, it is important to consider that global warming might turn cold areas (where the low temperature is nowadays a abiotic mortality factor of A. gemmatalis) more suitable to A. gemmatalis outbreaks. Therefore, more than a future reduction of A. gemmatalis occurrence due to global warming, we might expect changes regarding its area of occurrence on a global landscape perspective. It is also important to point out that, in this study, the A. gemmatalis populations used were from a long-term laboratory rearing program, and although periodic introductions of new stock have been carried out, additional field research is necessary. Under natural conditions, the species undergoes oscillations in diurnal and nocturnal temperatures and the genetic variability of the insects is high, allowing rapid adaptation along the generations.
Acknowledgements
The authors thank Embrapa for the supporting this research. Thanks are also due to ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)’ for the supporting grant (process 23038.035744/2008-89) and to Janet W. Reid, PhD, JWR Associates, for the English revision. This paper was approved for publication by the Editorial Board of Embrapa Soja as Manuscript number 03/2011.