Introduction
Sugarcane (Saccharum officinarum) (Poaceae) is an important agricultural crop worldwide, with Brazil as the largest producer and exporter of sugarcane products (Maga et al., Reference Maga, Thonemann, Hiebel, Sebastião, Lopes, Fonseca and Gírio2019). In Brazil, the highest sugarcane production is concentrated in São Paulo state (CONAB, 2020). The importance of this crop is mainly related to two factors, the profitable products from sugarcane, such as sugar and ethanol, and the sustainability of the crop (Brinkman et al., Reference Brinkman, da Cunha, Heijnen, Wicke, Guilhoto, Walter, Faaij and van der Hilst2018).
This sustainability is supported by sustainable practices, such as, according to Brazilian Federal Laws 4771/65 and 9605/98. Sugarcane plantations must guarantee at least 20% of forest area with native trees. As well, it is prohibited to dispose of vinasse (product from ethanol) into rivers. This vinasse is used in the fertigation of the sugarcane crop itself (Goldemberg et al., Reference Goldemberg, Coelho and Guardabassi2008). Other factors are related to the lower use of irrigation, as the sugarcane crops is mainly irrigated by rain (Matioli, Reference Matioli1998), and the reduction of pollutant emissions (carbon), through the mechanical harvesting of green cane (Cerri, Reference Cerri2007).
Saccharicoccus sacchari (Cockerell) (Pseudococcidae) is known as the pink sugarcane mealybug owing to its coloration. Rae (Reference Rae1993) indicated that its biological cycle consists of four nymph stages and one adult stage. In contrast, Girón et al. (Reference Girón, Lastra, Gómez and Mesa2005) described two nymph stages and one adult stage. Until now, records indicate that this mealybug species infests only host plants belonging to the families Arecaceae and Poaceae (García Morales et al., Reference García Morales, Denno, Miller, Miller, Ben-Dov and Hardy2016). Cruz et al. (Reference Cruz, Peronti, Martinelli, Costa, Ignácio and Almeida2019) reported the mealybug S. sacchari as an emerging pest of sugarcane, with a notorious appearance in São Paulo state.
Mealybug species occur in sugarcane crops of all producing countries (Box, Reference Box1953; Salama and Rizk, Reference Salama and Rizk1969; Inkerman et al., Reference Inkerman, Ashbol, Carver and Williams1986), including Brazil (Borges et al., Reference Borges Filho, Sturza, Nava, Guedes, Silva, Montero and Santos2016). The increase in the population of S. sacchari in sugarcane areas in Brazil is related to the total prohibition of sugarcane burning in accordance with State Federal Law No. 11.241 from 09/19/2002. Previous recommendations for the control of S. sacchari have involved the use of fire in infested areas (Gallo et al., Reference Gallo, Nakano, Silveira Neto, Carvalho, Batista, Berti Filho, Parra, Zucchi, Alves, Vendramin, Marchini, Lopes and Omoto2002). Considering the current management of the crop in not burning the sugarcane, it results in the abrupt jump of occurrence of this mealybug.
S. sacchari injures host plants by phloem-feeding and honeydew production (Abd-Rabou, Reference Abd-Rabou2007). Infestation occurs both on the stem and roots (Beardsley, Reference Beardsley1959), and are more aggressive severe when associated with infestations of the sugarcane borer Diatraea saccharalis (Crambidae) (Alam, Reference Alam1972). Heavy infestation causes the death of the shoots and atrophy and yellowing of the plant, consequently, reducing its growth (Abd-Rabou, Reference Abd-Rabou2007). Furthermore, it significantly reduces sugar yield (Willcocks, Reference Willcocks1925). In addition, viruses can be associated with this mealybug species (Cooper and Zhang, Reference Cooper and Zhang1992; Franke et al., Reference Franke, Fegan, Hayward, Leonard, Stackebrandt and Sly1999), including the sugarcane bacilliform virus and sugarcane mild mosaic virus (Lockhart et al., Reference Lockhart, Autrey and Comstock1992). In sugarcane fields in southern Brazil, infestation occurs mainly in the hot season of the year, when 80–90% of the plants are attacked (Borges et al., Reference Borges Filho, Sturza, Nava, Guedes, Silva, Montero and Santos2016).
In Sri Lanka, it is stated that effective control can be achieved through biological control and cultural practices (Rajendra, Reference Rajendra1974). The adoption of biological control of pests in sugarcane (Botelho et al., Reference Botelho, Parra, Chagas Neto and Oliveira1999; Van Lenteren and Bueno, Reference Van Lenteren and Bueno2003; Parra et al., Reference Parra, Botelho, Pinto and Cortez2014) certainly contributes to improvement of the environment (soil, water, and air) and contributes to sustainability of sugarcane farming (Shukla et al., Reference Shukla, Solomon, Sharma, Jaiswal, Pathak and Singh2019). In some sugarcane-producing countries, the parasitoid Anagyrus saccharicola Timberlake (Encyrtidae) has been evaluated for biological control of S. sacchari: Australia (Carver et al., Reference Carver, Inkerman and Ashbolt1987) and Egypt (Abd-Rabou, Reference Abd-Rabou2002).
In Brazil, the first record of A. saccharicola parasitizing S. sacchari in the field was reported by Cruz et al. (Reference Cruz, Peronti, Martinelli, Costa, Ignácio and Almeida2019). A. saccharicola has an uncertain origin, but probably comes from Malaysia (Timberlake, Reference Timberlake1932). Species of the genus Anagyrus are considered to be significant biological control agents (Noyes, Reference Noyes2000), with through their parasitization of mealybugs in the family Pseudococcidae (Gibson et al., Reference Gibson, Huber and Woolley1997). When parasitized, the host is stated to survive for 3 days, and on the 5th day, it is completely mummified – based on study in Barbados, West Indies (Alam, Reference Alam1972).
However, there is little information to date about this parasitoid and the pink sugarcane mealybug in Brazil, including survival of A. saccharicola, host preference, and parasitism rate, all of which are fundamental to understanding the role of the parasitoid in the biological control of the pink sugarcane mealybug. Such information will facilitate mass rearing of the parasitoid in the laboratory, which is a critical step in developing the biological control program (Parra et al., Reference Parra, Botelho, Corrêa-Ferreira and Bento2002; Van Lenteren, Reference Van Lenteren2000).
In this context, this study aimed to (1) evaluate the survival of females and males of A. saccharicola at three constant temperatures; (2) determine the host preference of the parasitoid and longevity of the pink sugarcane mealybug; and (3) estimate the parasitism rate based on feeding, mating, and time factors.
Materials and methods
Saccharicoccus sacchari colony
The study was undertaken at the Hemiptera Biosystematics Laboratory of the Agricultural Production Sciences Department, School of Agricultural and Veterinary Sciences, São Paulo State University (FCAV/UNESP), Jaboticabal, São Paulo, Brazil. S. sacchari was collected from the sugarcane variety RB867515, one of the most cultivated in the state of São Paulo (Barbosa et al., Reference Barbosa, Resende, Dias, Barbosa, Oliveira, Peternelli and Daros2012), in the municipality of Jaboticabal (21°14′52.8″S, 48°17′01.2″W), and taken for rearing to the laboratory. The mealybugs were removed from the stems with a fine brush. They were inspected for the presence of parasitism during a 5-day quarantine period, checking for the appearance of mummification by the expected change in the pink color. The non-parasitized mealybugs were transferred to new sugarcane shoots. For the preparation of the slips, the plant was taken to the screening room. The leaves were removed from the base of the leaf sheath with a machete and cut in such a way that two buds of approximately 25 cm in length remained. These were washed under running water with detergent, sterilized with 70% ethanol, dried with paper towels, and exposed to the natural environment until totally dried. To delay water loss from the shoots, the ends were sealed with liquid paraffin, as described by Beardsley (Reference Beardsley1962). After infesting the shoots with mealybugs, they were held at a temperature of 25 ± 1°C, 70 ± 10% relative humidity (RH), and 14 h photophase, until their use in bioassays.
Anagyrus saccharicola colony
Adult A. saccharicola, emerged from S. sacchari mummies, were collected at the same site as the mealybugs. The mummies were individualized in Petri dishes (150 mm diameter) containing honey droplets on their inner sidewall, to feed the emerging adult parasitoids. The Petri dishes were sealed with PVC film that was retained until the emergence of the parasitoids. After emergence, a pro number of the parasitoids was separated for identification using a Leica M205C stereomicroscope microscope (Leica Microsystems Switzerland Ltd., 2015) and identified with taxonomic keys (Gibson et al., Reference Gibson, Huber and Woolley1997; Noyes, Reference Noyes2000; Zu et al., Reference Zu, Zhang, Li and Zhang2018), and then used for further studies in the bioassays. The remainder of the parasitoids was kept in glass cages (50 × 70 × 30 cm) with voile tissue covers in the laboratory, at a temperature of 25 ± 1°C, 70 ± 10% RH, and 14 h photophase. The internal sidewalls of the cages contained honey, and the underside contained sugarcane shoots infested with S. sacchari. The infested sugarcane shoots were replaced by new slips every week, and honey was replaced whenever necessary. The parasitoids from the sixth generation onward were used in the bioassays; the process involved removing the mummies from inside the cage, placing them in Petri dishes, and after emergence, rereleasing the parasitoids into the cage.
Parasitoid survival at different temperatures
The survival of A. saccharicola females and males was compared between those maintained with and without honey feed at three temperatures, 20, 25, and 30°C, all kept at 70 ± 10% RH and 14 h photophase, with 15 replicate females and males per treatment. Each temperature was maintained in a climatic chamber, totaling three chambers. The newly emerged parasitoids were individualized in Petri dishes (150 mm diameter) and sealed with PVC film. The honey supplied to the parasitoids was spread in droplets on the inner sidewall of the Petri dishes. Daily evaluations were performed to verify parasitoid mortality.
Host preference of the parasitoid and biological parameters of the pink sugarcane mealybug
A newly emerged A. saccharicola female was kept with a male for 24 h for mating, with each couple individualized in glass tubes containing honey droplets on their inner sidewall. After this duration, only a female without oviposition experience was released in a Petri dish (150 mm diameter) containing ten S. sacchari mealybugs. To evaluate the host preference of the parasitoid, mealybugs of three ages were used (15-, 20-, and 30-day-old), with 50 replicates for each age. The age was counted from the stage of neonatal nymphs. All Petri dishes contained honey droplets for feeding the parasitoids and were sealed with PVC film. They were kept in a climatic chamber at a temperature of 20 ± 1°C, 70 ± 10% RH, and 14 h photophase, until mummy formation. A female parasitoid was kept in the Petri dish for 72 h. Daily observations were made as follows: mealybugs' oviposition; days to mummy formation; longevity of the parasitized mealybugs; longevity of the non-parasitized mealybugs; days to parasitoid emergence; emergence rate; sex ratio; and parasitism rate. Mealybugs' oviposition included the number of times the female produced egg mass. The mealybugs were evaluated until their death.
After 30 days from the beginning of the bioassay, all mealybugs were dissected to check for adult parasitoids that did not emerge. The emergence rate was calculated using the formula: [(number of mealybugs from which parasitoids had emerged/total number of mealybugs) × 100]. The sex ratio was obtained using the formula: [number of females/(number of males + number of females)]. The parasitism rate was calculated based on the formula adapted from Nava et al. (Reference Nava, Pinto and Silva2009): [(number of parasitized mealybugs/total number of released mealybugs) × 100].
Effect of feeding and mating on the parasitism rate of A. saccharicola
A newly emerged female was released in a Petri dish (150 mm diameter) containing five mealybugs of 20–30 days of age. Ten replicates of the following six treatments, totalizing 60 tests, were evaluated:
(1) fed, unmated, and newly emerged female;
(2) fed, unmated, and 24 h-old female;
(3) fed, mated, and 24 h-old female;
(4) unfed, unmated, and newly emerged female;
(5) unfed, unmated, and 24 h-old female; and
(6) unfed, mated, and 24 h-old female.
The feed was provided as honey droplets on the inner sidewall of the Petri dish. For the treatments of the mated females, a male and a female were kept in glass tubes after emergence for 24 h, and then, only the female was used. The female parasitoid was kept in the Petri dish for 96 h. The bioassay was maintained at a temperature of 20 ± 1°C, 70 ± 10% RH, and 12 h photophase. After the emergence of the new generation of parasitoids, the parasitism rate was calculated using the formula [(total of parasitized mealybugs/total of mealybugs offered) × 100] and evaluated in the same way as in the previous bioassay.
Statistical analysis
The Kaplan–Meier curve was constructed for the survival bioassay and analyzed by log-rank using PROC LIFETEST. Since the survival bioassay effect was significant (P < 0.05), subsequent separations of the mean were done by comparing each bioassay pair using a Sidak adjustment. This statistical analysis was performed using the SAS Institute (2007). Data on the host preference of the parasitoid, biological parameters of the mealybug, and parasitism rate were analyzed using R version 3.6.2 (www.R-project.org) for one-way analysis of variance followed by Tukey's test. When the data did not follow homogeneity (Bartlett test) and/or the normal distribution (Shapiro–Wilk normality test) even after transformation, the nonparametric Kruskal–Wallis test was used to evaluate the observations, followed by the Dunn test and Holm method for the pairwise comparisons. The parasitism rate and the mealybug age were evaluated by the regression model, represented by the following equation: y = ax + b, where y = variable response; a = maximum estimated value for the response variable; x = age; and b = variable response corresponding to the minimum point of the line.
For assessing the effect of feeding and mating of the parasitoid, there was no homogeneity or normality of the data, even when transformed; therefore, the nonparametric Kruskal–Wallis test was used, followed by the Dunn test and Holm method for the pairwise comparisons, using the PMCMR package. Data were processed using R version 3.6.2 (www.R-project.org).
Results
Parasitoid survival at different temperatures
Feeding with honey and temperature had a significant effect on the survival rate of A. saccharicola females (fig. 1, χ2 = 106.9, d.f. = 5, P < 0.0001) and males (fig. 2, χ2 = 70.5, d.f. = 5, P < 0.0001). Both the females and males that were fed and maintained at a temperature of 20°C had a higher survival rate than those maintained at 25 or 30°C. Furthermore, the survival rates of the fed female parasitoids were higher than those of the unfed females when pairwise comparisons were analyzed for the three temperatures.

Fig. 1. Survival of A. saccharicola female. °C indicates degree Celsius. NH indicates non-honey. Different letters show significant treatment differences following Sidak adjustment: 20 vs. 30°C, P = 0.0014; 20 vs. 20°C NH, P < 0.0001; 20 vs. 25°C NH, P < 0.0001; 20 vs. 30°C NH, P < 0.0001; 25 vs. 20°C NH, P = 0.0177; 25 vs. 25°C NH, P = 0.0081; 25 vs. 30°C NH, P = 0.0006.

Fig. 2. Survival of A. saccharicola male. °C indicates degree Celsius. NH indicates non-honey. Different letters show significant treatment differences following Sidak adjustment: 20 vs. 20°C NH, P < 0.0001; 20 vs. 25°C NH, P < 0.0001; 20 vs. 30°C NH, P < 0.0001; 25 vs. 30°C NH, P = 0.017; 30 vs. 20°C NH, P = 0.0424; 30 vs. 25°C NH, P = 0.0336; 30 vs. 30°C NH, P = 0.0102.
The unfed female parasitoids had a higher survival rate at 20°C than at the other temperatures (fig. 1). For males, the temperatures of 20 and 25°C had the same survival rate, these being higher than survival at 30°C (fig. 2). Regarding the unfed females, on day 2 and at 30°C, all parasitoids were dead. On day 4, none survived at 25°C. Moreover, on day 7, all were dead at 20°C. For the fed females, on day 25 at 30°C, all were dead, at 25°C on day 37, all were dead, and at 20°C parasitoids lived up to 61 days (fig. 1).
The males had a low survival rate. On day 2 at 30°C, all unfed males were dead on day 3 at 25°C and 20°C. The fed males were all dead on days 20, 30, and 34 at 25, 30, and 20°C, respectively (fig. 2).
Host preference of the parasitoid and biological parameters of the pink sugarcane mealybug
A. saccharicola is a gregarious endoparasitoid approximately 1.0 mm in length when adult. All tested host ages (15, 20, and 30 days) were parasitized by A. saccharicola. The number of progeny produced by mealybugs differed between treatments (table 1, H = 61.23, d.f. = 2, P < 0.01), with 30-day-old females having a higher number of oviposition episodes than those aged 20 and 15 days. It is important to highlight that some parasitized mealybugs generated offspring before mummification.
Table 1. Average ± SE of five biological parameters of the S. sacchari mealybug and reproduction of the parasitoid A. saccharicola at different ages of the mealybug

Means followed by different letters indicate a significant difference between treatments based on the nonparametric Kruskal–Wallis test (P < 0.05) and subsequent pairwise comparisons using Dunn test and holm method. Days until the formation of mummies, days until emergence and sex ration, were analyzed only in parasitized mealybugs. No.: number of individuals observed.
Regarding the parasitized mealybugs, the appearance of mummy formation (fig. 3) was different between the ages assessed (table 1, H = 2, d.f. = 2, P = 0.017), appearing approximately at 9, 10, and 10 days for hosts aged 15, 20, and 30 days, respectively. The longevity of parasitized S. sacchari remained constant between the different ages of mealybugs (table 1, H = 2, d.f. = 2, P = 0.4157). However, the non-parasitized mealybugs had different longevity at the ages evaluated (table 1, H = 2, d.f. = 2, P = 0.00936). Despite the age differences, parasitoids emerged after the same duration (table 1, H = 2, d.f. = 2, P = 0.3304); however, the emergence rate was different according to age (table 1, H = 2, d.f. = 2, P < 0.001); it was higher for from hosts that were 30 days of age than for those aged 20 days, followed by those aged at 15 days.

Fig. 3. Aspects of S. sacchari parasitized and non-parasitized by A. saccharicola.
The sex ratio of the parasitoid differed according to the age of the host mealybug (table 1, H = 2, d.f. = 2, P < 0.001), with sex ratios of 1, 0.9 and 0.7, for hosts aged 15, 20, and 30 days, respectively. A. saccharicola had a significant direct relationship with mealybug age, as observed in the regression linear for the parasitism rate, in which the data were adjusted to the second-degree equation (R 2 = 0.9) (fig. 4).

Fig. 4. Regression linear adjusted for parasitism rate from A. saccharicola at different ages of mealybug.
Effect of feeding and mating on the parasitism rate of A. saccharicola
There was a difference in parasitism rate (fig. 5, H = 18.303, d.f. = 5, P < 0.0026) between the tested combinations. The female parasitoids, which had fed, mated at 24 h-old were able to parasitize and produce a higher number of offspring (36%) than the unfed parasitoids that did not mate at 24 h of age (6%), and the fed parasitoids that did not mate at 24 h presented the lowest parasitism rate (2%).
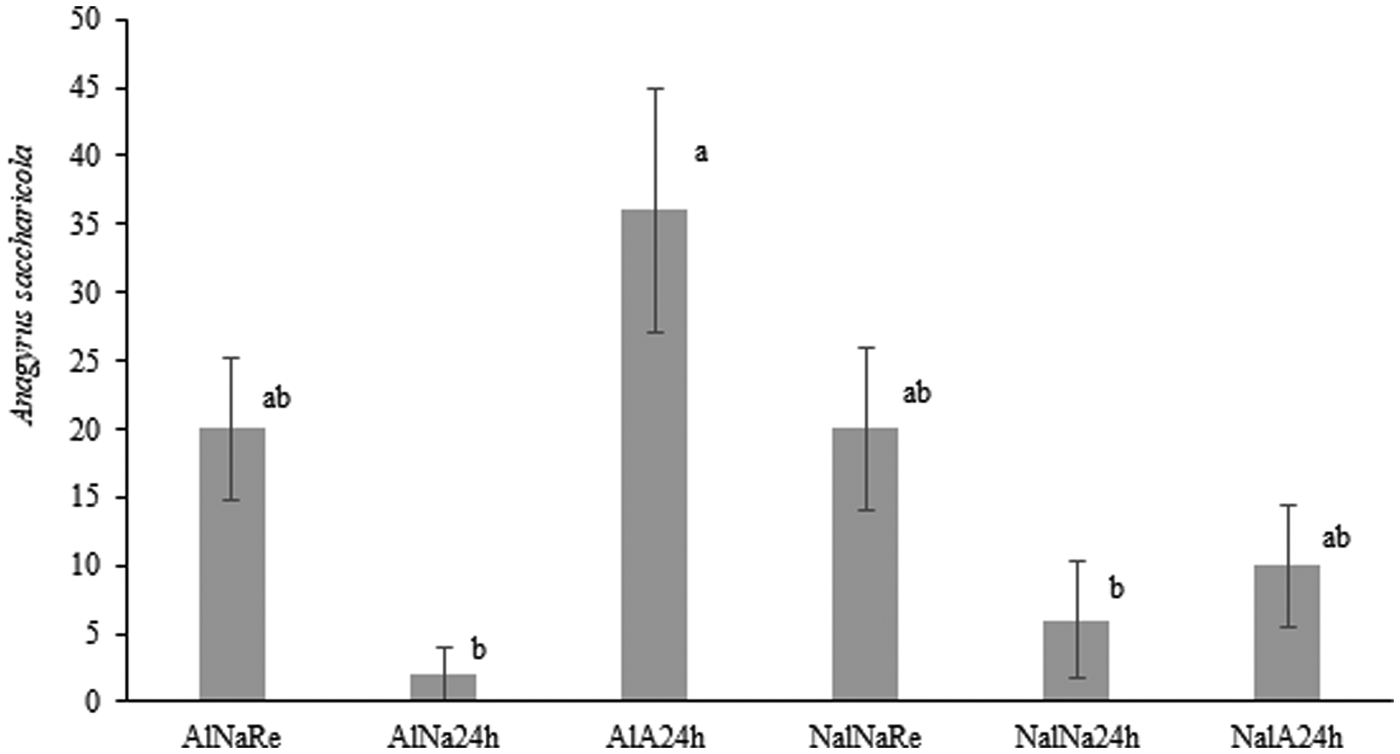
Fig. 5. Average (± SE) parasitism rate of A. saccharicola in S. sacchari. ALNaRe: fed, unmated and newly emerged; AlNa24h: fed, unmated and 24 h old; AlA24H: fed, mated and 24 h old; NalNaRe: not fed, unmated and newly emerged; NalNa24h: not fed, unmated and 24 h old; NalA24h: not fed, mated and 24 h old. Letters indicate a significant difference between treatments with nonparametric Kruskal–Wallis test (P < 0.05) and subsequent pairwise comparisons using Dunn test and holm method.
Discussion
This study showed that feeding A. saccharicola with honey and maintaining temperature at approximately 20°C play an important role in prolonging its survival, and that the mortality rates increased at the higher temperatures tested, i.e., 25 and 30°C. Temperature is a critical factor in insect development (Hance et al., Reference Hance, van Baaren, Vernon and Boivin2007), insects are among the organisms most likely to be affected by climate change, as the temperature influences their development, reproduction, and survival (Sangle et al., Reference Sangle, Satpute, Khan and Rode2015). Owing to their small size, parasitoids are more sensitive to climate change (Yadav et al., Reference Yadav, Baloda and Jakhar2019).
These results are consistent with those of previous studies. Sagarra et al. (Reference Sagarra, Vincent and Stewart2000) showed that the survival of the hymenopteran Anagyrus kamali (Encyrtidae) at 20°C was around 1.7 and 1.3 days when the parasitoids were not fed, and 40.3 and 31.7 days when they were fed (females and males, respectively). Davies et al. (Reference Davies, Ceballo and Walter2004) found that the survival of the Coccidoxenoides perminutus (Encyrtidae) decreased at temperatures at 35°C. In another laboratory-based study, Anagyrus sp. nov. nr. sinope (Encyrtidae) survived for 23.4 days when fed with honey and for 1.9 days without feeding, at 25°C (Chong and Oetting, Reference Chong and Oetting2006); in addition, Blepyrus clavicornis (Encyrtidae) survived for 20 days if fed and 4 days when not fed at the same temperature (Silva et al., Reference Silva, Garcia and Botton2017). Finally, Alam (Reference Alam1972) showed that at the normal temperature (the exact temperature was not reported in the study), females and males of A. saccharicola fed with honey survived for 9–11 days in studies undertaken in Barbados, West Indies.
The importance of parasitoids is recognized in relation to the functions they perform in nature, when adult, parasitoids need carbohydrate sources such as nectar and pollen substances (Fernández and Sharkey, Reference Fernández and Sharkey2006) and lack of food harms their survival. As shown in this study, the fed parasitoids survived at the three temperatures evaluated; but, a higher survival rate of females and males was observed at 20°C, that is at lower temperatures A. saccharicola live longer. Furthermore, females had a higher survival rate compared to males at all evaluated treatments, a finding similar to that of the study by Mozaddedul and Copland (Reference Mozaddedul and Copland2002) with the Leptomastix nr. Epona (Encyrtidae).
When parasitized, an organism activates defenses against the invader; thus, the available energy is allocated for its protection (Guzo and Stoltz, Reference Guzo and Stoltz1987), and in insects this can disrupt instar progression, reduce feeding (Slansky, Reference Slansky1978), moreover they can spend less energy on mating (Trail, Reference Trail1980) and reproduction (Forbes, Reference Forbes1993). Oviposition of S. sacchari occurs at the beginning of the reproductive period, before any significant onset of mortality (Rae and De'ath, Reference Rae and De'Ath1991) but, as in the current study, these authors found that older mealybugs (30 days) had the highest however, number of oviposition (3.46) compared to younger mealybugs of 15 and 20 days, with 0.04 and 1.92, respectively. A previous study showed that at 20°C, non-parasitized and fed S. sacchari had an oviposition proportion of 60.83 (Rae and De'ath, Reference Rae and De'Ath1991). Islam and Copland (Reference Islam and Copland1997) showed that the number of oviposition also increased in hosts with larger body sizes when analyzing parasitized or non-parasitized individuals of the mealybug Planococcus citri (Pseudococcidae). In our study, even parasitized females oviposited, in contrast to those in the study by Alam (Reference Alam1972), which indicated that parasitized S. sacchari produced fertile eggs but died before completing oviposition.
In this study, parasitized and non-parasitized S. sacchari individuals had the same coloration and morphology, which made it difficult to distinguish between them before the process of mummification. May be associated with cerosity found around the body of the mealybug (Rae and De'ath, Reference Rae and De'Ath1991; Rae, Reference Rae1993), making it difficult to perceive the mummy aspect. According to Müller et al. (Reference Müller, Adriaanse, Belshaw and Godfray1999), it is possible to recognize if the host is parasitized after the initiation of mummy formation. Mummy formation was noticed in 30-day-old mealybugs, i.e., at this age, the mealybug remained alive for 9 days after being parasitized. This result is different from that reported for the same species by Alam (Reference Alam1972), in which the mealybugs remained alive for 3 days after being parasitized.
The non-parasitized mealybugs had longer longevity, even those 15 days of age lived longer (13 days) than parasitized mealybugs of the same age. And those aged 20 and 30 days survived for an additional period of approximately 20 days; it is worth noting that the mealybugs used in the bioassay were not fed. Alam (Reference Alam1972) observed 16–17 days longevity for this same mealybug species. In contrast, Yang and Sadof (Reference Yang and Sadof1995) reported longevity of 60 days for another non-parasitized mealybug species, P. citri; however, it was fed with plants Solenostemon scutellarioides (Lamiaceae).
A. saccharicola parasitized the mealybugs at all assessed ages, and its emergence occurred around 24 days, 5 days more than that reported in a previous study (Sagarra et al., Reference Sagarra, Vincent and Stewart2001). The highest parasitism rate was observed in hosts aged 30 days, and hosts with the largest body size will probably have more resources available to the parasitoid offspring (Jervis and Copland, Reference Jervis, Copland, Jervis and Kidd1996). Similarly, in a study on Anagyrus pseudococci (Encyrtidae), the highest emergence rate was observed from older individuals of P. citri (Islam and Copland, Reference Islam and Copland1997). Another study found that the average emergence rate of A. pseudococci was 67.6% (Islam and Copland, Reference Islam and Copland2000). In contrast, Sagarra et al. (Reference Sagarra, Vincent and Stewart2001) observed a higher emergence rate of the hymenopteran A. kamali (Encyrtidae) in younger mealybugs. In this context about body mealybug, Rae and De'ath (Reference Rae and De'Ath1991) discuss the allometric growth (morphological change parallel to the increase in size) of the dorsum of S. sacchari; where after adulthood, this body part becomes more globular in relation to that in the previous instars, thus a shape that may be easier to parasitize.
Tabadkani et al. (Reference Tabadkani, Ashouri, Rahimi-Alangi and Fathi-Moghaddam2012) suggest that laboratory-based estimations of the sex ratios of arthropods can be accurate representations of field events; thus, informing a key factor in integrated pest management programs. Almost all females emerged from hosts aged of 20 and 15 days; however, the sex ratio was lower at 30 days, owing to the higher number of males in comparison with the other treatments. Islam and Copland (Reference Islam and Copland1997) found a larger number of males of A. pseudococci in younger specimens of P. citri. Koinobiont parasitoids are those that partially paralyze their hosts; however, these continue its development and only die when the parasitoid reaches maturity (Godfray and Shimada, Reference Godfray and Shimada1999). Koinobionts can adjust the sex ratio of their progeny in response to host quality or size (King, Reference King1989; Cloutier et al., Reference Cloutier, Lévesque, Eaves and Mackauer1991).
The presence of the parasitoid can be associated with a decrease in the mealybug population; for instance, parasitism by Anagyrus lopezi (Encyrtidae), however, reduces numbers of the mealybug Phenacoccus manihoti (Pseudococcidae) in the field (Wyckhuys et al., Reference Wyckhuys, Hughes, Buamas, Johnson, Vasseur, Reymondin, Deguine and Sheil2019). Our study showed the parasitism rate of mealybugs was enhanced when individuals of A. saccharicola were fed, mated, and within 24 h of age. According to Sagarra et al. (Reference Sagarra, Vincent and Stewart2002) A. kamali Moursi (Hym., Encyrtidae) managed to generate a greater number of progenies when they were mated, consequently interfered with the Maconellicoccus hirsutus Green (Hom., Pseudococcidae) parasitism.
Similar results were observed in the hymenopterans Paratelenomus saccharalis (Platygastridae) (Takano and Takasu, Reference Takano and Takasu2019) and Aphidius platensis (Braconidae) (Souza et al., Reference Souza, Marucci, Silveira, de Paulo and Lee2018); increased progeny was detected with the same three factors as in this study (food, mating, and 24 h of age). Adult parasitoids need a carbohydrate source such as nectar or pollen (Jervis et al., Reference Jervis, Kidd, Fitton, Huddleston and Dawah1993; Heimpel and Jervis, Reference Heimpel, Jervis, Wackers, Van Rijn and Bruin2005), or honeydew (Wäckers, Reference Wäckers2001), and the availability of these resources can increase the parasitism rate. For example, Microplitis mediator (Braconidae) showed a high parasitism rate when fed with floral nectar (Géneau et al., Reference Géneau, Wäckers, Luka, Daniel and Balmer2012). Honeydew can be a substitute source in the absence of floral nectar (Stapel et al., Reference Stapel, Cortesero, De Moraes, Tumlinson and Lewis1997). Thus, it proves the importance of the parasitoid being fed, because it directly influences the increase in the rate of parasitism.
In fact, S. sacchari had advantages in survival and oviposition at high temperature, at 30°C and on the contrary it occurred for the parasitoid, which was benefited at lower temperatures.
This fact may cause the mealybug to have an advantage over the parasitoid at high temperatures. However, when working with integrated pest management, it is at the level of control, before population outbreaks of pests, that one enters with the action of the control tactic (Gallo et al., Reference Gallo, Nakano, Silveira Neto, Carvalho, Batista, Berti Filho, Parra, Zucchi, Alves, Vendramin, Marchini, Lopes and Omoto2002). Therefore, with the results obtained can be used to determine the period in which the parasitoid is available to parasitize and increase its population before population outbreaks. In this case, higher temperatures, which will fit perfectly with the temperature range of the parasitoid, acting the parasitoid in this level of control, for when there are high temperatures the mealybug will be controlled, not reaching high population peak.
The results showed that 20°C is a suitable temperature for A. saccharicola and that the host preference is for S. sacchari aged 30 days or older, which therefore are factors that result in a higher parasitism rate. The fed parasitoids that mated at 24 h of age achieved a higher parasitism rate under laboratory conditions. These factors are critical for developing a pest management program as they contribute to a better understanding of the parasitoid rearing conditions, aiming at a future control system of the S. sacchari. Understanding the role of parasitoids in pest control has important implications for biological control (Cuny et al., Reference Cuny, Traine, Bustos-Segura and Benrey2019).
In real-field situations the temperature varies, which means that studies on parasitoids and their hosts at constant temperatures will not necessarily produce data that are easily transferable to the field. Mass rearing of the parasitoids at constant temperatures can be effective only in areas where temperatures are ideal. In Jaboticabal city, evaluated region, the temperature can vary from 13 to 33°C (Marcari et al., Reference Marcari, Souza Rolim and Oliveira Aparecido2015); it is interesting to evaluate the thermal requirement of the parasitoid, for a better understanding of the ideal moment of release to control the pest. Parasitoids under high temperatures in the laboratory may be less affected in the field, where high temperatures may be followed by low temperatures (Romo and Tylianakis, Reference Romo and Tylianakis2013).
Differences occur between constant and fluctuating temperature for parasitoids and their hosts. Mated females of Trissolcus brochymenae (Scelionidae) were evaluated in the field, longevity had no influence under fluctuating temperatures, however the longest life was recorded at a constant temperature of 27°C; in addition, the parasitism rate was four times higher at fluctuating temperatures, the host Podisus nigrispinus (Pentatomidae) was benefited at constant (17°C) and fluctuating (10–20°C) temperatures, presenting inversely proportional results (Torres et al., Reference Torres, Musolin and Zanuncio2002). For Eretmocerus hayati (Aphelinidae), at the lowest temperatures evaluated (22 and 26°C), increased parasitoid reproduction and longevity were observed (Zhang et al., Reference Zhang, Zhang, Liu and Wan2019).
The thermal performances of Pachycrepoideus vindemiae (Pteromalidae) and Trichopria drosophilae (Diapriidae) have been ascertained, P. vindemiae was more tolerant of low temperature variation in terms of survival and reproduction and T. drosophilae behaved to the contrary, it was tolerant at high temperatures (Wang et al., Reference Wang, Serrato, Son, Walton, Hogg and Daane2018). According to Bahar et al. (Reference Bahar, Soroka and Dosdall2012), the highest parasitism rate of Diadegma insulare (Ichneumonidae) was found at 22°C constant and 7°C fluctuating, and the lowest at 30°C fluctuating, its host on the contrary, benefited at higher temperatures of 30°C.
Studies are needed to identify periods of population pest outbreaks and release of the parasitoid at the most appropriate time, avoiding the impact of the parasitoid on the field (Torres et al., Reference Torres, Zanuncio, Cecon and Gasperazzo1996), and thus influence the suppression of the plague. This study was carried out under laboratory conditions and it is essential to evaluate its performance in the field. Further studies should be focused on A. saccharicola regarding the control of the pink sugarcane mealybug including methods related to its mass rearing and release of the parasitoid in the field.
Acknowledgements
This study was supported by the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) (grant number 88887.337790/2019-00) at the São Paulo State University (Unesp), School of Agricultural and Veterinarian Sciences, Jaboticabal (UNESP/FCAV). The authors thank MSc Patrice Jacob Savi for clarifying the statistics and performing statistics on survival data.









