Introduction
Chorthippus (Glyptobothrus) vagans (Eversmann, 1848) is a grasshopper (Orthoptera, Acrididae) belonging to the Subfamily Gomphocerinae, widely distributed on the Palaearctic Region. Nevertheless, its distribution area in Central Europe is regressive and quite fragmented because this species has suffered a large decline over recent decades due to extensive changes in land use and eutrophication and degradation of heathlands. For this reason, the species is included in the red list of several European countries (Proess & Meyer, Reference Proess and Meyer2003; Lock et al., Reference Lock, Adriaens, Devreise, San Martin and Decleer2011; Maas et al., Reference Maas, Detzel and Staudt2011; Bakker et al., Reference Bakker, Bouwman, Brekelmans, Colijn, Felix, Grutters, Kerkhof and Kleukers2015; Sutton, Reference Sutton2015). In addition, this species is considered ‘vulnerable’ in Austria (Berg et al., Reference Berg, Bieringer, Zechner and Zulka2005).
On the Iberian Peninsula generally, as well as in the studied area, it still is a rather common and widely distributed species (Llucià-Pomares & Fernández-Ortín, Reference Llucià-Pomares and Fernández-Ortín2009). It is active throughout the year, except in the coldest months (García, Reference García1984; García & Presa, Reference García and Presa1985). This flexible life cycle enables C. vagans to colonize very different biotopes such as sands and dunes with bushes, pine forests, cork and holm oak forests, wet forests, grasslands and cleared shrub, among others (Presa et al., Reference Presa, Montes and Ramírez-Díaz1983; Llucià-Pomares, Reference Llucià-Pomares2002; Olmo-Vidal, Reference Olmo-Vidal2002), and also ecotones or transitional zones (Hochkirch et al., Reference Hochkirch, Gärtner and Brandt2008).
There is information about some aspects of the genetics (Cabrero & Camacho, Reference Cabrero and Camacho1987; Cerro & Santos, Reference Cerro and Santos1995) and population dynamics in response to environmental management (Hochkirch et al., Reference Hochkirch, Gärtner and Brandt2008), and nutritional ecology of C. vagans in diverse areas from the European continent (Treiber, Reference Treiber and Detzel1998, Reference Treiber2001; Steiner, Reference Steiner2006).
The current knowledge of the biology of C. vagans in Mediterranean ecosystems is mainly based on field observations of the habitat or the activity pattern, and there is no available information about other significant aspects such as trophic preferences or reproductive biology. Food selection seems to determine distribution in the biotope of many phytophagous species (Sheldon & Rogers, Reference Sheldon and Rogers1978; Joern & Lawlor, Reference Joern and Lawlor1981), although this factor is less important for Orthoptera, which are usually polyphagous and more specialized on certain vegetation structures and microclimates. In addition, plant selection depends on their palatability which, in turn, is determined by leaf qualities (physical, chemical or nutritional; Otte & Joern, Reference Otte and Joern1977; Muralirangan & Muralirangan, Reference Muralirangan and Muralirangan1985; Southwood, Reference Southwood1987; Bright & Bernays, Reference Bright and Bernays1991), affecting the biological cycle and behaviour of the species (Mulkern, Reference Mulkern1967; Uvarov, Reference Uvarov1977). Very few studies use plants that resemble those of the natural scenarios from which grasshoppers come (Franzke et al., Reference Franzke, Unsicker, Specht, Köhler and Weisser2010), although bioassays provide knowledge of the use of habitat (occupation, feeding) and also on some competitive interactions (Litvaitis, Reference Litvaitis, Boitani and Fuller2000), apart from the aforementioned specific biological aspects.
Surveys on the feeding behaviour of Orthoptera also indicate that variation in the local environment is one of the most crucial factors in the establishment of specific associations between grasshoppers and vegetation (Guendouz-Benrima et al., Reference Guendouz-Benrima, Doumandji-Mitiche and Petit2011). In consequence, research on changes in the pattern of distribution of Orthopteran species in response to the application of restoration techniques should consider the trophic component together with analysis of environmental heterogeneity.
On the other hand, development of organisms in their respective habitats involves confrontation with environmental changes due to seasonal and inter-annual fluctuations of physical and biotic factors. Therefore, to survive such changes, they adjust their biology by modifying their breeding cycle, pattern of development and even their population dynamics (Karpakakunjaram et al., Reference Karpakakunjaram, Kolatkar and Muralirangan2002; Da Silva et al., Reference Da Silva, Marques, Battirola and Lhano2010; Savitsky, Reference Savitsky2010).
The adaptation of the life cycle of the species to the local environment involves developing a series of physiological and behavioural characteristics that affect multiple aspects such as fertility, growth rate, the annual number of generations, the processes of dormancy and quiescence, the duration of different stages of development, adult longevity and synchronization between the breeding period and environmental variables (Neumann, Reference Neumann, Taylor and Karban1986). Therefore, research on the reproductive biology of species is particularly important. Such studies typically include detailed monitoring of ovarian development, because females of Orthoptera, like many other insects, retain in their ovaries evidence of their ovipositional history (Sundberg et al., Reference Sundberg, Luong-Skovmand and Whitman2001; Hodin, Reference Hodin, Whitman and Ananthakrishnan2009). This legacy of past events in egg-laying takes the form of cell-rests, named corpora lutea, which are very informative for interpreting the biological cycle of species (Quesada-Moraga & Santiago-Álvarez, Reference Quesada-Moraga and Santiago-Álvarez2001).
The majority of available information on the biology of this grasshopper derives from Central Europe (Ramsay Reference Ramsay1964; Ingrisch, Reference Ingrisch1983; Ingrisch & Köler, Reference Ingrisch and Köhler1998). Regarding the Mediterranean Region, the literature on the ecology of C. vagans is quite extensive (García & Presa, Reference García and Presa1985; Llucià-Pomares, Reference Llucià-Pomares2002; Olmo-Vidal, Reference Olmo-Vidal2002). However, the biological aspects (food selection, reproduction) remain unknown at the lower latitude.
This work is aimed at investigating the biology of C. vagans through parameters including autoecology, feeding preferences and reproduction in the Southern Iberian Peninsula, in order to determine how it adjusts to the environmental features of this particular ecosystem and to interpret how it could be affected by the environmental changes. This species is one of the most common grasshoppers in the study area; potentially, it constitutes a major component of the diet of many vertebrate species (birds, wall lizards, amphibians, etc.). Studies on the biology of C. vagans are of considerable interest to understand their life history, ecology and adaptability to the environmental changes.
Materials and methods
Study area
This study was carried out in the surroundings of the Breña dam in the Hornachuelos Natural Park (SW Iberian Peninsula). This area is included in the Environmental Recovery Programme linked to the construction of this new dam (in compliance with the Directive Habitat, Council Directive 92/43/EEC), and it includes a total of twelve restoration plots (table 1).
Table 1. Nomination, code, locality and Geographic Coordinates for the sampling plots.

As a whole, the area shows a wavy relief, with altitudes ranging from 100 to 725 m and, from a lithological point of view, metamorphic rocks predominate, particularly quartzite, slates and semi-acidic intrusive rocks. Sandy or clayey substrates can also be found. The soils are chemically and physically homogeneous, mostly acids, with low levels of organic material and carbon (Pinilla, Reference Pinilla2006). The climate is of Mediterranean type, with mean temperatures of 25–26°C in summer and 8–10°C in winter, and rainfall levels ranging from 500 to 800 mm (Gallardo et al., Reference Gallardo, Cárdenas and Gaju2010).
The landscape is dominated by a Mediterranean mixed sclerophyllous forest, sited on the thermo- and meso-Mediterranean bioclimatic belts (Cárdenas & Bach, Reference Cárdenas and Bach1989).
The vegetation in the area belongs to the Duriilignosa formation, represented in the Iberian Peninsula by the Quercetea ilicis type. The sclerophyllous forests are characterized by the predominance of holm oaks (Quercus ilex L.) and cork oaks (Q. suber L.) (Cárdenas & Gallardo, Reference Cárdenas and Gallardo2012), as well as Pistacia lentiscus L., Asparagus albus L., Arbutus unedo L., and different species of Erica and Cistus in the bushes (Torres & Ruiz, Reference Torres and Ruiz2009).
Field work
The field work related to C. vagans autecology was performed between 2007 and 2010, from February to November each year, coinciding with the activity period of this grasshopper in the area (own data). During the sampling period, a monthly visit was made to each of the twelve sampling plots. At each plot, linear transects (Gardiner & Hill, Reference Gardiner and Hill2006) with zig-zag paths were performed over a sampling time of 30 min per sampling day. Direct manual capture and sweep nets were used to catch specimens. Later, they were identified, censused and released after recording sex and maturation stage (nymph or adult). Specimens that could not be identified in the field were collected, preserved in 70% ethanol, and transported to the laboratory for classification. For each individual observed, the environmental temperature and humidity at the observation time were also registered. To standardize field data, the monthly average number of individuals (males, females and nymphs) was calculated for each sampling plot.
Laboratory work related to trophic response tests
For the food selection tests, a total of ten nymphs of the last stages (IV or V) and ten adults of C. vagans were collected in the field (May 2011). Live individuals were brought to the laboratory in plastic insectaries measuring 23 × 13 × 18 cm3, perforated in the upper wall and provided with 2 cm of moistened vermiculite (1.5 ml distilled water/1 g vermiculite), a water dispenser and some shelters (leaves, branches, stones, etc.) for accommodation.
Specimens were maintained in the laboratory following a modification of the methods used by Quesada-Moraga & Santiago-Álvarez (Reference Quesada-Moraga and Santiago-Álvarez2001) and Michel & Terán (Reference Michel and Terán2006). In particular, each of the specimens of C. vagans was placed into wood insectaries of 35 × 35 × 35 cm3, with a metallic mesh on the upper and lateral walls to facilitate ventilation and observation. A daylight bulb of 60 W was placed 15 cm above the upper wall. As substrate, a 2-cm thick layer of vermiculite moistened with 1.5 ml of distilled water was used. In addition, some shelters (stones and branches) previously sterilized (at 100°C for 48 h) were added as well as a water dispenser of 20 ml and food supplied in each case (fresh plants).
The insectaries were maintained under controlled conditions (27 ± 2°C; 66 ± 7% relative humidity; photoperiod: 14 h light; 10 h dark).
Two types of food were selected to explore food preferences and to quantify the consumption rate.
Type 1: gramineous: Lolium sp.
Type 2: shrubs: Cistus monspeliensis L. for adults or C. salviifolius L. for nymphs. The difference in the type of food supplied to nymphs and adults depended on the Cistus species from which they were captured in the field.
The criteria for selecting species were as follows: Lolium the main component of the grassland in the sampling plots and diverse Cistus species (C. salviifolius and C. monspeliensis) were planted in sampling plots in order to restore the scrub.
Forced feeding tests (monospecific diet) and choice feeding tests (mixed diet) were performed. The plants were supplied ad libitum in both diets.
The method for estimating the amount of food consumed was a modification of that devised by Bardi et al. (Reference Bardi, Mariottini, De Wysiecki and Lange2011), and is described below.
To obtain the initial dry weight of each type of food, ten samples of 2 g (each) of each plant species (type 1 – Lolium sp.; type 2 – C. monspeliensis or C. salviifolius) were placed into the oven at 80°C. After 24 h, they were weighed and the average initial dry weight corresponding to 2 g of fresh weight was calculated.
In the monospecific diet test, 2 g of one food type was placed in each insectary. The plants were supplied vertically (Williams, Reference Williams1954; Kaufmann, Reference Kaufmann1965), and after 3 days the remaining food was removed. To obtain the consumption in dry weight, these vegetal rests were placed in an oven for 24 h at 80°C, and then they were weighed again. Once the test of type 1 food was completed, the same procedure was followed with type 2 food (2 g of C. monspeliensis or C. salviifolius).
For the mixed diet test, 2 g of each type of food (Lolium and Cistus) were simultaneously supplied and the same protocol as that described above was followed. A total of ten replicates were made for each type of food experiment.
The weight data were obtained with a CB-Junior 0.01 g precision balance.
For the statistical comparison of data on the mean consumption of each type of food supplied in treatments of forced feeding and choice feeding, the statistical Student's t test was applied, provided that the data met the assumptions of normality. Otherwise, the nonparametric Mann–Whitney U test was applied. The assumptions of normality were previously verified by the Shapiro–Wilk test. All statistical tests were conducted with α = 0.05. Calculations were performed with SP statistical software (SPSS Inc., 2011).
Laboratory work related to reproductive biology tests
To study the reproductive biology of C. vagans different procedures were used as described below.
Anatomical observations
From February to November 2012, a total of 29 females was captured and preserved in 70% ethanol for at least 7 days to fix internal structures. The females were anatomically inspected, and were dissected according to the protocol described by Elamin et al. (Reference Elamin, Abdalla and El Naim2014) to determine their age and reproductive stage. Age was determined by testing the softness of the integument and the wear extent of structures such as bristles, tibial spines and tarsal claws (Van Huizen, Reference Van Huizen1980; Cárdenas & Bach, Reference Cárdenas and Bach1992) and, particularly, the mandibles (Köhler et al., Reference Köhler, Jentzsch and Reinhardt2000). The females were classified as follows:
Immature or newly emerged females
Showing no wear in the integumentary structures or in the molar and incisor regions of the mandible. Mostly, they had no differentiated ovaries or they were in the early stages of differentiation.
Mature
Females showing oocytes in different stages of differentiation and with incipient wear of the external anatomical structures.
Gravid
Females containing mature eggs and egg-pods in development; bristles, spines and mandibles with moderate wear.
Old
Females with regressed ovaria, corpora lutea and worn integumentary structures.
Old females starting reproduction
Females showing developed ovaries with differentiated oocytes and with corpora lutea. Integumentary structures very worn.
In addition, in line with Uvarov (Reference Uvarov1966) and Elamin et al. (Reference Elamin, Abdalla and El Naim2014), potential fecundity was defined as the average number of eggs per fertile female dissected. A female was considered fertile if she had at least one mature egg in the ovaria.
Laboratory test
Laboratory tests were performed with 15 pairs of adults of C. vagans. They were captured in April and the rearing experiments were conducted until October 2012. The specimens were kept in the same conditions described above for the trophic preferences test. In addition, to provide a suitable substrate for oviposition, a cylindrical plastic container measuring 10 cm Ø and 6 cm in height with a substrate of sand and soil (50:50) was placed into the insectary. The soil originated from the same place as the specimens and had previously been sieved (0.55 mm mesh) and sterilized (100°C/48 h). The containers were examined twice a week and the egg-pods were removed and measured (width and length). Later, the egg-pods were opened to quantify the total number of eggs.
Results
Autecology of C. vagans
A total of 1217 specimens (502 ♂♂, 459 ♀♀ and 256 nymphs) of C. vagans was recorded during the sampling period. Field data indicate that, in the study area, C. vagans is a fairly abundant and widely distributed species, except for in plot 1 (Mesas Bajas) (fig. 1) where the species was not recorded. The most suitable sampling places for C. vagans species were plots 9 (Raso del Conejo) and 5 (Los Baldíos), with average values of abundance higher than 100 specimens/year each. In addition, noticeable abundance levels were obtained in plots 11 (Mezquitillas), 4 (Llanos de la Iglesia) and 3 (Cerro del Trigo), where we recorded 57, 37 and 50 individuals/year, respectively (figs. 2a and b). The species were scarcer in the remaining sampling plots.

Fig. 1. Location of sampling plots in the research area. The plots with presence of C. vagans are marked in grey.
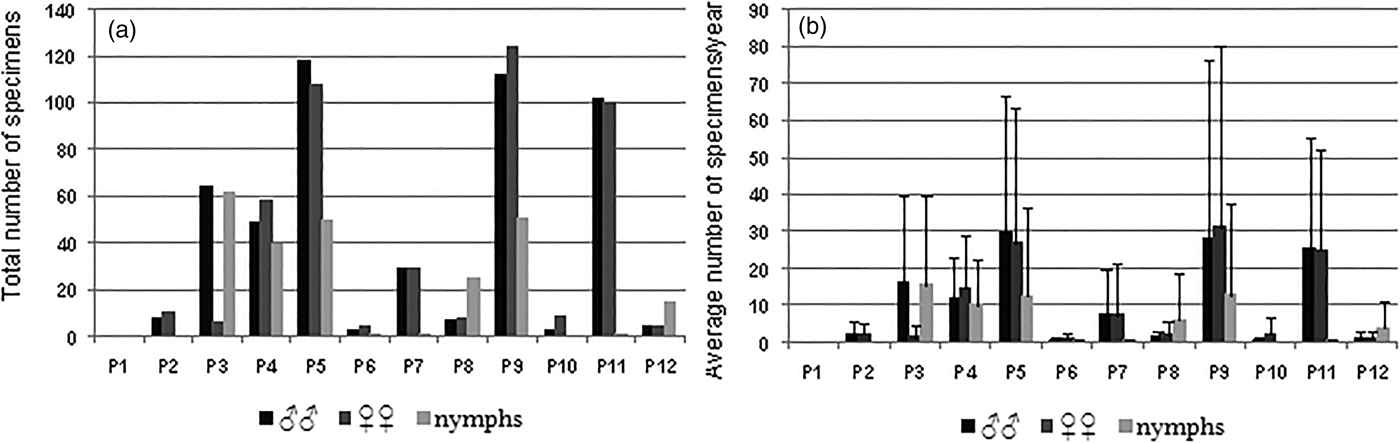
Fig. 2. (a) Abundance (total number of specimens) of males, females and nymphs of C. vagans recorded in each sampling plot during the sampling period 2007–2010. (b) Average number (and standard deviation, SD) of males, females and nymphs of C. vagans/year in each sampling plot during de sampling period 2007–2010.
The species was mostly located in different maturation stages of grasslands and medium-sized scrubs composed predominantly of Cistaceae (C. salviifolius and C. monspeliensis). Some individuals were also observed in transitional zones, open areas with scarce herbaceous or shrub vegetation or in litter substrate of cork and holm oaks.
This species showed a wide phenological pattern (fig. 3), displaying activity throughout the year excluding the winter time, regardless of which developmental post-embryonic stage was considered. The first to appear were the nymphs (April) which reach their maximum abundance in early summer and disappear towards mid-August. The adult population had a lag of approximately 1 month compared with the immature insects (May), peaking towards the end of summer. Their activity gradually decreases in the following weeks until late November, when adults were completely absent.

Fig. 3. Field activity-pattern of nymphs and adults of C. vagans.
On the other hand, males and females were quite synchronized in their activity periods and had a balanced population size, with a sex ratio close to 1 (52.6% ♂♂:47.4% ♀♀).
Trophic response tests
The statistical comparisons of data on the mean consumption (table 2) indicate a noticeable preference of adults for the herbaceous plants when a mixed diet is supplied. The average amount of Lolium consumed was significantly higher than that of Cistus. However, nymphs and adults subjected to a monospecific diet showed no significant differences between the average consumption of the two types of food offered. In the case of nymphs, the statistical comparison did not find significant differences in consumption in any of the treatments considered.
Table 2. Results of the comparison test of average consumption of C. vagans.

S–W, normality test Shapiro–Wilk; P, statistical significance for α ≥ 0.05; Z, statistical significance Mann–Whitney U test; T, statistical significance T-Student's test; N, number of samples of each type of food;
![]() ${\bar{\rm X}}$
, average consumption (g); SD, standard deviation.
${\bar{\rm X}}$
, average consumption (g); SD, standard deviation.
Reproductive biology
Anatomical observations related to the ovarian development of the 29 females collected in 2012, throughout the adult activity period, enabled us to establish the course of their reproductive stage (fig. 4). Each of the females dissected was assigned to their respective maturation stages (immature, mature, gravid, old and old females starting reproduction).

Fig. 4. Temporal course of reproductive stage of females (olds, gravids, matures and inmatures) of C. vagans.
The start of ovarian development coincides with the beginning of the adults’ activity. First, females with completely developed eggs (mature) and gravid females appeared in April, although some immature specimens were observed at this time and were present until June. In early summer, the female population included both females at different stages of maturity and those which have already oviposited and that showed clear signs of senescence (wearing in the anatomical structures). The egg-laying activity continued in summer and ended in September.
To estimate the reproductive potential of C. vagans, the monthly average number of mature eggs by dissected females was analysed (fig. 5). According to the results, the egg-laying period ranges from April to August, the average number of mature eggs/♀ oscillates between 5 and 11, and the average size of mature eggs is 1 ± 0.1 mm Ø and 4 ± 0.3 mm in length.

Fig. 5. Temporal course of the average number (and standard deviation, SD) of eggs/fertile females of C. vagans.
The reproductive biology was monitored in the laboratory (fig. 6). The first pods occurred at the end of May and continued to the end of August, reaching the maximum in July, when the species was at its reproductive optimum (fertility of 1.33 egg-pods/♀). The total number of egg-pods collected during the experiment was 34 and the ratio of egg-pods/♀ was 2.26.

Fig. 6. Temporal course of the relation number of egg-pods/number of females of C. vagans.
In addition, the results provide the following information:
-
1. The egg-pods were buried between 2 and 5 cm deep.
-
2. The average size of egg-pods was 4 ± 0.9 mm Ø and 9.8 ± 1.7 mm in length.
-
3. The average number of eggs per egg-pod was 7 ± 1.6; the average size of viable eggs was 1 ± 0.1 mm Ø and 4 ± 0.2 mm in length.
Discussion
Autecology of C. vagans
According to our results, C. vagans is a ubiquitous and abundant grasshopper in the studied area, which is consistent with previous data relative to the Iberian Peninsula (Presa et al., Reference Presa, Montes and Ramírez-Díaz1983; Llucià-Pomares, Reference Llucià-Pomares2002; Olmo-Vidal, Reference Olmo-Vidal2002) reporting that the species can colonize a great diversity of biotopes including sand grassland, steppe habitats, heathland, pasture and scrubs and their transitional ecotones (Hochkirch et al., Reference Hochkirch, Gärtner and Brandt2008). Research performed in Central Europe indicates that C. vagans colonizes any type of vegetation (woody plants, herbs, grasses, etc.) avoiding bare soil and areas with a large fraction of gravel (Wunder, Reference Wunder2001; Kurth, Reference Kurth2007). Data referred to the Iberian Peninsula also link this species to a great variety of environmental types, including beaches, dunes, pine and oak forests, grassland and bush (Llucià-Pomares, Reference Llucià-Pomares2002; Olmo-Vidal, Reference Olmo-Vidal2002).
Nevertheless, C. vagans was not observed in the sampling plot P1 (Mesas Bajas). The study site P1 was an extremely uniform wasteland due to intensive farming and grazing, which led to a complete removal of forest vegetation and hampered any spontaneous regeneration of the understory (Gallardo, Reference Gallardo2011). These circumstances restrict the presence of insect species that prefer to colonize grass and shrubs (Braschler et al., Reference Braschler, Marini, Thommen and Baur2009). Similar results were also observed by Kurth (Reference Kurth2007) in the Southern Alps. Intensive grazing on natural ecosystems affects even species, which are able to colonize open environments and bare soils, like C. vagans, because of the reduction of the amount of food available, the micro-climate change and the disruption and disturbance of spawning areas (O'Neill et al., Reference O'Neill, Olson, Rolston, Wallander, Larson and Seibert2003). In sampling plots with higher environmental heterogeneity and hunting exploitation (e.g., P9 and P11), the populations of C. vagans were noticeably more numerous. This result confirms the importance of maintaining a mosaic of habitats for the conservation of Orthoptera (Schirmel et al., Reference Schirmel, Blindow and Fartmann2010). By contrast, the most homogeneous and extensive environments are not suitable for the proliferation of these insects, which prefer more ecologically diverse environments (Tscharntke et al., Reference Tscharntke, Steffan-Dewenter, Kruess and Thies2002; Tews et al., Reference Tews, Brose, Grimm, Tielböger, Wichmann, Schwager and Jeltsch2004).
Regarding microhabitat, our observations suggest that C. vagans occurs in xeric grasslands and dry ecotones and transitional zones, which is consistent with results reported by Hochkirch et al. (Reference Hochkirch, Gärtner and Brandt2008) and Llucià-Pomares et al. (Reference Llucià-Pomares, Íñiguez and Quiñones2009), respectively.
Phenologically, the species displays a broad period of activity, as described by most research on Mediterranean ecosystems (García, Reference García1984; García & Presa, Reference García and Presa1985; Olmo-Vidal, Reference Olmo-Vidal2002; Voisin, Reference Voisin2003). A few authors have attributed a more restricted activity period to C. vagans (Gómez & Presa, Reference Gómez and Presa1990; Gómez et al., Reference Gómez, Presa and García1992; Pardo & Gómez, Reference Pardo and Gómez1995; Hernández et al., Reference Hernández, Clemente, García and Presa1998; Llucià-Pomares & Fernández-Ortín, Reference Llucià-Pomares and Fernández-Ortín2009). To explain this it must be taken into account that the life cycle of Orthoptera is also influenced by environmental factors including climatic variables, relief, soil substrate, vegetation and food availability that largely determine activity in both nymphal and imaginal phenology of species, as well as other parameters (population density, birth and death rates, nymphal development, etc.; Carter et al., Reference Carter, Macrae, Logan and Holtzer1998) that induce local variations in the full phenology of the species. In fact, in samplings performed to study trophic response and reproductive biology (2011 and 2012, respectively) the first adults were already recorded in April, which extends its activity period. According to our data, C. vagans could be considered a eurytopic species that is capable of activity in wide ranges of temperature and environmental humidity.
The immature population shows a clear trend of concentrating activity in the spring–summer transition, when climatic conditions seem to be more suitable for development (Badih et al., Reference Badih, Hidalgo, Ballesta, Ruano and Tinaut1997). This result is consistent with those of Kleukers et al. (Reference Kleukers, Van Nieukerken, Odé, Willemse and Van Wingerden1997) for C. vagans, and is commonly observed for Orthopteran communities from the Mediterranean Basin (Cebada & Novoa, Reference Cebada and Novoa1985; Aguirre & Pascual, Reference Aguirre and Pascual1988; Voisin, Reference Voisin2003; Massa et al., Reference Massa, Fontana, Buzzetti, Kleukers and Odé2013).
Adults are abundant during the summer. Nevertheless, it should be mentioned that the decrease in adult activity observed in August could be explained by the extreme environmental conditions (dryness and temperature) of this month, which forces the insects to seek shelter in the vegetation, making observation and sampling difficult. In fact, this decrease has not been observed at the highest latitudes and with more consistent climates (Steiner, Reference Steiner2006; Jaulin & Baillet, Reference Jaulin and Baillet2007; Kurth, Reference Kurth2007).
Overall, C. vagans is an univoltine species in the research area, in accordance with Hochkirch et al. (Reference Hochkirch, Gärtner and Brandt2008), with the presence of nymphs in spring and adults mostly in summer. The activity of adults extends to late autumn and overwintering occurs during the embryonic stage (Ingrisch, Reference Ingrisch1983; Beckmann et al., Reference Beckmann, Purse, Roy, Roy, Sutton and Thomas2015), as in other Chorthippus species (Wingerden et al., Reference Wingerden, Musters and Maaskamp1991). Nevertheless, our data also suggest that some adults may survive the winter, reappearing in the following spring based on the observance of some reproductively mature females in April. Most grasshoppers from temperate and sub-tropical regions usually overwinter as diapausing eggs (Tanaka & Zhu, Reference Tanaka and Zhu2008), while some species may overwinter as diapausing adults (Tanaka & Okuda, Reference Tanaka and Okuda1996). Each diapause phase may be influenced by environmental factors as well as by insect genetics (Tauber et al., Reference Tauber, Tauber, Obrycki, Gollands and Wright1988; Han & Denlinger, Reference Han and Denlinger2009). Zhu et al. (Reference Zhu, Cui, Fan and Liu2013) suggest that the females of some grasshopper species (Stenocatantops splendens Thunberg; Orthoptera, Catantopidae) are colder hardy than their male counterparts, which may result from a trade-off between investments in cold tolerance and reproduction. This is consistent with our observation of reproductive females already in the middle of spring.
Trophic response
The fact that, of the four treatments tested, only significant differences were found between the consumption of grass and Cistus in the mixed diet for adults indicates that this species is polyphagous organism in the Mediterranean ecosystem with some preference for grass in the case of adults. This result is in consistent with the statements of Treiber (Reference Treiber2001) and Steiner (Reference Steiner2006), which considers C. vagas mainly graminivorous, being also able to consume other plant species such as Ulex or Astragalus.
The mandibular typology of this species is consistent with those of a grass-feeding type and also coincides with the information in the literature related to the mouthparts of Gomphocerinae (Joern & Lawlor, Reference Joern and Lawlor1980; Isern, Reference Isern1992; Chen et al., Reference Chen, Zhao and Kang2003; Smith & Capinera, Reference Smith and Capinera2005; Savitsky, Reference Savitsky2010). Nonetheless, in a more recent survey other non-gramineous but Fabaceae plants such as the dwarf gorse (Ulex minor Roth.) are considered a main food support for this species (Edwards, Reference Edwards2011). The euryphagy of C. vagans mentioned in the literature and obtained in our data could be explained by a behavioural plasticity or ecological opportunism common in Orthoptera (Smith & Capinera, Reference Smith and Capinera2005) with a genetic background (Steiner, Reference Steiner2006).
More specifically, and with reference to the Iberian fauna, there is evidence of a graminivorous diet for other Chorthippus species including C. dorsatus (Zetterstedt, 1821), C. binotatus (Charpentier, 1825), C. apricarius (Linnaeus, 1758), C. biguttulus (Linnaeus, 1758), C. albomarginatus (De Geer, 1773) and C. parallelus (Zetterstedt, 1821) (Vieira, Reference Vieira1989; Gangwere, Reference Gangwere1990; Isern, Reference Isern1992). In accordance with our results, these Gomphocerinae species may also feed on shrub plants as a food supplement when the availability of grass is restricted (Picaud et al., Reference Picaud, Bonnet, Gloaguen and Petit2003).
Reproductive biology
The information obtained from our laboratory tests about the reproductive life cycle of C. vagans confirms that this is a univoltine species, which is also consistent with the data of Rauh (Reference Rauh, Schlumprecht and Waeber2003); Jaulin & Baillet (Reference Jaulin and Baillet2007) and Hochkirch et al. (Reference Hochkirch, Gärtner and Brandt2008). Ovarian development of C. vagans begins in the spring, coinciding with the activity of the adults. The appearance of the first gravid females in April is consistent with the results obtained in the laboratory dating the first egg-pods in May. In summer, the sample of females is composed of individuals in different stages of ovarian maturation, together with a small fraction of females that have already reproduced, as revealed by the presence of corpora lutea and also the clear signs of senescence (wearing integumentary structures).
Anatomical studies also reveal that the females of this species may have an extended period of egg-pod development (April–August), based on the presence of egg-pods between late May and August in the laboratory; the optimal period was in July with 1.33 egg-pods/♀. This result is not conclusive because research indicates that the number of egg-pods for specimens of the same species can vary depending on the environmental conditions (natural or experimental; Köhler & Brodhun, Reference Köhler and Brodhun1987; Kriegbaum, Reference Kriegbaum1997), usually increasing under controlled laboratory conditions (Uvarov, Reference Uvarov1966).
In the laboratory, egg-pods were found between 2 and 5 cm in depth. Treiber (Reference Treiber and Detzel1998) indicates that C. vagans oviposit more superficially (0.5–1.5 cm). However, it has been also observed that other Chorthippus species, such as C. brunneus, usually lay their eggs up to 6 cm deep (Atkinson & Begon, Reference Atkinson and Begon1987; Treiber, Reference Treiber and Detzel1998), which is more consistent with our results.
Furthermore, the average number of eggs/female on anatomical dissections ranged between 5 and 11, and is consistent with the laboratory results (7 ± 1.6 egg number/egg-pod) and with data provided by Waloff (Reference Waloff1950) and Richards & Waloff (Reference Richards and Waloff1954) (6 ± 0.2), also for C. vagans. This result is also consistent with observations of other species of Chorthippus (9.5 eggs for C. parallelus and 9.26 for C. biguttulus (Kriegbaum, Reference Kriegbaum1997); 8–14 eggs for C. brunneus (Atkinson & Begon, Reference Atkinson and Begon1987; Wall & Begon, Reference Wall and Begon1987)).
There is fairly comprehensive knowledge of the number of nymphal instars in studies on C. vagans performed in Central Europe: four for males and four to five for females (Richards & Waloff, Reference Richards and Waloff1954; Ramsay, Reference Ramsay1964; Ingrisch & Köhler, Reference Ingrisch and Köhler1998).
Finally, it should be mentioned that there are multiple issues requiring consideration in future research relative to the life traits of this species in the Mediterranean ecosystems, such as the number and duration of nymphal stages, because it has been demonstrated that environmental variations affect the life cycle of Chorthippus species (Telfer & Hassall, Reference Telfer and Hassall1999).
Other issues to be considered in the future are mating frequency, which can influence the oviposition rate (Arnqvist & Nilsson, Reference Arnqvist and Nilsson2000) and reproductive potential (Bellinger & Pienkowski, Reference Bellinger and Pienkowski1985; Taylor & Whitman, Reference Taylor and Whitman2010). When these questions are clarified, a more accurate understanding of the adaptation of C. vagans to the Mediterranean ecosystems will be possible. This information would be very valuable to perform an accurate environmental management in order to prevent the regression of this species that has occurred in more septentrional areas of Europe.
Acknowledgements
We thank our colleague Mr. Juan M. Hidalgo for the assistance in field. This research has been financially supported by ACUAES (Aguas de la Cuenca de España, S.A., Ministry of Agriculture, Food and Environment, Government of Spain) and by Ingeniería y Gestión del Sur, S.L. (Grupo IG-IPA).











