Introduction
Fundamental research on the biology, ecology, behaviour and host plant interactions have contributed significantly in formulating effective fruit fly pest management and control strategies (Allwood, Reference Allwood, Allwood and Drew1997; Koyama et al., Reference Koyama, Kakinohana and Miyatake2004; Roitberg, Reference Roitberg2007; Clarke et al., Reference Clarke, Powell, Weldon and Taylor2011). An advanced understanding of the tephritid fruit fly behavioural response to phytochemical lures, such as methyl eugenol (ME), cue-lure and medlure, has led to their successful integration into fruit fly area-wide management through the male annihilation technique, or incorporated in sterile insect technology programmes as ‘aromatherapy’ male pre-release treatments to increase sterile male mating competitiveness (Qureshi et al., Reference Qureshi, Bughio and Siddiqui1981; Vargas et al., Reference Vargas, Stark, Kido, Ketter and Whitehand2000; Reference Vargas, Piňero, Mau, Jang, Klungness, McInnis, Harris, McQuate, Bautista and Wong2010; Shelly et al., Reference Shelly, Edu and McInnis2010). However, in the case of the dusk-mating Bactrocera umbrosa, the information pertaining to its biology, sexual development and mating behaviour that are mediated by ME remain unknown.
B. umbrosa (Fabricius) (Diptera: Tephritidae), also known as the Artocarpus fruit fly, is an oligophagous fruit fly species that infests mainly fruits from the Moraceae family such as jackfruit (Artocarpus heterophyllus), chempedak (A. integer) and breadfruit (A. altilis) (Allwood et al., Reference Allwood, Chinajariyawong, Drew, Hameck, Hancock, Hengsawad, Jipanin, Kon Krong, Kritsaneepaiboon, Leong and Vijaysegaran1999). The species occurs over a wide geographic range extending from the tropical South Pacific (New Caledonia, Papua New Guinea, Solomon Islands, Vanuatu) westwards into Southeast Asia (Philippines, Indonesia, Malaysia and southern Thailand) (Hardy, Reference Hardy1973; Vijaysegaran, Reference Vijaysegaran1988; Michaux & White, Reference Michaux and White1999). This species is a primary pest of breadfruit, a starch crop, which is cultivated as a staple food, especially in Pacific countries such as Vanuatu (Vagalo et al., Reference Vagalo, Hollingsworth, Tsatsia, Allwood and Drew1997; Haq, Reference Haq2006). An increase in jackfruit cultivation as a high quality commercial food crop in Southeast Asia, especially Malaysia (Mengersen et al., Reference Mengersen, Quinlan, Whittle, Knight, Mumford, Wan Ismail, Tahir, Holt, Leach, Johnson, Sivapragrasam, Lum, Sue, Othman, Jumaiyah, Tu, Anh, Pradyabumrung, Salyapongse, Marasigan, Palacpac, Dulce, Panganiban, Soriano, Carandang and Hermawan.2012), has significantly elevated the pest status of B. umbrosa.
Whilst B. umbrosa has long been identified as an economically important pest (Drew et al., Reference Drew, Hooper and Bateman1982), this species is very much understudied. This is in part due to difficulties in raising laboratory colonies (Walker et al., Reference Walker, Vueti, Hamacek, Allwood, Allwood and Drew1997), as well as the restricted seasonal availability of many Artocarpus fruits. Thus published work on the biology of B. umbrosa remains restricted to a very few field studies, most based on trapping records obtained using ME baited traps (Tan, Reference Tan1985; Tan & Jaal, Reference Tan and Jaal1986; Clarke et al., Reference Clarke, Allwood, Chinajariyawong, Drew, Hengsawad, Jirasurat, Kong Krong, Kritsaneepaiboon and Vijaysegaran2001). ME is a naturally occurring plant secondary compound, to which the males of many Bactrocera species, including B. umbrosa, respond strongly and positively (Tan, Reference Tan1985; Tan & Jaal, Reference Tan and Jaal1986; Clarke et al., Reference Clarke, Allwood, Chinajariyawong, Drew, Hengsawad, Jirasurat, Kong Krong, Kritsaneepaiboon and Vijaysegaran2001; Tan & Nishida, Reference Tan and Nishida2012).
Studies on the Oriental fruit fly, Bactrocera dorsalis (Hendel), carambola fruit fly Bactrocera carambolae Drew & Hancock, Queensland fruit fly, Bactrocera tryoni (Froggatt), and melon fly Zeugodacus cucurbitae (Coquillett) have demonstrated that their positive response and associated compulsive feeding behaviour on a small group of plant derived phenylpropanoids (of which ME is one) is linked to increasing rates of male mating success. Ingested chemicals are either sequestered unchanged or converted into derivative products, stored in the male pheromone glands, and released as pheromonal components that enhance male mating competitiveness (Shelly & Dewire, Reference Shelly and Dewire1994; Tan & Nishida, Reference Tan, Nishida, McPheron and Steck1996; Hee & Tan, Reference Hee and Tan1998; Khoo & Tan, Reference Khoo and Tan2000; Wee et al., Reference Wee, Tan and Nishida2007; Kumaran et al., Reference Kumaran, Balagawi, Schutze and Clarke2013). Additionally, the compounds may be associated with the mating behaviour of Bactrocera not just through male pheromone modification, but they have also been associated as acting as mating rendezvous sites (Raghu & Clarke, Reference Raghu and Clarke2003), or with increasing male–male competitive advantage (Kumaran et al., Reference Kumaran, Prentis, Mangalam, Schutze and Clarke2014a ). As more Bactrocera species are studied with respect to these chemicals it is clear that while the generality that the compounds are always associated with the mating system remains, the specific mechanism by which this occurs may vary between Bactrocera species, or may even vary within one fly species depending on the chemical (Kumaran et al., Reference Kumaran, Hayes and Clarke2014b ). Such findings are an important justification for repeating similar trials on different Bactrocera species.
Given the background literature, the aim of this study is to investigate the ecological significance of ME in the reproductive ecology of the Artocarpus fruit fly. Specifically, we evaluated: (i) the species’ sexual development, i.e. age at which first mating occurred in relation to; (ii) males’ dose- and age-related response to the lure and the effects of ME consumption by males by examining; (iii) the intraspecific female–male and male–male attractions in flight tunnel assays as well as; (iv) male mating performance in semi-field assays.
Materials and methods
Insects
Mature larvae of B. umbrosa from infested Artocarpus fruits, i.e. jackfruit and chempedak, were collected from villages and fruit orchards in Selangor and Terengganu states, Peninsular Malaysia. Larvae were allowed to pupate in moist and sterilized fine sand. Emergent adults were sexed within 5 days and maintained separately in large screen cages (cage dimension: 45 × 45 × 45 cm3) under a 12 h light:12 h dark regime at 25–29 °C with 76–82% relative humidity. All flies were provided with an artificial adult diet containing yeast, sugar, protein (Wee & Tan, Reference Wee and Tan2000) and water ad libitum. Sexually mature, virgin male and female flies of age 25–35 days after adult emergence (DAE) were used in experiments unless otherwise stated.
Chemical
ME (1,2-dimethoxy-4-[2-propenyl]benzene; >99.8% purity) (Agrisense-BCS Ltd., London, UK) was serially diluted with absolute ethanol to obtain concentrations of 0.001, 0.01, 0.1, 1.0, 5.0, 10.0, and 100 mg ME per 100 µl solution.
Preparation of ME-fed males
In the morning between 08:00 to 11:00 h, when B. umbrosa males are highly responsive to ME (Tan, Reference Tan1985), 0.5 µl of neat ME was dispensed via a glass syringe (Hamilton) onto a Whatman no. 1 filter paper (9.0 cm diam.) that was placed in a disposable Petri dish. An individual male fly was exposed to the ME source. The attracted male fly was allowed to feed on ME for 15 min. The procedure was repeated to produce 10 ME-fed males, which were transferred into a small cylindrical screen enclosure (7 cm diam. × 15 cm height) containing food and water ad libitum. The following day the treated males were transferred to the wind-tunnel laboratory (see below) 3 h prior to experimentation for acclimatization.
Dose- and age-related responses to ME
A tubular flight tunnel (30 cm diameter × 200 cm length) made of transparent polyacetate sheet as described by Wee & Tan (Reference Wee and Tan2000) was used in this experiment. Trials were conducted in the morning between 08:00–11:00 h in a laboratory, which received natural sunlight from the north. Room temperature and relative humidity were maintained between 25–29°C and 83–90%, respectively.
In the flight tunnel, sexually mature virgin B. umbrosa males were exposed to a series of ME doses to determine an optimum response dosage for use in the age-related response bioassay. This is to avoid underestimation of the species’ age-related response to ME if a lower-than-optimum dose is used for the assay. Starting from the lowest quantity at 0.001 mg, 100 µl of ME solutions was dispensed, using a 100 µl Hamilton® syringe, onto a piece of filter paper (4.25 cm diam.), which was placed on an inverted glass petri dish supported by a tripod (height: 15 cm). The tripod was placed ca. 10 cm away from the upwind grill. Two electric fans, placed at both ends of the tunnel and regulated by voltage regulators, generated a continuous and steady airflow (15–18 cms−1) that carries the ME plume from upwind to downwind.
During each trial, a group of 10 male flies were assayed for their response to increasing quantities of ME. A positive response was when an individual male fly at the downwind end took off, flew in zigzag motion against the airflow (Kennedy & Marsh, Reference Kennedy and Marsh1974) and finally landed and fed on the ME source at the upwind end within 10 min. The respondent fly was then immediately removed from the flight tunnel with an aspirator. After the 10-min assay, the remaining flies were recaptured and fresh batches of ten flies were used for subsequent doses. For controls, 100 µl absolute ethanol was used instead of a ME solution. The tubing was cleaned with 70% alcohol after each trial and the tubing was changed regularly to avoid contamination. A total of five replicates of multiple cohorts, with each replicate consisting of ten males, were performed for each ME dose tested.
For subsequent age-related response bioassay, similar procedures were employed except that males of different ages were assayed separately to the optimum dose of ME (=5.0 mg, see the section Results). Five replications, with each replicate consisting of ten males, were tested at 5, 7, 10, 20, 23, 25, 27, and 30 DAE without any flies being re-used.
Adult sexual maturation
On the DAE and between 1400 and 1600 h, 30 B. umbrosa male/female pairs were placed in a medium size screen cage (30 × 30 × 30 cm3) and provided with food and water ad libitum. Under low red light conditions, daily observations for mating pairs were conducted at the onset of dusk from 1 to 60 DAE. As soon as a pair was in copula, the pair was coaxed into an individual, labelled glass specimen vial (2 cm diam. × 8.5 cm). Their mating duration was monitored every 5 min thereafter until the pair separated. The experiment was replicated six times, with a total of 180 pairs observed. At the end of 60-day observation, age of flies when first mating occurred was plotted as accumulated mating percentages against DAE. In this paper we make the explicit assumption that mating is directly correlated with the reaching of sexual maturation, as has been done elsewhere (Ooi & Wee, Reference Ooi and Wee2016). This approach may slightly over-estimate the time required for flies to reach sexual maturity (i.e. if flies are sexually mature for one or more days before mating), but it cannot underestimate the time taken to reach sexual maturity as sexually immature Bactrocera do not mate (Fletcher, Reference Fletcher1987).
Effect of ME-feeding by males for intraspecific attraction
Ten sexually mature males, at 1-day post ME-feeding, were used as a live attraction source and assayed continuously for their attractancy to conspecific females in a flight tunnel. A mini cylindrical screen cage containing the ten ME-fed males was then positioned 10 cm away from the upwind end and perpendicular to the direction of air flow. Airborne volatiles released by the live ME-fed males were carried by the continuous air flow to the downwind end. At the downwind end of the flight tunnel, ten sexually mature virgin females were then released from a similar mini screen cage. A positive response was scored when the females flew in zigzag anemotactic flight (Kennedy & Marsh, Reference Kennedy and Marsh1974) for at least 50 cm and eventually landed at the upwind region, either directly on the attraction source or on the side wall of the flight tunnel. After the 10-min assay, 5 min was used to recapture the female test flies before a fresh batch of female flies were introduced. The experiment was conducted during dusk from 18:00 to 20:00 h with a total of eight time intervals for assays. Prior to each assay, the light intensities at the upwind and downwind areas were measured using a Lutron LX-101 light meter. For controls, similar procedures were repeated except that sexually matured ME-deprived males were used as the source of attraction. For each of the time intervals, seven and eight replications were conducted for the ME-fed and ME-deprived treatments, respectively.
To investigate the effect of ME-feeding by males in intraspecific male–male attraction, similar experimental procedures were employed except that sexually mature virgin males were released and assayed against the live ME-fed male source. ME-deprived male source was used as control measures. For each of the time intervals, four and five replications were conducted for ME-fed and ME-deprived treatments, respectively.
Effect of ME-feeding by males on male mating competitiveness
The effect of ME feeding by males on mating performance was evaluated in a semi-field outdoor cage (2.1 m × 2.1 m × 2.1 m). After light cold immobilisation, young virgin B. umbrosa males (<10 DAE) were individually marked with a small dot of either green or white enamel paint on its dorsum, using an entomological pin head. A day prior to the mating trial, sexually mature males (bearing green thoracic marking), in a group of ten, were allowed to feed on 10 µl (=10.5 mg) of pure ME dispensed on a filter paper (Whatman® no. 1) for 30 min. ME-deprived males (with white thoracic marking) were used as controls. To eliminate bias, colour markings were switched between the two treatments in alternate replications.
A non-host star-fruit tree (Averrhoa carambola L.) (canopy: 2.0 m height × 0.5 m width) was placed at the centre of the field cage to provide shade and resting sites for flies upon release. For male mating competitiveness assessment, an equal number of ME-fed and ME-deprived males were allowed to compete for the same number of females (n = 20–30 flies each, depending on the availability of flies). For acclimatization, all fly groups, contained in separate screen cages with food and water, were placed in the field cage 4 h before the onset of dusk. Males were released 1 h earlier than females to allow time for male territory establishment (McInnis et al., Reference McInnis, Rendon, Jang, van Sauers-Muller, Sugayama and Malavasi1999). During the dusk mating period, pairs were searched for and carefully coaxed into individual glass specimen vials. The following morning, the identity of the male of each mating pair was determined based on the thoracic colour marking. A total of eight replications were performed on different days using flies from different cohorts.
Statistical analysis
Data on proportion of responding and mated flies over the total number tested was transformed to a modified arcsine square root (Anscombe, Reference Anscombe1948) before subjected to further statistical analyses. For wind tunnel assay, one-way analysis of variance was used and means were separated by Tukey's test (P = 0.05). Student's t-test was used to evaluate (i) female (or male) attraction between ME-fed and ME-deprived males at each time interval during courtship period, and (ii) mating competitiveness between ME-fed and ME-deprived males (P = 0.05). Data analyses were performed using Sigma Plot 12.0.
Results
Adult sexual maturation
The first mating of B. umbrosa occurred at 11 DAE, but matings remained low until 17–20 days of age, at which time the rate of matings increased sharply such that by 26–27 DAE about 50% of the test population had mated (fig. 1). By the end of the 60-day observation period, 84.7% of test flies had mated, with an average mating duration of 55.2 ± 1.7 min.
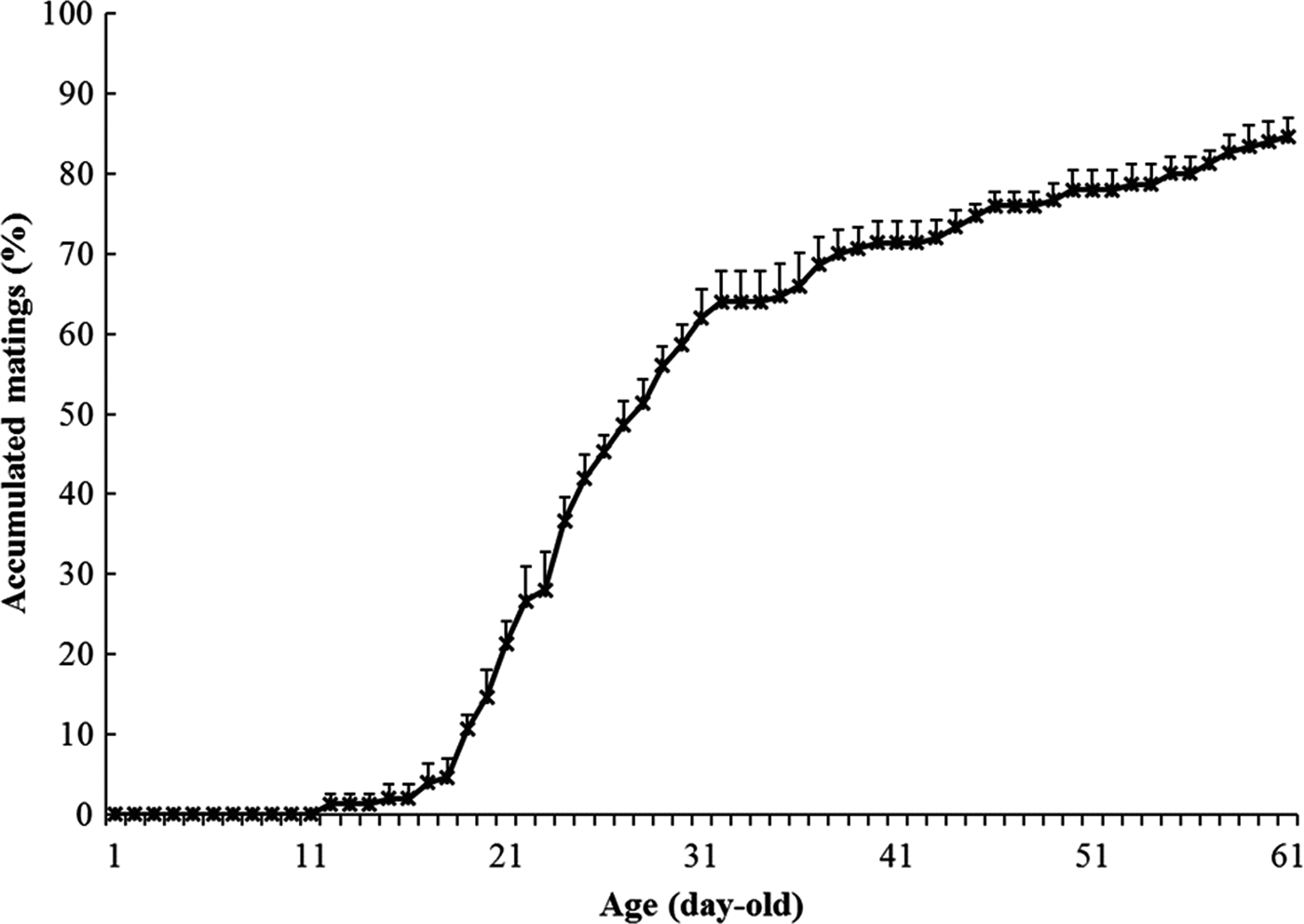
Fig. 1. Accumulated first mating percentages (mean ± SE) of the Artocarpus fruit fly, Bactrocera umbrosa based on a 60-day observation period in screen cages since adult emergence. A total of six replications (n = 30 each, with a total of 180 pairs observed) from different cohorts was conducted.
Dose- and age-related response to ME
Response behaviour
Once the presence of ME was detected, an attracted male would fly upwind in a zig-zag manner to the ME source. In these instances, some males demonstrated strong and non-stop continuous flight and landed on ME source in less than 10 s. There were also some males that made several stops during the upwind flight, but still landed at the source within the experimentally stipulated time. In most cases, immediately after arrival, the males were observed to extend their proboscis and started to feed on the ME source. Males that alighted early at the ME source would often exhibit territorial behaviour with head-butting actions towards later arriving males, particularly when lower quantities of ME were offered.
Dose response
Males of B. umbrosa responded differently to different doses of ME tested (F = 34.597, df = 7, 32; P < 0.001). A solution of 0.001 mg ME did not elicit any response from B. umbrosa males, while a small number of males, less than 10%, responded at 0.01 mg ME. Male response peaked at between 40 and 50% at 1.0 mg, and was then stable at that response level to the maximum treatment dosage of 100.0 mg (P > 0.05, Tukey's test) (fig. 2). With no significant treatment difference between 1.0 and 100.0 mg ME, 5.0 mg was arbitrarily selected as the optimum ME dosage for subsequent age-related response bioassays.

Fig. 2. Zigzag anemotactic flight of sexually mature Artocarpus male fruit flies, Bactrocera umbrosa Fabricius to different quantities of methyl eugenol assayed in a wind tunnel. Means (bars = ± SE) followed by different alphabets are significant different (P = 0.05, Tukey's test).
Age response
When B. umbrosa males of different ages were tested against the optimum ME dose, a significant effect of age-related responses was observed (F = 29.418, df = 7, 32; P < 0.001). Bactrocera umbrosa males did not respond to ME until they were 7 DAE, but by 10 DAE the males’ attraction to ME increased rapidly to >50% (fig. 3). Although there were fluctuations in the ME responses between 10 and 27 DAE, the differences were not statistically significant (P > 0.05, Tukey's test). When the male flies were older at 30 DAE, their attraction to ME decreased significantly (P < 0.05; Tukey's test) (fig. 3).
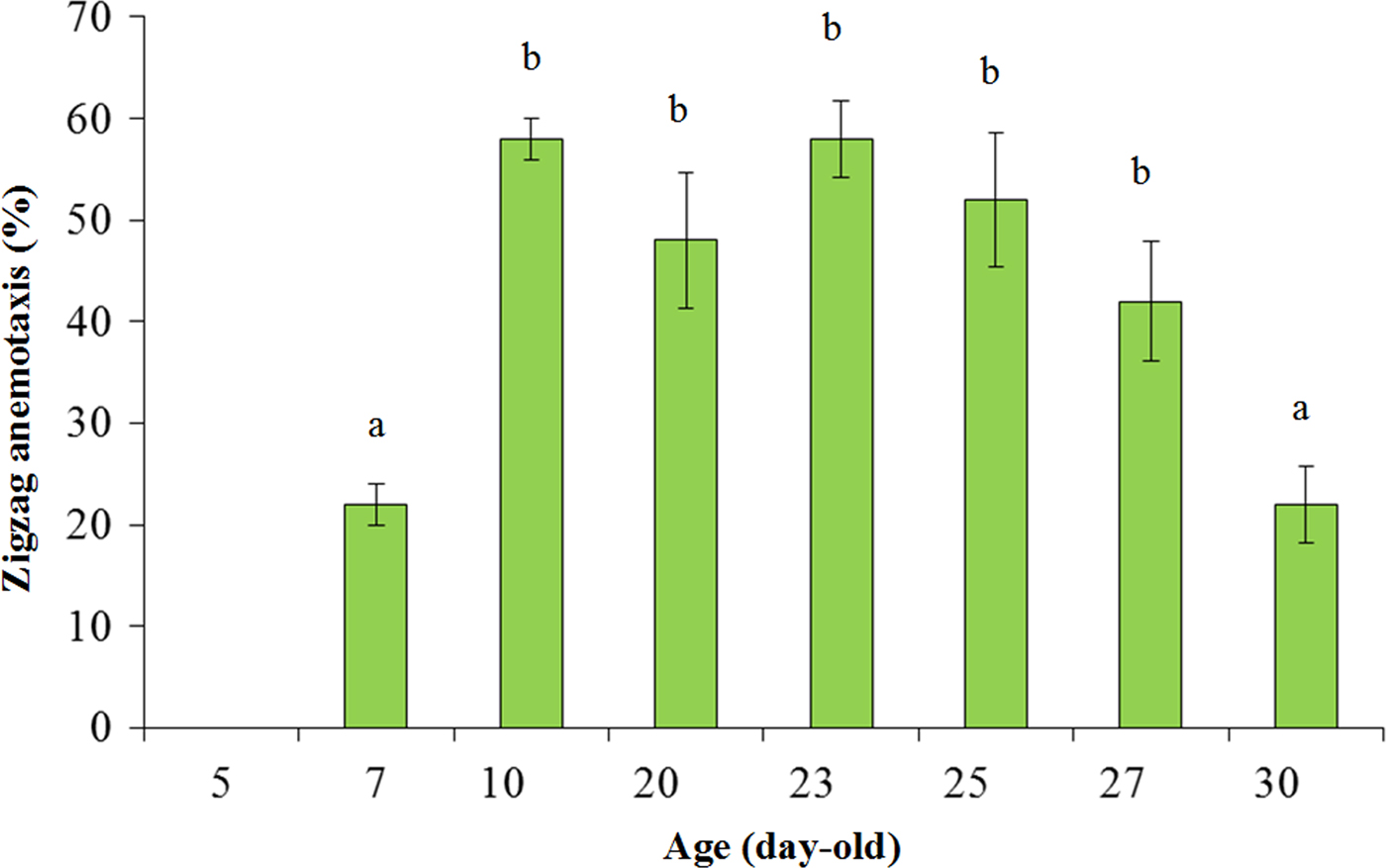
Fig. 3. Age-related zigzag anemotactic response (mean ± SE) by males of Artocarpus fruit fly, Bactrocera umbrosa Fabricius to an optimum dose of methyl eugenol ( = 5 mg) in wind tunnel assays. Means (bars = ± SE) followed by different alphabets are significant different (P = 0.05, Tukey's test).
Effect of ME-feeding by males on intraspecific attraction in wind tunnel assays
In the wind-tunnel assays, B. umbrosa females demonstrated significant attraction to the live male sources, both ME fed (F = 14.811, df = 7,48; P < 0.001) and control (F = 10.921, df = 7,56; P < 0.001) (fig. 4). Females’ attraction to untreated males peaked at 18:45 h when light intensity approaching 300 lx, but decreased as light intensity declined below that (fig. 4a). However, ME-fed males were significantly more attractive to females with ca. 30% attraction as early as 18:30 h at a light intensity of 700 lx (t = 2.427, df = 13; P = 0.031). A two-fold increase in female attraction to ME-fed males was also observed at 18:45 h during peak courtship period (Mann–Whitney U test, P = 0.006) (fig. 4a).

Fig. 4. Zigzag anemotactic flights by sexually mature virgin (a) females and (b) males of the Artocarpus fruit fly, Bactrocera umbrosa to both methyl eugenol (ME)-fed (treatment) and ME-deprived (control) conspecific males during courtship period at dusk. Means (bars = ± SE) followed by different alphabets within the same treatment are significant different (P = 0.05, Tukey's test). Asterisks (*) indicate the level of statistical significance between ME-fed and ME-deprived treatment by Student's t-test at a particular time interval (*P < 0.05, **P < 0.01, ***P < 0.005).
B. umbrosa males responded positively to live ME-fed and ME-deprived males during dusk, with significant differences in attraction over time (Control: F = 9.519, df = 7,32; ME-fed: F = 27.519, df = 7,24; P < 0.001) (fig. 4b). The trend of male–male attraction was consistent to that of the female–male attraction, with peak attraction occurring at 18:45 h. However, a significant increase of male–male attraction following ME consumption was only observed at 18:45 (t = 5.54, df = 7, P < 0.001). At 19:30 h, when light intensity was approaching zero (<5 lux), the control male source was no longer attractive to test males. However, the ME-fed male source remained attractive to some males (ca. 5%) and some of the ME-fed males were still actively engaged in wing fanning at 19:30 h (fig. 4b).
Effect of male ME consumption on male mating competitiveness
At 1-day post ME consumption, significantly more ME-fed B. umbrosa males were successfully mated with females than ME-deprived males in the field cage assays (t = 3.848, df = 14, P = 0.0018) (fig. 5). When the light intensity was still high (>1500 lux), most of the male flies were seen grooming (i.e. preening antennae, wings and legs) with minimal physical contact with other males in the field cage. When light intensity dropped to slightly lower than 1500 lux at 18:00 h, several groups of ME-deprived males were observed to feed on the anal region of ME-fed males. At 18:30 h (ca. 500 lux), some males were observed to initiate wing fanning, which is a mechanism of pheromone dispersal in dacine tephritids. Wing fanning behaviour was observed to begin slightly earlier in ME-fed (18:20 h) than ME-deprived males.

Fig. 5. Mean (±SE; %) mating success by methyl eugenol (ME)-fed and ME-deprived (control) Bactrocera umbrosa males in field cage bioassays. Bars followed by different alphabets are significantly different (P < 0.001; Student's t-test).
At 18:35 h, a first mating pair was spotted, with the male being an ME-fed male. As the light intensity decreased further to 100 lux, more mating pairs were observed. Some males and females were also seemed to be attracted to mating pairs and tried to dislodge the mating couple. At 19:00 h, whilst most of the ME-deprived males were already at resting posture, the ME-fed males were still engaged in wing fanning as they continued to court the females despite low light intensity (<5 lux). At 19:15 h, when light intensity approaching 0 lux, most of the males had ceased from courtship activity.
Discussion
We demonstrate for the first time that ME plays a significant role in the mating system of B. umbrosa by promoting intraspecific sexual communication and enhancing male mating performance. The phenomenon of male pharmacophagous acquisition of ME (intake of non-nutritive compounds that are advantageous to the individual e.g. increased sexual attractiveness) (Boppré, Reference Boppré1984) occurs in most ME-attracted Bactrocera species examined to date, e.g. in B. dorsalis (Shelly & Dewire, Reference Shelly and Dewire1994; Tan & Nishida, Reference Tan, Nishida, McPheron and Steck1996; Hee & Tan, Reference Hee and Tan1998), B. carambolae (Wee et al., Reference Wee, Tan and Nishida2007), B. correcta (Orankanok et al., Reference Orankanok, Chinvinijkul, Sawatwangkhoungm, Pinkaew and Orankanok2009) and B. zonata (Sookar et al., Reference Sookar, Alleck, Ahseek, Khayrattee and Permalloo2009), with the exception of B. cacuminata (Raghu & Clarke, Reference Raghu and Clarke2003).
After ME consumption, males of B. dorsalis, B. carambolae, B. correcta and B. zonata converted ME into different analogues and stored them in the rectal gland (Tan & Nishida, Reference Tan, Nishida, McPheron and Steck1996; Wee et al., Reference Wee, Tan and Nishida2007; Tan et al., Reference Tan, Tokushima, Ono and Nishida2011). These additional sex pheromone components were incorporated into the natural pheromone repertoire and released along with the other endogenous pheromones during courtship (Wee & Tan, Reference Wee and Tan2005; Wee et al., Reference Wee, Tan and Nishida2007). Subsequently, it has significantly boosted the mating performance of these fruit fly species in intraspecific sexual competitions (Shelly & Dewire, Reference Shelly and Dewire1994; Tan & Nishida, Reference Tan, Nishida, McPheron and Steck1996, Reference Tan and Nishida1998; Wee et al., Reference Wee, Tan and Nishida2007; Orankanok et al., Reference Orankanok, Chinvinijkul, Sawatwangkhoungm, Pinkaew and Orankanok2009; Sookar et al., Reference Sookar, Alleck, Ahseek, Khayrattee and Permalloo2009). The chemical analyses of B. umbrosa male rectal gland following ME consumption has not been resolved. Preliminary investigations have shown ME-analogues were produced by male flies following ME-consumption (Nishida, Tan & Wee, unpublished data). Future chemical analyses on the pheromone composition in male B. umbrosa following ME consumption and subsequent assays of conspecific female response to the identified chemicals is expected to shed more light on the ecological role of ME in B. umbrosa.
In B. umbrosa, the earlier onset of calling activity may be associated with increase male metabolic activity following lure consumption. Kumaran et al. (Reference Kumaran, Prentis, Mangalam, Schutze and Clarke2014a ) reported that zingerone- and cue lure-fed males of B. tryoni increased locomotor activity and inter-male aggression as part of the mechanism that increase male competitiveness. This, together with a more attractive pheromone signals from ME-fed males may serve to increase overall female attraction and subsequently mating success.
The effect of lure consumption by Bactrocera males is often focused on female–male interactions. Only on a few occasions have male–male interactions following lure consumption been documented (ME: B. dorsalis – Hee & Tan, Reference Hee and Tan1998; B. caramboale – Wee et al., Reference Wee, Tan and Nishida2007; Cue lure: Z. cucurbitae – Khoo & Tan, Reference Khoo and Tan2000). Male–male interactions are particularly important when males advertise for potential mates. It is known that male aggression and/or territoriality and courtship displays that are factors of ‘lek’ behaviour are advantageous for successful matings (Shelly & Whittier, Reference Shelly, Whittier, Choe and Crespi1997). Numerous studies have been attempted to ascertain the role of leks in mating systems of tephritids (Shelly & Kaneshiro, Reference Shelly and Kaneshiro1991; Kaspi & Yuval, Reference Kaspi and Yuval1999; Shelly, Reference Shelly2000, Reference Shelly2001). A lek is a communal display area in which male congregate to engage in competitive displays before and during the breeding or courtship period that may entice visiting females who are surveying prospective partners and leading to copulation (Emlen & Oring, Reference Emlen and Oring1977; Fiske et al., Reference Fiske, Rintamaki and Karvonen1998). Results from present studies suggest that characteristics of lek may also be present in male B. umbrosa e.g. higher magnitude of male–male attraction in contrast to female–male attraction just before and during the peak courtship period. The attraction of more males to a male congregation may serve to amplify the pheromone signals to more female arrivals leading to higher chances of mating. Hence, we suggest that ME may also enhance the courtship display in male B. umbrosa.
In the field cage, ME-deprived B. umbrosa males were often seen aggregating and fed on the anal region of the ME-fed males. The ecological significance of such phenomena and its occurrence in nature has not been well studied. Similar interaction between ME-fed and ME-deprived males was also observed in B. carambolae during the courtship period (Wee et al., Reference Wee, Tan and Nishida2007). Further chemical analysis on the anal secretion of ME-fed male by Wee et al. (Reference Wee, Tan and Nishida2007) revealed the presence of (E)-coniferyl alcohol, a ME metabolite, which is highly attractive to females (Hee & Tan, Reference Hee and Tan1998). Although Wee et al. (Reference Wee, Tan and Nishida2007) suggested that the ME-fed males would benefit from this aggregation behaviour by diverting the attention of ME-deprived males from mating with arriving females, Shelly (Reference Shelly2010) argued that such behaviour may also distract potential mates and hamper the courtship and copulation process of the ME-fed males. In both cases, the focus has been on the ME-fed males, and no explanation was offered to the possible positive/negative effect on the ME-deprived males. We speculate that the ME-deprived males may likely gain direct benefit from the anal-feeding behaviour by incorporating the ME metabolites obtained directly into its pheromone repertoire without any need/cost of chemical conversion, thus being a more cost-effective way to acquire the much sought-after sex booster. Such male–male interactions following ME consumption and their ecological significance certainly warrant further investigation.
Virgin males responded to ME as early as 7 DAE, when no mating had occurred. By 11 DAE, i.e. at the age when first mating occurred, more than 50% of the virgin males would have already responded to ME. While the reason behind ‘immature’ B. umbrosa male attraction to ME is not known and not within the scope of this study, we cannot discount the possibilities that (i) males mature earlier than female but do not mate until a few days later; or (ii) male mature earlier than females, which is why they respond to the lure but mating is not seen. Given that all flies used were direct emergent from field-collected fruits and it is common for wild strain fruit flies to mate much later in age than laboratory strain (due to high protein diet supply in the laboratory), the results obtained would realistically represent the sexual maturation although it may be slightly overestimated. This is a significant finding and important input in the development of a control strategy against B. umbrosa. It has been acknowledged that one of the essential pre-requisites for a successful and effective male annihilation technique (MAT) against the fruit fly species lies in the ability of the males to respond to the lure before sexual maturity attainment (Wong et al., Reference Wong, Couey and Nishimoto1982, Reference Wong, McInnis and Nishimoto1989). By targeting and mass-kill the sexually immature males, this technique would deprive the female population of mating partners and eventually lead to eradication of the pest population (Hendrichs et al., Reference Hendrichs, Vreysen, Robinson, Kenmore, Vreysen, Robinson and Hendrichs2007). Therefore, MAT could serve as an effective birth control strategy against B. umbrosa as almost 50% of the males would have been trapped and killed before the males are sexually able to inseminate females leading to effective population suppression. This technique is especially helpful as the species has a narrow host range. Thus, when combined with other integrated fruit fly management strategies, such as protein bait spray, it is possible to greatly suppress if not eradicate these species from establishing in a new country.
Taken together, the current finding suggests that ME plays a prominent role in the reproductive ecology of B. umbrosa in that the species have developed fine-tuned affinity to ME, which is highly associated with its sexual maturation attainment and obtained mating advantage following the acquisition of ME by males. The fact that the attracted B. umbrosa males often demonstrated aggression or territorial behaviour on other alighted males at the same ME source such as chasing away and/or head-on fighting against other intruding males can be translated as if they are protecting a resource of great value. Hence, these precise and specific responses demonstrated by male fruit flies towards male lures at a certain state of physiological readiness, must be of great adaptive significance from the ecological and evolutionary point of views. This certainly warrants future investigations.
Acknowledgements
We are grateful to Professor Anthony R. Clarke (Queensland University of Technology, Australia) for his valuable comments on the revised manuscript. This project is supported by Research Incentive, Ministry of Higher Learning Malaysia (IP-2014-066) awarded to S.L. Wee.








