Introduction
Larvae of the screwworm, Cochliomyia hominivorax (Coquerel) (Diptera: Calliphoridae), are obligate parasites of endothermic vertebrates that caused economic losses estimated to exceed $1 billion annually before their eradication from the United States, Mexico and most of Central America by using the sterile insect technique (SIT) (Vargas-Terán et al., Reference Vargas-Terán, Hofmann, Tweddle, Dyck, Hendrichs and Robinson2005). Screwworms remain a threat to domestic animals and wildlife, as well as humans, in regions of their current distribution, part of the Caribbean and all of South America (with the exception of Chile) (Wyss & Galvin, Reference Wyss and Galvin1996). Major challenges to the eradication effort include monitoring the progress of eradication in zones under treatment with sterile flies, detecting reinfestations in screwworm-free zones and monitoring the area surrounding the mass production facilities for the escape of fertile flies. Consequently, effective monitoring, surveillance and quarantine methods should be adopted because sporadic outbreaks and reintroduction of this pest to the previously eradicated regions (southwestern United States and Mexico), as well as new geographical regions (Libya, Australia, and France), by livestock trade or human travel have threatened the efficacy of SIT (Chermette, Reference Chermette1989; Hall & Beesley, Reference Hall and Beesley1990; Searson et al., Reference Searson, Sanders, Davis, Tweddle and Thornber1992; Narang & Degrugillier, Reference Narang and Degrugillier1995).
Additionally, the secondary screwworm, Cochliomyia macellaria (Fabricius) (Diptera: Calliphoridae), shows morphological similarity and overlap in geographic distribution with C. hominivorax. C. macellaria also coexists with C. hominivorax in the same infested wound, thereby contributing to numerous misidentifications (Leite & Guevara, Reference Leite and Guevara1993; Litjens et al., Reference Litjens, Lessinger and de Azeredo-Espin2001). Cochliomyia macellaria is a saprophagous species that feeds primarily on carrion and is a secondary agent of myiasis (Litjens et al., Reference Litjens, Lessinger and de Azeredo-Espin2001). Therefore, it is important to investigate novel and reliable methods for correct identification of these two species because of the possibility of sabotage or fraud for outbreak samples (in one case, emergency release of sterile flies had been made on samples that proved negative on subsequent analysis: Anonymous, 1990). A rapid, simple and easily interpretable screening system that can distinguish closely related insect species would speed research on wild populations and improve insect control decisions. A blocking enzyme-linked immunosorbent assay, based on monoclonal antibodies against screwworm antigens, was developed that differentiated screwworm eggs, larvae, pupae and adults from those of the secondary screwworm, as well as Phormia regina (Meigen), Lucilia sericata Meigen, Calliphora vicina Robineau-Desvoidy and Chrysomya rufifacies (Macquart) (Figarola et al., Reference Figarola, Skoda, Berkebile and Foster2001). However, apart from the development of a monoclonal antibody-based assay, the ability of DNA markers for identification also needs to be explored.
Currently, screwworm eradication programmes rely heavily on the use of SIT. A major constraint to the eradication effort is the limited amount of information regarding the population structure for implementing SIT. Therefore, assessment of the genetic population structure of the screwworm pest will be valuable to the success of the eradication program (Bram, Reference Bram and Graham1985; FAO, 1992). It is not known whether the populations of C. hominivorax in South America are the ‘same’ as in Central America, i.e. whether the populations are related or isolated. These questions are important because, if there is mating isolation, eradication could not occur if an inappropriate strain was reared and released. Therefore, if the wrong strain was being reared the program would fail because of assortative mating and failure of sterile males to mate with native females.
The questions of population structure, identification and differentiation of C. hominivorax from C. macellaria and other related blow fly larvae can, to a large extent, be resolved using molecular genetic tools. Molecular genetic markers represent a powerful tool for identifying source populations in cases of reintroduction, outbreak, sabotage or biological warfare/terrorism. The overall objective of this study was to assess the genetic variability and to develop reliable DNA fingerprints for C. hominivorax (an obligatory pest) and C. macellaria (a facultative parasite) by using polymerase chain reaction (PCR)-based molecular markers.
Studying the population structure of an insect species helps in understanding the influence of genetic processes such as mutation, migration and selection on insect biology, behavior, ecology and evolution (Roderick, Reference Roderick1996). In insects where autocidal biological control has been implemented to control pest populations, knowledge of insect population structure and distributional ecology can contribute to the development of elite strains for large-scale deployment. Population structure consists of deciphering the genetic relationships among individuals within and between subpopulations and, thus, assessing the genetic variation among individuals in a population (Leung et al., Reference Leung, Nelson and Leach1993). Advances in DNA marker technology in the past three decades have provided the necessary tools for systematists and population geneticists to use common approaches to study both intraspecific and interspecific variation in insect populations (Avise, Reference Avise1994). Amplified fragment length polymorphism (AFLP) is a powerful technique that detects molecular genetic variations in DNA of any source or complexity without a priori knowledge of genome structure or sequence (Vos et al., Reference Vos, Hogers, Bkeeker, Reijans, van de Lee, Horner, Frijters, Pot, Peleman, Kuiper and Zabeau1995). The ‘AFLP fingerprints’ combine the reliability of restriction fragment length polymorphisms (RFLPs) and the power of PCR in a single technique, i.e. they are actually RFLPs visualized after selective PCR amplification of DNA restriction fragments (Vos et al., Reference Vos, Hogers, Bkeeker, Reijans, van de Lee, Horner, Frijters, Pot, Peleman, Kuiper and Zabeau1995). Therefore, analysis of AFLPs has the potential to become a powerful DNA fingerprinting technique for studying genetic relationships and genetic diversity in arthropods (Reineke et al., Reference Reineke, Karlovsky and Zebitz1999; Bensch & Åkesson, Reference Bensch and Åkesson2005). AFLP analysis has been used to study geographic populations of gypsy moth, Lymantria dispar (Linnaeus) (Reineke et al., Reference Reineke, Karlovsky and Zebitz1999), to assess the biodiversity of the midge Orseolia oryzae (Wood-Mason) (Katiyar et al., Reference Katiyar, Chandel, Tan, Zhang, Huang, Nugaliyadde, Fernando, Bentur, Inthavong, Constantino and Bennett2000), to determine differences in fall armyworm, Spodoptera frugiperda (J.E. Smith), based on host strains (McMichael & Pashley, Reference McMichael and Pashley1999) and its genetic variation (Clark et al., Reference Clark, Molina-Ochoa, Martinelli, Skoda, Isenhour, Krumm and Foster2007); and to study honey bee, Apis mellifera L., population genetics (Suazo & Hall, Reference Suazo and Hall1999). Here, we explore the utility of AFLP both to assess the degree of similarity and differentiate populations of C. hominivorax from different geographic regions and also to distinguish them from the morphologically similar calliphorid C. macellaria.
Materials and methods
Insect samples
All screwworm and secondary screwworm populations/strains used in this study were obtained from laboratory colonies reared at the USDA–ARS Midwest Livestock Insects Research Unit (MLIRU) Biosecure Screwworm Rearing Laboratory in Lincoln, Nebraska, USA. Specimens included third instars of C. macellaria samples from Nebraska, USA (CM), and third instars of C. hominivorax larvae representing four locations (Panama, PN and C9; Costa Rica, CR; Jamaica, JA; and Mexico, MX, CE, LH, LM, RL, and CN) (table 1). Samples were frozen and stored at −80°C before DNA extraction.
Table 1. Country of origin, approximate year of collection, code and important characteristics of screwworm populations studied.

a Flies from Mexico had the following geographic origins: PA34 – Chiapas, MX; CECH – Quintana Roo, MX; LH – Oaxaca, MX; Limon – Palenque, MX; RL – cross of LH and Limon; CN – cross between Chiapas and Oaxaca, MX.
DNA isolation
DNA was isolated from individually frozen insect samples by using a modified Black & Duteau (Reference Black, Duteau, Crampton, Beard and Louis1997) cetyltrimethylammonium bromide (CTAB) extraction protocol. The gut was removed and the larvae were homogenized in 500 μl of extraction buffer (100 mM Tris-HCl, pH 8.0, 1.4 M NaCl, 0.02 M EDTA, 2% CTAB and 0.2% β-mercaptoethanol) by using a disposable micropestle. The homogenate was treated with proteinase K (200 μg ml−1 extraction buffer) for 2 h at 65°C, followed by RNase (500 μg ml−1) treatment for another 2 h at 37°C. The homogenate was then centrifuged at 12,000 rpm for 4 min at room temperature, and the supernatant was extracted with an equal volume of chloroform: isoamyl alcohol (24:1) by centrifugation at 12,000 rpm for 10 min to separate the phases. The aqueous phase was transferred to a clean tube, and the chloroform:isoamyl alcohol step was repeated. DNA was precipitated by the addition of an equal volume of chilled isopropanol to the aqueous phase and incubation at 4°C for at least 30 min. The precipitate was collected by centrifugation at 12,000 rpm at 4°C for 15 min, rinsed with 70% ethanol, air-dried and dissolved in 100 μl of 1X TE buffer (10 mM Tris-HCl, pH 8.0, and 0.1 mM EDTA). DNA quality and concentration were determined on 0.8% Tris borate-EDTA agarose gel (Sambrook et al., Reference Sambrook, Fritsch and Maniatis1989) by using a known λ concentration standard (Invitrogen, Carlsbad, California, USA).
AFLP–PCR
Template preparation and AFLP assay were performed using a modified protocol of Vos et al. (Reference Vos, Hogers, Bkeeker, Reijans, van de Lee, Horner, Frijters, Pot, Peleman, Kuiper and Zabeau1995) with the incorporation of infrared fluorophore (IRD-700) (LI-COR, Lincoln, Nebraska, USA) labeled EcoRI primers in the PCR.
Template preparation
One microgram of genomic DNA was incubated with restriction endonucleases EcoRI (Invitrogen) and MseI (New England Biolabs, Beverly, Massachusetts, USA) for 2.5 h at 37°C in a total volume of 12.5 μl that contained 1.25 μl of 10X One-Phor-All PLUS buffer (GE Healthcare, Little Chalfont, Buckinghamshire, UK), 0.32 μl of 4 U μl−1 MseI enzyme (1.25 U per reaction), 0.08 μl of 15 U μl−1 EcoRI enzyme (1.25 U per reaction), and 0.125 μl of 10 mg ml−1 bovine serum albumin (New England Biolabs); autoclaved nanopure water was added to make up the final volume of 12.5 μl. The restriction fragments were incubated for 6 h at room temperature (~25°C) with 5 μl of ligation mixture containing 0.5 μl of 10X T4 DNA ligase buffer (Invitrogen), 0.5 μl of 5 pmol μl−1 EcoRI adapter, 0.5 μl of 50 pmol μl−1 MseI adapter, 0.150 μl of T4 DNA ligase (catalog no. M0202S, New England Biolabs) and 3.350 μl autoclaved nanopure water for ligating the adapters. Double-stranded adapters for the corresponding restriction enzymes were prepared by incubating equimolar amounts of both strands (EcoRI-F and EcoRI-R for EcoRI adapter and MseI-F and MseI-R for MseI adapter) (QIAGEN Operon, Alameda, CA) (table 2; sequences taken directly from Vos et al., Reference Vos, Hogers, Bkeeker, Reijans, van de Lee, Horner, Frijters, Pot, Peleman, Kuiper and Zabeau1995) for 10 min at 65°C, 10 min at 37°C and 10 min at 25°C.
Table 2. Oligonucleotide adapters and primers used for AFLP analysis.
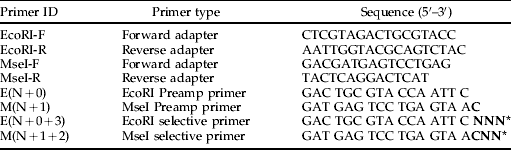
* N represents the position of (+3 and +2) selective nucleotides in the primer and may be either A, T, G or C.
AFLP assay
Ligation was followed by 20 cycles of preselective PCR amplification (30 s at 94°C, 1 min at 56°C and 1 min at 72°C) by using 1 μl of adapted DNA diluted 10-fold with 1X TE buffer (10 mM Tris-Cl and 0.1 mM EDTA, pH 8.0), 10 μl of pre-amp primer mix II (contains two oligonucleotide primers, one primer corresponding to the EcoRI adapted-ends and one primer corresponding to the MseI-ends) (Invitrogen), 1.25 μl of 10X PCR buffer plus 15 mM MgCl2 and 0.25 μl of 5 U μl−1 AmpliTaq DNA polymerase (1.25 U per reaction) (Applied Biosystems, Foster City, California, USA). The oligonucleotide primers in the pre-amp primer mix II are complementary to the adapter/restriction site with MseI primer containing one selective nucleotide (M+1 primer; table 2.) and EcoRI primer containing no selective nucleotide (E+0 primer; table 2). Products from preamplification reactions were diluted 20-fold with autoclaved nanopure water and were used as templates for further selective amplifications by using IRD-700 fluorophore-labeled EcoRI primer (LI-COR) with three selective nucleotides at the 3′ end (E+0+3) and MseI primer with two additional nucleotides added at the 3′ end of M+1 primer used earlier (M+1+2) (table 3).
Table 3. Selective primers used for AFLP analyses.

Selective PCR amplifications were performed in a 12-μl volume and contained 2.5 μl of diluted preamplification product, 5.8 μl of autoclaved nanopure water, 1.2 μl of 10X PCR buffer containing 15 mM MgCl2 and 0.06 μl of 5 U μl−1 AmpliTaq DNA polymerase (Applied Biosystems), 2.0 μl of MseI primer (M+1+2) (6.7 ng μl−1; dNTPs) (Invitrogen) and 0.5 μl of 1.0 pmol μl−1 IRD-700-labeled EcoRI primer (LI-COR). Selective amplifications were performed in a DNA thermal cycler 9600 (Applied Biosystems) by using the following touch-down PCR profile (Vos et al., Reference Vos, Hogers, Bkeeker, Reijans, van de Lee, Horner, Frijters, Pot, Peleman, Kuiper and Zabeau1995): one cycle of 30 s at 94°C, 30 s at 65°C and 1 min at 72°C, 12 cycles of subsequently lowering annealing temperature (65°C) by 0.7°C per cycle while keeping denaturation (30 s at 94°C) and extension (1 min at 72°C) temperature constant, 23 cycles of 30 s at 94°C, 30 s at 56°C, and 1 min at 72°C, and soak at 4°C. After amplification, reactions were stopped by adding 2.5 μl of blue stop solution (LI-COR), and the samples were denatured at 94°C for 5 min and flash cooled on ice immediately before electrophoresis. One microliter of the sample along with 1 μl of IRD-labeled 50–700-bp size marker (LI-COR) was electrophoresed through KBPlus 6.5% ready-to-use gel matrix (LI-COR), and the infrared fluorescent bands were detected by the laser scanning system (model 4200S-2, LI-COR).
Data scoring and analysis
For statistical analysis of inter- and intraspecific genetic variation, we acknowledge the following assumptions: the genotypes at marker loci are in Hardy–Weinberg proportions; AFLP products segregate as dominant alleles in a Mendelian manner, with most variation at the AFLP loci being biallelic (+/−) with one band allele and one bandless allele (Haymer, Reference Haymer1994; Borowsky, Reference Borowsky2001). Image data (16-bits) were automatically collected and simultaneously recorded during electrophoresis. Sizes of AFLP fragments were estimated by comparing to IRD-700-labeled 50–700-bp AFLP ladder (LI-COR). AFLP fragments were scored and analyzed using Gene ImagIR software, version 3.52 (LI-COR) and converted into numerical data as a matrix by assigning 1 (band present) and 0 (band absent).
Data were analyzed with the software NTSYS-pc (Numerical Taxonomy and Multivariate Analysis System, version 2.02) (Rohlf, Reference Rohlf1997). Similarity, matrices were generated according to the methods of Jaccard (=Grower's; a/(a+b+c)), as well as Dice (=Nei and Li; 2a/(2a+b+c)) – where ‘a’ is the number of fragments shared by two populations, ‘b’ is the number of fragments present only in one population and ‘c’ is the number of fragments present only in the other population – and results were compared (Sneath & Sokal, Reference Sneath and Sokal1973; Rohlf, Reference Rohlf1997). Coefficients were computed using the SIMQUAL (similarity based on qualitative differences) program and a phenogram was generated by unweighted pair group method [UPGMA] by using arithmetic averages (Sokal & Michener, Reference Sokal and Michener1958). The resulting cluster was represented as a dendrogram by using the sequential, agglomerative, hierarchical and nested (SAHN) clustering method. Bootstrapping (1000 replications) was performed using the Winboot program (Yap & Nelson, Reference Yap and Nelson1996) to assess the strength of the clusters generated by the tree-making program and to test the consistency of the estimated relationships among individuals and populations (Felsenstein, Reference Felsenstein1985).
Results
Interspecific discrimination of Cochliomyia spp
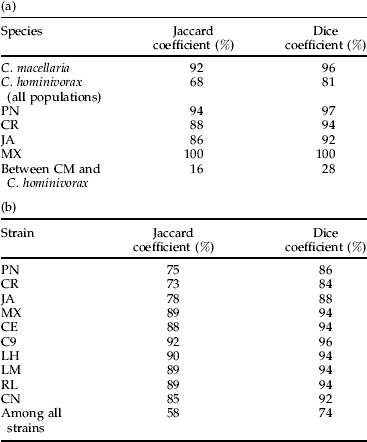
Four populations of C. hominivorax (PN, CR, JA and MX) and one population of C. macellaria (CM) (table 1) were used to screen ten primer combinations (E1+M7, E1+M6, E2+M7, E2+M2, E2+M3, E2+M1, E2+M4, E2+M6, E2+M5 and E3+M8) (table 3). The two species C. hominivorax and C. macellaria were readily distinguished by their well-resolved AFLP banding patterns generated by all of the ten primer combinations tested (fig. 1). Two kinds of parameters were considered for this study: (i) the presence or absence of diagnostic bands (particularly those present only in C. macellaria and absent in C. hominivorax and/or vice versa); and (ii) the existence of a characteristic, reproducible pattern of AFLP–PCR products, revealing a potentially diagnostic profile for a particular geographical population. The ten primer combinations used generated a large number of fragments (10–35 bands per individual) that depended on the type of selective nucleotides used. For statistical analyses, 52 consistent and discrete, scorable bands were used: seven bands were monomorphic and were found in both species; 22 bands were specific for C. macellaria populations; of the remaining 23 bands, ten were found only in C. hominivorax and 13 bands were diagnostic for different geographical populations of C. hominivorax. The species-specific, as well as population-specific, diagnostic bands for Cochliomyia spp. discrimination and identification are listed in table 4. Cluster analysis of similarity matrix (Jaccard coefficient) by UPGMA unambiguously separated the flies of each species into two major clusters that were only 16% similar. Also, bootstrap analyses showed 100% support for the branch separating the two species (fig. 2). Although both Jaccard and Dice coefficients generated similar clustering patterns, the resulting similarity values were slightly different (table 5a). Cochliomyia macellaria samples formed a distinct cluster, grouped together at the 92% level. Within this cluster, the similarity values between individuals in the population varied (range 92–100%). Cochliomyia hominivorax samples formed four distinct clusters, and bootstrap values among individuals within a population and between populations were variable (fig. 2). Populations from PN grouped together at the 94% level (range 94–100%), populations from MX grouped at the 100% level, CR samples clustered together at the 88% level (range 88–100%) and samples from JA grouped together at the 86% level. Within the C. hominivorax groupings, flies from PN and MX at the 72% level and CR and JA at 79% level were apparent. Populations of C. hominivorax from the four geographical locations grouped at the 68% level. Fly specimens from MX showed a high degree of similarity to each other (100%), whereas flies from JA showed relatively higher heterogeneity compared with the other flies (fig. 2).

Fig. 1. AFLP fingerprints generated using primer combinations (a) E-AGG+M-CAA and (b) E-AGG+M-CAC from the genomic DNA of one population of C. macellaria, lanes (1–8) CM, and four populations of C. hominivorax, lanes (9–16) PN, (17–24) CR, (25–32) JA and (33–40) MX. Lane M contains the IRD-700 ladder (50–700 bp).

Fig. 2. Dendrogram showing interspecific relationship between screwworms, C. macellaria (CM) and C. hominivorax (PN, CR, JA and MX) based on data from ten AFLP primer combinations. Similarity values were calculated using the Jaccard coefficient, UPGMA clustering. Values on branches indicate bootstrap values.
Table 4. Diagnostic AFLP bands for (a) interspecific and (b) intraspecific identification of screwworms.
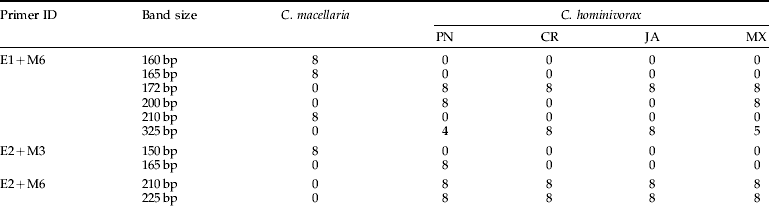
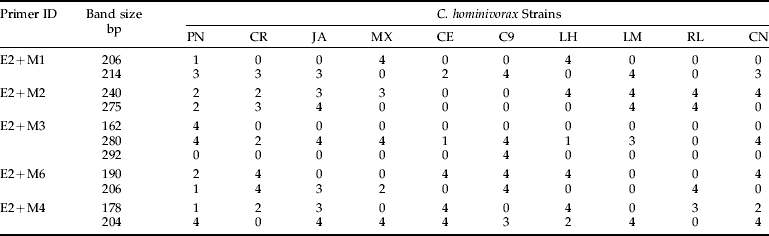
bp represents band size in base pairs; values below each strain represent the number of individuals displaying a band.
Table 5. Comparison of the genetic similarity levels in populations of C. macellaria (CM) and C. hominivorax (PN, CR, JA, MX, CE, C9, LH, LM, RL and CN) as calculated by the Jaccard and Dice coefficients for the interspecific (a) and intraspecific (b) studies.
Intraspecific discrimination of C. hominivorax strains
PCR amplification of DNA from the ten C. hominivorax strains (table 1) produced a discrete banding pattern (fig. 3) for each of the ten primer combinations tested (E2+M1, E2+M2, E2+M3, E2+M6, E2+M4, E2+M5, E2+M7, E2+M8, E3+M7 and E3+M8) (table 3). The number of bands generated by each primer pair ranged between ten and 20 and depended largely on the selective nucleotides used. For estimating intraspecific relationships among different screwworm strains, statistical analyses were performed using 72 bands composed of 19 monomorphic fragments (found in all C. hominivorax strains) and 53 polymorphic bands. Cluster analysis of similarity matrix (Jaccard coefficient) by UPGMA grouped all of the strains with a similarity level of 58%. At the 85% similarity level, seven clusters (MX, LM, CN, LH, C9, CE and RL), representing mutant strains, were resolved; and, at the 72% similarity level, all ten clusters (representing ten strains) were resolved (fig. 4). PN flies grouped at the 75% similarity level, JA flies at the 78% level, MX flies at the 89%, LM flies at the 89% level, CN flies at the 85% level, LH flies at the 90% level, C9 flies at the 92% level, CR flies at the 72% level, CE flies at the 88% level and RL flies at 89% level. Within the C. hominivorax strains, PN, JA and CR flies seemed to have a relatively lower level of similarity compared with the mutant strains from MX (table 5b) that had similarity levels greater than 85%. Bootstrap values are presented for each strain and not for individuals within a strain (fig. 4). The bootstrap values for clusters representing individual strains were high, suggesting a strong cluster. However, clusters of different strains had a lower bootstrap value (fig. 4), suggesting a weak cluster. Additionally, compared with the previous study of interspecific relationships in screwworms, here we observed that JA strain flies grouped with MX (and not CR strain) at the 72% similarity level; and these two groups further clustred with PN flies at the 67% level. Strain-specific marker bands generated by AFLP primers are presented in table 4b.

Fig. 3. AFLP fingerprints generated using primer combination E-ACA+M-CTC from the genomic DNA of ten strains of C. hominivorax: lanes (1–4) PN, (5–8) CR, (9–12) JA, (13–16) MX, (17–20) CE, (21–24) C9, (25–28) LH, (29–32) LM, (33–36) RL and (37–40) CN. Lane M contains IRD-700 ladder (50–700 bp).

Fig. 4. Dendrogram showing intraspecific relationship among C. hominivorax populations (PN, CR, JA, MX, CE, C9, LH, LM, RL and CN) based on data from ten AFLP primer combinations. Similarity values were calculated using the Jaccard coefficient, UPGMA clustering. Values on branches indicate bootstrap values.
Discussion
Genome fingerprinting with arbitrary primers is a versatile method for obtaining highly informative genetic markers in geographical populations of insects, and the information generated from such studies can be used for tracking individuals, genes or both (Parker et al., Reference Parker, Snow, Schug, Booton and Fuerst1998). As with random amplified polymorphic DNA (RAPD) (Welsh & McClelland, Reference Welsh and McClelland1990; Williams et al., Reference Williams, Kubelik, Livak, Rafalski and Tingey1990), the AFLP technique generates individual specific profiles from template DNA by amplifying anonymous fragments from sites scattered through the genome (Borowsky, Reference Borowsky2001). However, AFLP differs from the RAPD method in technical detail and has several advantages, such as higher temperature stringency, resulting in more reliable, reproducible data (Majer et al., Reference Majer, Mithen, Lewis, Vos and Oliver1996; Maugham et al., Reference Maugham, Saghai-Maroof, Buss and Huestis1996; Sanchez et al., Reference Sanchez, Restrepo, Duque, Fregene, Bonierbale and Verdier1998; Reineke et al., Reference Reineke, Karlovsky and Zebitz1999). Similar to RAPD markers, AFLP produces dominant markers and also is affected by small changes in reaction conditions, such as buffer concentrations, quality and concentration of DNA, source and concentration of Taq polymerase enzyme, and amplification conditions (Cervera et al., Reference Cervera, Cabezas, Simon, Martinex-Zapater, Beitia and Cenis2000); therefore, we optimized several parameters as defined in ‘Materials and methods’.
A primary requirement for the screwworm eradication program is the correct identification of species, because facultative and scavenger flies in the families Calliphoridae and Sarcophagidae may be attracted to necrotic wounds of animals, thereby confounding the identification dilemma in the early instars, especially by morphological observations (Hall & Wall, Reference Hall and Wall1995). On average, we identified at least 5–10 diagnostic bands per primer pair to discriminate C. hominivorax from C. macellaria. Observed differences in the banding patterns, low levels of similarity (16%) and 100% support for the branch separating C. hominivorax from C. macellaria indicate that the two species have considerable genetic differences and that AFLP is a good tool for rapid identification at the interspecific level (figs 1 and 2). Species-specific diagnostic bands were found (table 4a) that provide for discrimination of these species based on the presence or absence of some AFLP–PCR products. AFLP analysis yielded 10–35 bands per individual per primer combination, more than those detected either by RAPDs (Skoda et al., Reference Skoda, Pornkulwat and Foster2002) or PCR–RFLP of mitochondrial DNA (mtDNA) (Taylor et al., Reference Taylor, Szalanski and Peterson1996), many diagnostic bands that may ultimately be used for characterizing C. hominivorax strains (table 4b).
The population genetics of screwworms explored in the intraspecific studies showed that flies from MX were more homogenous and had high percent similarity values and bootstrap support (>95%). For example, MX flies grouped at the 100% similarity level (fig. 2) and 89% similarity level (fig. 4) in both studies, respectively. Also, other mutant strains originating from Mexico (CE, LH, LM, RL and CN) had >85% similarity level, and the branches were well supported (>95%) by bootstrap analysis, indicating low genetic variability (fig. 4). These results are consistent with the strains from MX (table 1) being the oldest of the screwworm strains examined (established as laboratory cultures in 1984–1985). Consistent with this observation is the relatively higher variability (lower percent similarity) of JA flies that represented the more recent (1998) establishment of the population from Jamaica as laboratory colonies. AFLP patterns for the earlier, interspecific studies indicated similarity between two populations, PN and MX; whereas CR showed similarity with JA populations (fig. 2). However, in the intraspecific studies clustering between populations was not strong (fig. 4). This difference in the clustering may be caused by the small sample size for intraspecific studies as well as the difference in the primer pairs screened.
In general, within-population variability was low compared with between-population variability, suggesting population substructure in C. hominivorax. The relatively higher variability between populations probably occurred because different alleles were fixed in different populations during culture. These results are similar to results reported using RAPD–PCR (Skoda et al., Reference Skoda, Pornkulwat and Foster2002) on laboratory populations, as well as by Infante-Malachias et al. (Reference Infante-Malachias, Yotoko and Azeredo Espin1999) in screwworms from southeastern Brazil and Argentina. Population genetic studies of screwworms using protein electrophoresis indicated a low genetic differentiation in flies (Krafsur & Whitten, Reference Krafsur and Whitten1993; Taylor & Peterson, Reference Taylor and Peterson1994), whereas mtDNA analyses revealed high genetic variability and low gene flow (Roehrdanz & Johnson, Reference Roehrdanz and Johnson1988; Roehrdanz, Reference Roehrdanz1989). As suggested by Taylor et al. (Reference Taylor, Szalanski and Peterson1996), the low level of genetic variation observed within screwworm populations can be caused by bottlenecking, especially when samples used in the analyses are obtained from colonies that were in the laboratory for ≥4 years, as in this case. Microsatellite markers for screwworms, co-dominant and potentially highly polymorphic (Torres & Azeredo-Espin, Reference Torres and Azeredo-Espin2005), used along with AFLP, dominant but providing high numbers of markers, may provide additional resolution for assessing genetic variability in screwworms.
In conclusion, our study demonstrates that, despite certain limitations, AFLP–PCR analysis is a good tool for differentiating species and has tremendous potential for studies of intraspecific genetic variation; it is capable of elucidating relationships and may provide diagnostic markers that identify geographical populations of C. hominivorax. Also, diagnostic bands could be isolated from the gels and sequenced so that specific primers can be generated and then used to determine the geographical origin of an outbreak or infestation. In addition, these markers can be used effectively to screen strains for quality control in mass-rearing facilities.
Acknowledgements
The authors wish to thank Dennis Berkebile for providing the samples used in this research and, along with David Carter, useful comments provided on an early draft. This work was part of Coordinated Research Project IAEA-314-D4-RC.851.1. This article reports the results of research; mention of a proprietary product does not constitute endorsement or recommendation for its use by USDA.











