Introduction
When pathogens or parasites invade a host, the initial and predominant defensive reaction is normally the hemocytic immune system. To survive, these hemocytes-mediated immunity, encapsulation, phagocytosis, bacteriostasis, phenoloxidase, and melanization, usually is suppressed by the endoparasitoids (Madanagopal & Kim, Reference Madanagopal and Kim2006; Nalini et al., Reference Nalini, Ibrahim, Hwang and Kim2009; Huang et al., Reference Huang, Shi, Chen and Zhang2011; Fang et al., Reference Fang, Wang, Zhu, Stanley, Chen, Hu and Ye2011b; Zhang et al., Reference Zhang, Huang, Zhu and Ye2012; Teng et al., Reference Teng, Xu, Gan, Chen, Fang and Ye2016), which are also the primary agents and processes involved in eliminating or mitigating microbial invasions in vivo (Zhong et al., Reference Zhong, Liu, Wang and Liu2017). Consequently, it has been hypothesized that the parasitized host immunity must be maintained at a functional level against pathogens or other metazoan parasites to offset the constant exposure to microbial organisms (Strand & Pech, Reference Strand and Pech1995; Akira, Reference Akira2009).
The main immunologically relevant processes against pathogenic bacteria are phagocytosis, nodulation, and the production of antimicrobial peptides (AMPs) (Strand, Reference Strand2008; Bell, Reference Bell2011; Laughton et al., Reference Laughton, Garcia, Altincicek, Strand and Gerardo2011). Phagocytosis is a widely conserved cellular defense response against a small quantity of bacteria, yeast, apoptotic bodies and abiotic particles of relatively small size. This is mainly achieved by hemocytes in a process that includes recognition, engulfment, and intracellular destruction (Giulianini et al., Reference Giulianini, Bertolo, Battistella and Amirante2003; Ling & Yu, Reference Ling and Yu2006; Giglio et al., Reference Giglio, Battistella, Talarico, Brandmayr and Giulianini2008; Huang et al., Reference Huang, Yang, Shi, Li, Chen, Chen and Chen2010; Aung et al., Reference Aung, Boldbaatar, Umemiya-Shirafuji, Liao, Tsuji, Xuenan, Suzuki, Kume, Galay, Tanaka and Fujisaki2012), while nodulation has been proven to be more prevalent in the degradation of larger quantities of bacteria (Dubovskiy et al., Reference Dubovskiy, Krukova and Glupov2008; Yi et al., Reference Yi, Chowdhury, Huang and Yu2014). Though, these two cellular responses are capable of degrading a large proportion of the bacteria produced during the early stages of an infection (Haine et al., Reference Haine, Moret, Siva-Jothy and Rolff2008a; Makarova et al., Reference Makarova, Rodriguez-Rojas, Eravci, Weise, Dobson, Johnston and Rolff2016), recognition molecules and AMPs are also important extracellular effector agents in destroying foreign invaders (Strand, Reference Strand2008; Nazario-Toole & Wu, Reference Nazario-Toole and Wu2017). In general, insects initiate cellular immunity such as phagocytosis and nodulation, the constitutive defenses which rely on the response of insect hemocytes and the rapidly activated enzyme cascades, immediately being infected, followed by the launching of the long-lasting production of AMPs (the induced response) (Haine et al., Reference Haine, Moret, Siva-Jothy and Rolff2008a; Makarova et al., Reference Makarova, Rodriguez-Rojas, Eravci, Weise, Dobson, Johnston and Rolff2016). The higher antimicrobial activity against microorganisms is usually realized by the collaboration of many AMPs, for example, the redundancy properties of attacins and some defensins combining to combat Escherichia coli (Hultmark et al., Reference Hultmark, Engström, Andersson, Steiner, Bennich and Boman1983; Yi et al., Reference Yi, Chowdhury, Huang and Yu2014).
The endoparasitoid chalcidoid wasp, Tetrastichus brontispae Ferrière (Hymenoptera: Eulophidae) is an effective biocontrol agent often used in controlling Octodonta nipae (Maulik) (Coleoptera: Chrysomelidae), a serious invasive beetle pest of palms in southern China (Hou & Weng, Reference Hou and Weng2010; Hou et al., Reference Hou, Wu, Wang, Xie, You and Hou2011, Reference Hou, Miao and Zhang2014a, Reference Hou, Miao and Zhangb; Wan et al., Reference Wan, Hou and Jiang2015; Tang & Hou, Reference Tang, Hou, Wan, Jiang and Zhan2017). Due to its importance in controlling this and other coleopterous pests of palms, various aspects involving the mechanism of immune regulation of O. nipae after being parasitized by T. brontispae were studied at the physiological and molecular level by our laboratory (Tang et al., Reference Tang, Xu and Hou2014a, Reference Tang, Chen, Hou and Mengb; Meng et al., Reference Meng, Tang, Hou, Chen, Chen and Yu2016). This study was undertaken because the process by which parasitism regulates these immune responses against microbes is still unclear. This was accomplished by comparing the number of live E. coli, the phagocytosis, the bacteriostatic activity, and the mRNA expression level of the AMPs reported mainly against E. coli between parasitized and unparasitized pupae after injection of enhanced green fluorescent protein (EGFP)-expressing E. coli. The choice of EGFP-expressing E. coli was based on the advantage of the being visually engulfed (Bicker et al., Reference Bicker, Höflich, Vogt, Volk and Katrin2008; Aung et al., Reference Aung, Boldbaatar, Umemiya-Shirafuji, Liao, Tsuji, Xuenan, Suzuki, Kume, Galay, Tanaka and Fujisaki2012), the induction of antimicrobial reaction (Shiratsuchi et al., Reference Shiratsuchi, Nitta, Kuroda, Komiyama, Gawasawa, Shimamoto, Tuan, Morita, Aiba and Nakanishi2016), and their propagative ability in infected hosts (Aung et al., Reference Aung, Boldbaatar, Umemiya-Shirafuji, Liao, Tsuji, Xuenan, Suzuki, Kume, Galay, Tanaka and Fujisaki2012). Moreover, the practicability of phagocytosis by the injection of EGFP-expressing E. coli in O. nipae pupae had been previously tested before by Meng et al. (Reference Meng, Tang, Hou, Chen, Chen and Yu2016). In addition, an in-depth analysis of the factors involved in such modifications including the role of maternal fluids, ovarian and venom proteins produced by the female endoparasitoids, on phagocytosis, the main immune reaction in microbial clearance, occurred, due to the effects of the maternal fluids in regulating host immunity, especially in those with hemocytes-mediated immunity (Richards & Parkinson, Reference Richards and Parkinson2000; Er et al., Reference Er, Uçkan, Rivers and Sak2011; Fang et al., Reference Fang, Wang, Gatehouse, Gatehouse, Chen, Hu and Ye2011a; Teng et al., Reference Teng, Xu, Gan, Chen, Fang and Ye2016). These results not only add to our understanding of the immune regulation of the O. nipae–T. brontispae system, but also help to lay a foundation for the potential application of insect AMPs in agricultural systems, disease vector control, and the development of pharmaceuticals (Yi et al., Reference Yi, Chowdhury, Huang and Yu2014).
Material and methods
Insect rearing, culture of EGFP-expressing E. coli, and hemolymph collection
The T. brontispae wasps and O. nipae beetles were reared at a constant 25 ± 1°C, 80 ± 5% RH, and a photoperiod of 12 h light:12 h dark as described by Xu et al. (Reference Xu, Lan, Hou, Chen, Chen and Weng2011), Xi et al. (Reference Xi, Zhang, Hou and Shi2013), and Tang et al. (Reference Tang, Xu and Hou2014a). To obtain parasitized individuals, 1-day-old O. nipae pupae were removed once they were parasitized by the mated female wasps, while individuals that pupated without exposure to the wasps were removed as unparasitized pupae.
The maternal fluids were obtained by dissecting 2–3 days old female adult wasps (newly emerged wasps were considered as 1-day-old) and directly separating the ovary and venom apparatus. The venom fluids were obtained by macerating the venom apparatus and then removing the supernatant after centrifugation at 12,000 g for 10 min at 4°C. The ovarian fluids were prepared by tearing the ovaries with forceps and then collecting the supernatant after centrifuging the mangled ovaries at 10,000 g for 15 min at 4°C. The concentration of the venom and ovarian fluids were prepared as two equivalent per microliter with one equivalent used for per injection using a 10 µl syringe (Hamilton, Switzerland). One equivalent was defined as the supernatant from single female wasp.
The EGFP-expressing E. coli (donated by Xiong Guan, Fujian Agricultural and Forestry University) was cultured for 12 h in LB broth (10 g l−1 tryptone, 5 g l−1 yeast extract, and 10 g l−1 NaCl, pH 7.4) with a final concentration of 100 µg ml−1 ampicillin at 37°C. When the optical density of the bacterial solution at 600 nm reached 0.6 (OD600 = 0.6), a final concentration of 1 mM isopropyl β-D-1-thiogalactopyranoside was added to the solution and allowed to incubate for an additional 11 h at 37°C with agitation (200 rpm). The EGFP-expressing E. coli was resuspended in the same volume of PBS (150 mM NaCl, 2.7 mM KCl, 1.8 mM KH2PO4, and 10.1 mM Na2HPO4, pH 7.2) after centrifuging at 2000 g for 2 min. A volume of the above prepared 101.2 nl EGFP-expressing E. coli suspension (OD600 = 0.6) was injected into pupae at 12, 24, 48, 72, and 96 h after parasitism or pupation and those pupae injected with venom/ovarian/PBS at 6, 12, 24, and 48 h, using a Nanoliter Reference Aung, Boldbaatar, Umemiya-Shirafuji, Liao, Tsuji, Xuenan, Suzuki, Kume, Galay, Tanaka and Fujisaki2010 injection system (World Precision Instruments, Sarasota, FL, USA). Hemolymph was then drawn at 12 h post-injection of the EGFP-expressing E. coli using a 2.5 µl pipette (Eppendorf Research, Hamburg, German) as described by Meng et al. (Reference Meng, Tang, Hou, Chen, Chen and Yu2016). The extracted hemolymph was used to assess the survivorship of E. coli and phagocytosis activity of the treated pupae.
Measurement of E. coli survival in vivo
To assess the effects of parasitism on the survivorship of the injected E. coli, comparisons of colony-forming units (CFUs) between unparasitized and parasitized pupae were performed using a plate count method. Hemolymph samples (2 µl) drawn from unparasitized and parasitized pupae previously injected with E. coli were diluted with 38 µl PBS and spread on ampicillin-resistant LB plates. The LB plates were kept in an incubator at 37°C for 12 h. After which, the bacteria colonies were counted and the CFUs per microliter calculated.
Measurements of hemocytes phagocytosis activity
To assess the effects of parasitism on phagocytosis activity of O. nipae, 1 µl samples of hemolymph were drawn from unparasitized or parasitized pupae with E. coli injection and diluted with 3 µl PBS. The hemocytes containing fluorescent bacteria were then counted using fluorescence and differential interference contrast microscopy (Nikon). The activity of phagocytosis was expressed as the percentage of hemocytes containing fluorescent bacterial cells (Rohloff et al., Reference Rohloff, Wiesner and Götz1994). The effects of ovarian and venom proteins on hemocyte phagocytosis activity at 6, 12, 24, or 48 h post-injection was also measured as above.
Measuring hemolymph bacteriostatic ability
Hemolymph samples (2 µl) drawn from pupae at 12, 24, 48, 72, or 96 h after parasitism or pupation were added to a centrifuge tube containing 38 µl PBS with 1 µl phenylthiourea (5 mM, Aladdin, Shanghai, China) and centrifuged at 8000 rpm for 10 min at 4°C. The supernatant was stored at −80°C until use.
The antimicrobial activity was assessed by the alteration in turbidity of E. coli cells (Trans T1, Beijing, China). The glycerol stock E. coli was recovered three times, before 20 µl of bacteria cells (OD600 = 0.6) were added to 2 ml LB culture medium and incubated for an additional 1 h at 37°C (200 rpm) to obtain an optical density of 0.3 (OD600 = 0.3). One hundred microliters of the above E. coli bacteria, 500-fold diluted, was cultured with 20 µl diluted hemolymph, PBS (negative control) or tetracycline (1 mg ml−1) (positive control) at 37°C for 12 h, and the absorbance at 600 nm was assayed using a SpectraMax 190 Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The bacteriostatic activity was determined by the alteration of optical density at a wavelength of 600 nm.
Expression profile of AMPs by quantitative real-time PCR
To determine the effects of parasitism on the mRNA expression level of AMPs in O. nipae, two types of AMPs, attacins and defensins, were selected due to their antimicrobial activities against E. coli (Seufi et al., Reference Seufi, Hafez and Galal2011; Bang et al., Reference Bang, Park, Yoo and Cho2012). The gene sequences of all AMPs belonging to attacins and defensins (NCBI accession number: attacin C1, MG099660; attacin C2, MG099661; attacin C3, MG099662; defensin 2A, MG099658; defensin 2B, MG099659) and reference gene ribosomal protein S3 (RPS3) (Supplementary table S1) from the transcriptome data of O. nipae were selected to design primers by primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/primer3/). The tissue-specific distribution and alteration of the AMPs induced by parasitism were determined by quantitative real-time PCR (qRT-PCR).
Total RNA was extracted from the head, hemocytes, fat body, epidermis, and gut of naïve unparasitized pupae to exploit the tissue-specific distribution of AMPs. Meanwhile, the role that parasitism has on altering AMPs mRNA expression levels was tested with the total RNA extracted from the whole bodies of unparasitized and parasitized pupae. All of the total RNA was then subjected to the Thermo Scientific Verso cDNA kit (Thermo Fisher Scientific Incorporation, Waltham, MA, USA) to synthesize the complementary DNA (cDNA) as a template, where the RT enhancers are used to remove contaminating genomic DNA.
Twenty microliters reactions containing 1 µl of 500 nM primers, 1 µl of tenfold diluted cDNA, 8 µl of sterilized water, and 10 µl of FastStart universal SYBR Green Master Mix (Roche) were performed on an ABI 7500 real-time PCR system in duplicate. The standard curve of each gene was prepared by serial dilutions (10×) of the cDNA samples. The qRT-PCR procedure was performed at 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min, followed by one cycle of 95°C for 15 s, 60°C for 1 min, 95°C for 30 s, and 60°C for 15 s (melting-curve stage). The expression level was presented as 2−△△CT (Pfaffl, Reference Pfaffl2001).
Statistical analysis
All the data were analyzed using SPSS 21.0 for Windows (IBM Corp. Armonk, NY, USA). The homogeneity of variances of all the analyzed data was examined by Levene's test before further analyses. Because of the heterogeneity of variance in the relative expression of attacin C1, C2, and C3 of the unparasitized, parasitized pupae, and of different tissues, and those of defensin 2A and 2B across different tissues, the transformed data by the logarithmic function were used for further data analysis. The unpaired two-tailed Student's t-test was performed to compare the survivorship of E. coli in vivo, the phagocytosis and antimicrobial ability, and the mRNA expression level of AMPs in unparasitized and parasitized at different time intervals. Except for the effects of time on antimicrobial activity revealed by Welch's analysis of variance (ANOVA), because of the heterogeneity of variance (Jan & Shieh, Reference Jan and Shieh2013), the one-way ANOVA was used to determine the effect of time on survivorship of E. coli, phagocytosis, and antimicrobial activity in unparasitized and parasitized or PBS/ovarian/venom-injected pupae, as well as the effect of ovarian or venom protein on phagocytosis ability. Multiple comparison procedures were compared by Tukey's or Games-Howell (A) test based on whether ANOVA or Welch's ANOVA analysis was used. Differences were considered significant when P < 0.05.
Results
Greater lethal susceptibility to bacterial infection after parasitism results primarily from enhanced bactericidal activity rather than phagocytosis activity
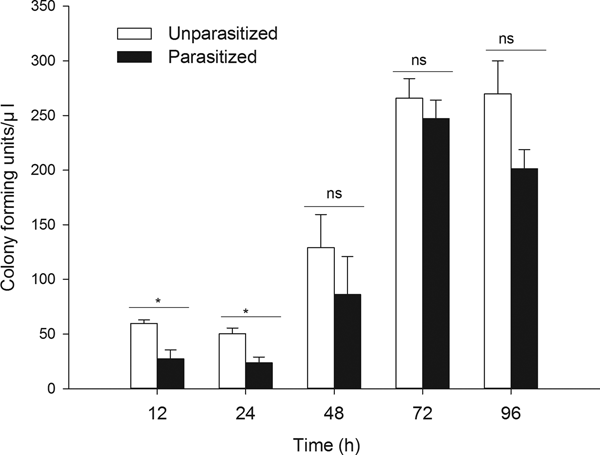
Parasitism by T. brontispae led to a higher bacterial degradation level at 12 and 24 h post-parasitism, i.e., the degradation activity was enhanced by parasitism during the early parasitism period (fig. 1, 12 h, t 6 = 3.698, P = 0.010; 24 h, t 8 = 3.521, P = 0.008). In addition, significant differences in bactericidal activity were found to correlate with ages in unparasitized and parasitized pupae (unparasitized, F 4,20 = 11.555, P < 0.001; parasitized, F 4,17 = 22.327, P < 0.001).

Fig. 1. The level of colony forming unit (CFUs) were determined in hemolymph of unparasitized and parasitized pupae 12 h after injection of EGFP-expressing E. coli and cultured with LB agar plate containing ampicillin. ‘Parasitized’ indicates the pupae of O. nipae parasitized by T. brontispae, while ‘unparasitized’ means pupae are not parasitized. The asterisk (*) indicates a significant difference between the means at the indicated time period (P < 0.05); while ‘ns’ indicates no significant difference between the parasitized and unparasitized pupae at P < 0.05. The data are the means ± standard error.
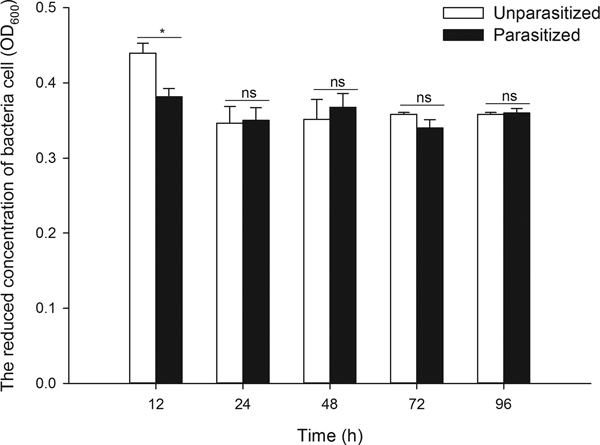
As the degradation of bacteria relies primarily on phagocytosis and bacteriostasic responses, a study was conducted focusing on the two processes. The data showed that parasitism significantly suppressed phagocytic activity (fig. 2, t 7 = 3.002, P = 0.020) and markedly stimulated antibacterial activity (fig. 3, t 4 = 3.396, P = 0.027) at 12 h post-parasitism, indicating the major role of bacteriostasic activity in degrading bacteria during this early parasitism period. No other significant differences in phagocytic and bacteriostasic activity were observed between unparasitized and parasitized pupae thereafter (Supplementary table S2), although parasitism did induce a notable fluctuation in phagocytosis activity as time progressed (unparasitized: F 4,15 = 0.477, P = 0.752; parasitized: F 4,18 = 3.674, P = 0.023). Time differences played no significant role on antimicrobial activity of unparasitized and parasitized pupae (unparasitized: F 3,5.14 = 1.203, P = 0.396; parasitized: F 4,15 = 1.176, P = 0.361).

Fig. 2. Effects of parasitism by Tetrastichus brontispae on phagocytosis activity of Octodonta nipae pupae in vivo. ‘Parasitized’ indicates the pupae of O. nipae parasitized by T. brontispae, while ‘unparasitized’ means pupae are not parasitized. The asterisk (*) indicates a significant difference between the means at the indicated time period (P < 0.05); while ‘ns’ indicates no significant difference between the parasitized and unparasitized pupae at P < 0.05. The data are the means ± standard error.

Fig. 3. The antimicrobial activity in the hemolymph of unparasitized and parasitized pupae of Octodonta nipae. ‘Parasitized’ indicates the pupae of O. nipae parasitized by T. brontispae, while ‘unparasitized’ means pupae are not parasitized. The asterisk (*) indicates a significant difference between the means at the indicated time period (P < 0.05); while ‘ns’ indicates no significant difference between the parasitized and unparasitized pupae at P < 0.05. The data are presented as the means ± standard error.
Increased mRNA expression levels of many AMPs induced by parasitism
Five AMP genes belonging to defensin and attacin were used to determine the expression profile of tissue-specific and parasitism-induced alteration. The results showed that these AMPs were expressed in all tissues examined, but were highly expressed in fat body (table 1, Supplementary table S3) except defensin 2A which is expressed similarly in these tissues. To be more specific, the mRNA expression levels of attacin C1 and defensin 2B in fat body and gut tissue were markedly higher than levels in the head, while attacin C3 was highly transcribed in the fat body and hemocytes, with the highest transcripts of attacin C2 only found in the fat body.
Table 1. The relative abundance of transcripts of attacins and defensins in different tissues of unparasitized O. nipae pupae.

Different lowercase letters indicate significant differences within the same column at P < 0.05 by one-way ANOVA and Turkey's test; while the same lowercase letters donate no statistically significant difference. The data are presented as the means ± standard error.
The qRT-PCR results showed that parasitism induced a significant fluctuation in mRNA expression level of attacin C1 (increased at 4 h post-parasitism) and defensin 2B (decreased at 24 h post-parasitism) during the early parasitism period, with a conspicuous increase in the abundance of transcripts of attacin C1 and C3 at 96 h post-parasitism, along with strikingly elevating transcripts of attacin C2 at 48, 72, and 96 h post-parasitism and defensin 2A at 72 and 96 h post-parasitism (fig. 4, Supplementary table S4).

Fig. 4. Expression profile analysis of attacins and defensins in parasitized and unparasitized pupae of Octodonta nipae. (a) Attacin C1; (b) attacin C2; (c) attacin C3; (d) defensin 2A; (e) defensin 2B. ‘Parasitized’ indicates the pupae of O. nipae parasitized by T. brontispae, while ‘unparasitized’ means pupae are not parasitized. The asterisk (*) indicates a significant difference between the means at the indicated time period (P < 0.05); while ‘ns’ indicates no significant difference between the parasitized and unparasitized pupae at P < 0.05. The data are presented as the means ± standard error.
Effects of T. brontispae ovarian and venom proteins on the phagocytosis ability of O. nipae pupae
Considering the significant role phagocytosis plays in removing microbial invaders, a more detailed study conducting on how maternal fluids of parasitoids affect phagocytosis demonstrated that ovarian fluid rather than venom protein had a remarkable effect on the phagocytosis ability at 48 h post-injection (fig. 5, F 2,11 = 6.986, P = 0.011). An increasing phagocytosis induced by ovarian protein rather than PBS and venom protein was observed after time-course analysis (PBS: F 3,8 = 1.353, P = 0.324; ovarian: F 3,14 = 5.840, P = 0.008; venom: F 2,14 = 0.725, P = 0.502).

Fig. 5. Effects of Tetrastichus brontispae ovarian and venom fluids on phagocytosis activity of Octodonta nipae pupae in vivo. PBS: PBS-injected pupae; ovarian: ovarian-injected pupae; Venom: venom-injected pupae. Different lowercase letters indicate significant differences among the means at the indicated time period (P < 0.05); while ‘ns’ indicates no significant difference among various treated pupae at P < 0.05. The data are presented as the means ± standard error.
Discussion
This study evaluated how parasitism affected on host immune response against bacteria, including phagocytosis and bactericidal activity (i.e., induced response, AMPs composition with lytic and/or agglutinating properties) (Dubovskiy et al., Reference Dubovskiy, Krukova and Glupov2008; Yi et al., Reference Yi, Chowdhury, Huang and Yu2014). It also demonstrated that parasitism induces a higher bacteria degradation activity, a decrease in phagocytosis activity and an enhanced bactericidal capacity, thereby sustaining the hypothesis that the enhanced ability of parasitized pupae to degrade E. coli was caused by bactericidal action. This observation contradicts the previous viewpoint that cellular immunity is largely responsible for the breakdown of the bacteria, which is proposed by Makarova et al. (Reference Makarova, Rodriguez-Rojas, Eravci, Weise, Dobson, Johnston and Rolff2016), postulating that parasitism, indeed, was responsible for altering the immune reaction of the host to invading pathogens.
Typically, phagocytosis as a constitutive defense is the main mechanism in clearing E. coli, and antimicrobial compounds (an induced response) act primarily against the bacteria that persist within the body (Haine et al., Reference Haine, Moret, Siva-Jothy and Rolff2008a, Reference Haine, Pollitt, Moret, Siva-Jothy and Rolffb). However, in most cases, decreased phagocytosis was induced by parasitism (Er et al., Reference Er, Uçkan, Rivers and Sak2011; Fang et al., Reference Fang, Wang, Zhu, Stanley, Chen, Hu and Ye2011b; Mahmoud et al., Reference Mahmoud, De Luna-Santillana and Rodríguez-Perez2012). Strand et al. (Reference Strand, Beck, Lavine and Clark2006) suspected that the decreased phagocytosis might function essentially for parasitism to be successful, because of the similar reaction phases, hemocyte adhesion, and spreading activity, between phagocytosis and encapsulation, the main immune reaction against parasitism (Rosales, Reference Rosales and Vonnie2017). Even so, the protein designated ‘encapsulation promoting lectin’ from Mythimna separate (Walker) (Lepidoptera: Noctuidae), which activates encapsulation while suppressing phagocytosis (Ishihara et al., Reference Ishihara, Maruyama and Furukawa2017), excludes this assumption to a certain extent, because numbers of researchers have shown that the higher encapsulation ability was against parasitism (Smilanich et al., Reference Smilanich, Dyer and Gentry2009; Kacsoh & Schlenke, Reference Kacsoh and Schlenke2012; Han et al., Reference Han, Huang and WANG2013). The decrease in phagocytic hemocytes may be due to a potential trade-off among host immune responses. This was demonstrated by the significant negative correlation between antibacterial activity (lysosome-like) and hemocyte density in Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) (Moret & Schmid-Hempel, Reference Moret and Schmid-Hempel2001; Cotter et al., Reference Cotter, Kruuk and Wilson2004). The enhanced bactericidal activity accompanied by reduced phagocytosis activity reinforces the validity of the trade-off assumption. Notably, a weakened microbial clearance ability was observed in both unparasitized and parasitized pupae during development, while a similar reduction in encapsulation, another constitutive defense, during development was previously observed in Pieris rapae (L.) (Lepidoptera: Pieridae) (Stoehr, Reference Stoehr2007). The effects of age on host immunity was also observed in other insects, such as in Dendroctonus valens LeConte (Coleoptera: Curculionidae, Scolytinae), Drosophila melanogaster Meigen (Diptera: Drosophilidae), and Forficula auricularia L. (Dermaptera: Forficulidae) (Lesser et al., Reference Lesser, Paiusi and Leips2006; Shi & Sun, Reference Shi and Sun2010; Vogelweith et al., Reference Vogelweith, Korner, Foitzik and Meunier2017).
The higher bactericidal activity noted in parasitized O. nipae pupae seemed to be of short duration and their rapid increase was ahead of the rise in the AMPs mRNA expression level. This may be due to the direct injection of some other antibacterial substances during parasitism. Antimicrobial activity has been reported in the maternal fluids of parasitic wasps Pimpla hypochondriaca (Retzius) (Hymenoptera: Ichneumonidae) (Dani et al., Reference Dani, Richards, Isaac and Edwards2003), Pteromalus puparum (L.) (Hymenoptera: Pteromalidae) (Shen et al., Reference Shen, Ye, Cheng, Yu, Yao and Hu2010), and Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) (Ye et al., Reference Ye, Zhao, Wang, Bian and Zheng2010). Costly prophylactic venom-based strategies, such as the injection of venom AMPs, are frequently used by parasitoids to protect the host from secondary infections (Moreau, Reference Moreau2013), although some host AMPs do show activity against parasites (McGwire et al., Reference McGwire, Olson, Tack and Engman2003; Fieck et al., Reference Fieck, Hurwitz, Kang and Durvasula2010). In Pseudoplusia includens (Walker) (Lepidoptera: Noctuidae), the ‘induced’ response mainly consists of AMPs which were found to be activated at 1–3 h post-injection and peak at 8–24 h post-challenge (Lavine et al., Reference Lavine, Chen and Strand2005); while in Tenebrio molitor (Coleoptera: Tenebrionidae), the AMPs activity in hemolymph reached their peak after 12–48 h and maintained for at least 14 days (Haine et al., Reference Haine, Pollitt, Moret, Siva-Jothy and Rolff2008b). In the present study, most AMPs increased during the later parasitism periods, corroborating the previous observation that AMPs were more appropriate as the last defense line in dealing with persistent bacterial infections (Haine et al., Reference Haine, Moret, Siva-Jothy and Rolff2008a). Despite of the increased AMP transcripts, no differences were found in the bacteriostasis between unparasitized or parasitized pupae during the late infection stage, which may reflect the atypism of the tissue employed, where additional time may be required to transfer AMPs from fat body to hemocytes (Gillespie & Kanost, Reference Gillespie and Kanost1997), or other types of AMPs also fight against the bacteria in O. nipae. For this, the bactericidal activity should be further studied in parasitized or pupated pupae that are over 4 days old, and some roles of other AMPs should be further investigated. The synchronous soaring of most mRNA expression level of O. nipae AMPs indicated that the collaboration among AMPs as a strong first line of defense against an initial pathogen invasion, which can retard the development of resistance in bacteria (Yi et al., Reference Yi, Chowdhury, Huang and Yu2014; Chernysh et al., Reference Chernysh, Gordya and Suborova2015), because the resistance development to single AMPs is more easier (Perron et al., Reference Perron, Zasloff and Bell2006).
The wasp-originated ovarian and venom proteins are involved in modifying the immune responses, especially those hemocyte-mediated reactions (Glupov & Kryukova, Reference Glupov and Kryukova2016). Contrary to the reduction noted in parasitized pupae, T. brontispae ovarian fluid induced an increase in phagocytosis activity. Such discrepancy may stem from the presence of antidote(s) to ovarian proteins in the venom apparatus, which were also found in Asobara japonica–Drosophila melanogaster (Mabiala-Moundoungou et al., Reference Mabiala-Moundoungou, Doury, Eslin, Cherqui and Prévost2010). On the other hand, the dose used for injection may also account for such discrepancy because most ovarian and venom proteins are dose-dependent in regulating immunity (Teng et al., Reference Teng, Xu, Gan, Chen, Fang and Ye2016; Li et al., Reference Li, Xu, Liu, Wu, Ren and Zhu2018). The elevated immune response (cuticular encystment) induced by ovarian fluids was also observed in the Autographa nigrisigna (Walker) (Lepidoptera: Noctuiidae)–Campoletis chlorideae Uchida (Hymenoptera: Ichneumonidae) association (Namba et al., Reference Namba, Nakamatsu, Miura and Tanaka2008). Unfortunately, the mechanisms responsible for frequently preventing these properties of ovarian protein from functioning during parasitism are still little understood.
In summary, the intensive bactericidal activity largely accounted for the enhanced bacteria degradation noted in parasitized O. nipae pupae. This study contributes to our understanding of the basic physiological and molecular regulation of immunity between parasites and their hosts, but overlooks the underlying mechanisms involved. Further study is necessary to elucidate the molecular mechanics involved in this process of alteration, e.g., to study the effects of parasitism on the genes regulating the synthesis of AMPs or those genes related to phagocytosis and the genes which participate in switching cellular and humoral immunity as reported in Drosophila by Tsuzuki et al. (Reference Tsuzuki, Matsumoto, Furihata, Ryuda, Tanaka, Sung, Bird, Zhou, Shears and Hayakawa2014). The effects of venom, ovarian protein on microbial clearance are also under investigation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485318000780.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31471829 and 31301727).