Introduction
Over the last few decades, human activities have altered the earth’s ecosystems and threatened the well-being of wildlife (Jetz et al. Reference Jetz, Wilcove and Dobson2007). Human disturbances are diverse and differ in intensity and frequency, constituting a stressor for animals (Benítez-Díaz and Bellot-Rojas Reference Benítez-Díaz and Bellot-Rojas2007). For instance, human-induced forest fragmentation decreases food availability (Zanette et al. Reference Zanette, Doyle and Trémont Steve2000), urban development reduces habitat suitability (Alonso et al. Reference Alonso, Martín, Palacín, Magaña and Martin2003, Reference Alonso, Martín, Alonso, Palacín, Magaña and Lane2004, Martín Reference Martín2008), and agriculture exposes animals to disturbance caused by farming activities (Sastre et al. Reference Sastre, Ponce, Palacín, Martín and Alonso2009). Human disturbance may lead wildlife to react by changing distributions (e.g. Gomes et al. Reference Gomes, Oostra, Nijman, Cleef and Kappelle2008, López-Jamar et al. Reference López-Jamar, Casas, Díaz and Morales2011, Chávez-Zichinelli et al. Reference Chávez-Zichinelli, Macgregor-Fors, Quesada, Rohana, Romano, Valdéz and Schondube2013), behaviour (e.g. Casas et al. Reference Casas, Benítez-López, Tarjuelo, Barja, Viñuela, García, Morales and Mougeot2016, Mougeot and Arroyo Reference Mougeot and Arroyo2017) and physiology (e.g. Barja et al. Reference Barja, Silvan, Rosellini, Piñeiro, Gonzalez-Gil, Camacho and Illera2007, Chávez-Zichinelli et al. Reference Chávez-Zichinelli, MacGregor-Fors, Rohana, Valdéz, Romano and Schondube2010, Fokidis and Deviche Reference Fokidis and Deviche2011, Chávez-Zichinelli et al. Reference Chávez-Zichinelli, Macgregor-Fors, Quesada, Rohana, Romano, Valdéz and Schondube2013), although the strategy to cope with alterations might be species-specific (e.g. Samia et al. Reference Samia, Nakagawa, Nomura, Rangel and Blumstein2015). Nevertheless, human activities may be also beneficial for wildlife on some occasions so that human-altered landscapes often show high levels of biodiversity (Walk and Warner Reference Walk and Warner2000, Wolff et al. Reference Wolff, Jean-Philippe, Martin and Bretagnolle2001).
Theoretical studies have suggested that an understanding of the specific physiological mechanisms underlying conservation problems might be critical for animal conservation as this may allow identifying the optimal range of habitat and stressor thresholds for threatened species (Cooke et al. Reference Cooke, Sack, Franklin, Farrell, Beardall, Wikelski and Chown2013). Under both short- and long-term alterations in their habitats, individuals initiate a stress response resulting in an activation of the hypothalamic-pituitary-adrenal (HPA) axis and then in an increase in the level of circulating plasma glucocorticoids (Romero Reference Romero2004). Released hormones manage the homeostatic energy balance and allocation of resources between vital processes and threat events in vertebrates (Buchanan Reference Buchanan2000, Sapolsky et al. Reference Sapolsky, Romero and Munck2000, Landys et al. Reference Landys, Ramenofsky and Wingfield2006). In the face of short-term perturbations in the environment, glucocorticoids can promote rapid physiological and behavioural changes in animals that help them to escape from unexpected survival challenges (Sapolsky et al. Reference Sapolsky, Romero and Munck2000). However, when perturbations continue over the long-term (i.e. chronic stress), the maintenance of high baseline levels of glucocorticoids may impair immunocompetence (Martin Reference Martin2009), reproductive functions (Sapolsky et al. Reference Sapolsky, Romero and Munck2000), and, ultimately, affect fitness (Boonstra Reference Boonstra2013).
In this study we analyse distribution, physiological and behavioural responses to variable levels of human activities associated with agriculture or human development in the south of Spain for two farmland birds: the European Roller Coracias garrulus (hereafter Roller) and the Eurasian Scops Owl Otus scops (hereafter Scops Owl). Farmland birds are among the most threatened bird group in Europe due to increasing human development of agricultural habitats (Donald et al. Reference Donald, Green and Heath2001, Reference Donald, Sanderson, Burfield and Bommel2006, Burfield and Van Bommel Reference Burfield and Van Bommel2004, Bota et al. Reference Bota, Morales, Mañosa and Camprodon2005), and hence, human activities may have a great impact on these species (e.g. Onrubia and Andrés Reference Onrubia, Andrés, Bota, Morales, Mañosa and Camprodon2005, Casas et al. Reference Casas, Mougeot, Viñuela and Bretagnolle2009, Sastre et al. Reference Sastre, Ponce, Palacín, Martín and Alonso2009, Steven et al. Reference Steven, Pickering and Castley2011, Tarjuelo et al. Reference Tarjuelo, Barja, Morales, Traba, Benítez-López, Casas, Arroyo, Delgado and Mougeot2015). Also, human development leads to the construction of linear infrastructure as roads which may cause detrimental habitat loss, fragmentation, degradation and noise for wild animals (e.g. Barber et al. Reference Barber, Crooks and Fristrup2010). European Roller and Scops Owl breeding populations have declined in the last few decades (BirdLife International 2018), due to habitat loss caused by agricultural intensification (Avilés and Folch Reference Avilés, Folch, Madroño, González and Atienza2004, Martínez et al. Reference Martínez, Zuberogoitia, Martínez, Zabala and Calvo2007) and loss of suitable nesting holes (Kiss Reference Kiss, Elek and Moskát2014, Reference Kiss, Tokody, Deák and Moskát2016). However, the spatial distribution, physiological and behavioural responses to variable levels of human disturbance in these two species have not yet been simultaneously analysed, which impedes a full understanding of the possible strategies of these declining species to buffer human alteration in farmland habitats. We specifically assessed whether individuals of the two species were distributed differently, and according to their qualities (estimated through laying date) in relation to human activities. In addition, we evaluate whether stress levels measured through blood (CORT) and feather (fCORT) corticosterone increased and condition of nestlings and parental behaviour (measured as hourly feeding rate) decreased with higher levels of human activities. Regarding distributional patterns we predicted that i) Rollers and Scops Owls were distributed differently in relation to human activities; and ii) that best quality individuals of each species avoided nesting in habitats with high level of human disturbance. We also expect that iii) nestlings of the two species reared in nests exposed to high levels of human activities would show higher corticosterone concentrations and lower weight at fledging than nestlings reared in more natural habitats. Finally, we predicted that iv) parents would lower their provisioning effort in nests exposed to high levels of human activities because they would devote more time to vigilance (Casas et al. Reference Casas, Mougeot, Viñuela and Bretagnolle2009, Wang et al. Reference Wang, Li, Beauchamp and Jiang2011, Yorzinski et al. Reference Yorzinski, Chisholm, Byerley, Coy, Aziz, Wolf and Gnerlich2015).
Methods
Study area
The study was conducted in the breeding season (April–July) of 2013 in south-eastern Spain (37818° N, 3811° W). The area is an extensive agricultural landscape with scattered holm oaks Quercus ilex and crossed by numerous dry riverbeds (ramblas). The area covers approximately 756 ha of holm oak wooded landscape where 443 cork oak nest-boxes (roof surfaces of 24 x 24 cm, 40 cm height and an opening of 6 cm diameter) were positioned on trees (see details in Rodríguez et al. Reference Rodríguez, Avilés and Parejo2011). At least once a week, nest-boxes were checked to determine occupancy and to record reproductive parameters in the context of a long-term monitoring project of cavity-nesting breeding birds (Parejo et al. Reference Parejo, Cruz-Miralles, Rodríguez-Ruiz, Expósito-Granados and Avilés2018). A nest-box was considered to be occupied if at least one egg was laid in it. In the year of study, 28 nests of Rollers were initiated and physiological measurements were taken from 60 nestlings from 16 nests that escaped predation. For Scops Owls, 21 nests were initiated and physiological measurements were taken from 74 nestlings from 18 available nests that escaped predation.
Environmental data
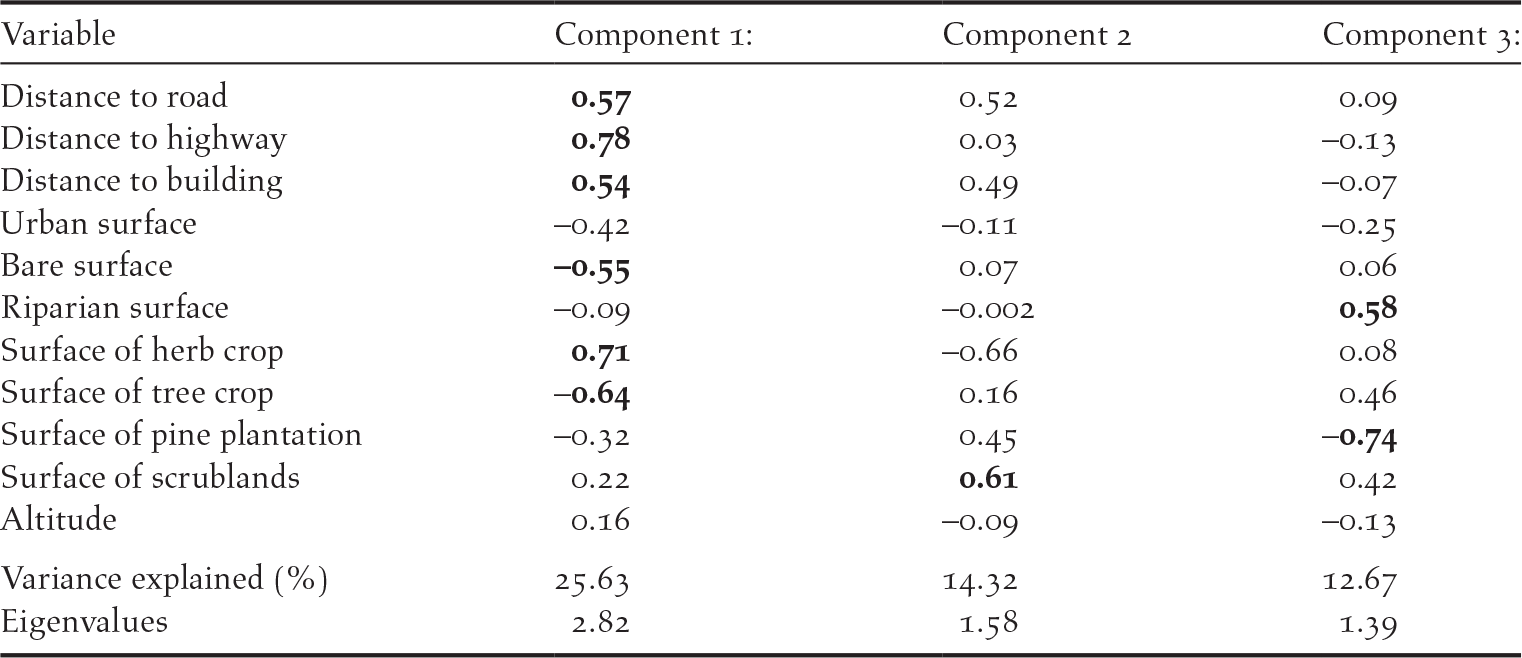
We used R software version 3.3.3 (raster Package v 2.6-7; Hijmans et al. Reference Hijmans, van Etten, Joe Cheng, Mattiuzzi, Sumner, Greenberg, Lamigueiro, Bevan, Racine, Shortridge and Ghosh2017) to process environmental data based on aerial photographs and 2005 Vegetation Cover and Land Use Databases for the Province of Granada that were freely available. Information from these sources was updated in 2011 (Documento técnico SIOSE 2011, Versión 1.2, Dirección General del Instituto Geográfico Nacional, Ministerio de Fomento). For each nest-box, we derived the following variables: (1) distance to the nearest road (m), (2) distance to the highway (m), (3) distance to the nearest building (m) and (4) altitude above sea level (m). The study area is crossed by a heavily used highway and other minor roads that could also potentially disturb animals (Reijnen and Foppen Reference Reijnen and Foppen1994; see Figure 1 in Rodriguez et al. Reference Rodríguez, Avilés and Parejo2011). Buildings are isolated farmhouses, most of them abandoned, and one small village. Also, in order to qualify variability in human pressure/alteration, we estimated the percentage of urban surface, bare ground (non-cultivated or covered area), the surface occupied by grassland and herb crops (i.e. cultivated areas), tree crops, pine plantation, scrubland and surface occupied by riparian surfaces (i.e. watercourses) within a circular area with a radius of 100 m centred on each nest-box (Rodríguez et al. Reference Rodríguez, Avilés and Parejo2011). Preliminary analyses revealed that some environmental variables were highly inter-correlated (Table S1 in the online Supplementary material), so we simplified the data by performing a principal components analysis (PCA) on the set of environmental variables. The first three PCA axes explained 25.63, 14.32 and 12.67% of the variance, respectively (Table 1). The first PCA axis was identified as a gradient of farming and human development as it classified nest-boxes according to distance to highway, roads and buildings and the surface covered by herb crops (positive loadings) versus the surface covered by tree crops and bare surface (negative loadings) (Table 1). Given that in our study area most of tree crops are almond trees, which are not managed during spring and summer, tree crops can be considered as a less human-disturbed habitat than herb crops. Thus, nest-boxes with positive loadings were in areas with high levels of farming activity but low levels of human disturbance induced by infrastructure. The second axis classified the nest-boxes into a gradient of scrublands with positive values on this axis indicating nest-boxes in areas with high density of scrublands (Table 1). Finally, the third axis classified nest-boxes according to the density of pine plantations versus density of watercourses (i.e. riparian areas). Hence, this axis can be regarded as a gradient from areas with high pine cover to areas with high riparian vegetation cover (Table 1). Component scores derived from the PCA were used in subsequent analyses.
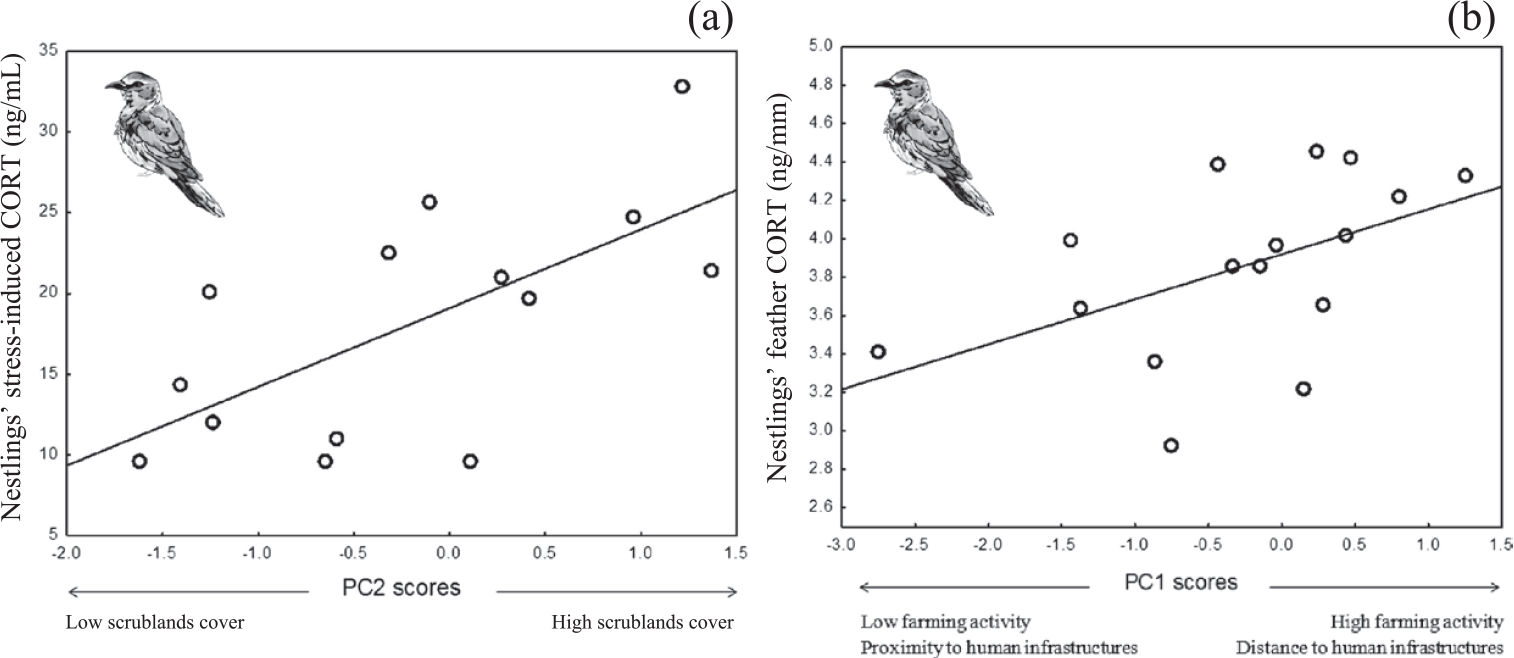
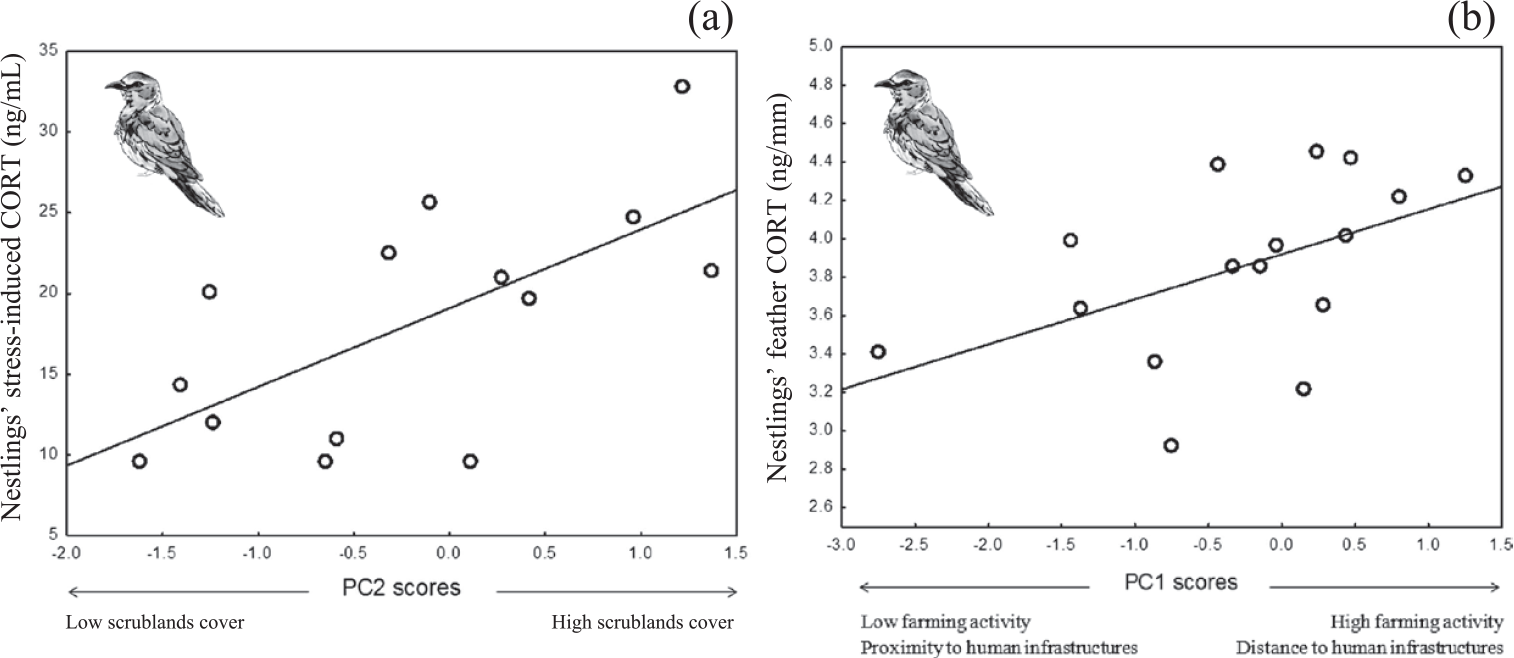
Figure 1. Mean stress-induced corticosterone (a) and feather corticosterone (b) of Roller nestlings in relation to second (a) and first (b) PC scores of a PCA on habitat features (see table 1).
Table 1. Loadings of the environmental variables in the three principal components (n = 443 nest-boxes). Important loadings (>0.50) within each component are depicted in bold.

Nestling sampling
Blood from nestlings of the two species was extracted on two different days but at similar stage of nestling development for both species. Twenty days after the hatching of the first chick in Rollers and 15 days after the hatching of the last chick in Scops Owls, nestlings were bled to obtain CORT measurements in plasma (stress-induced CORT). Sampling was always done in the morning (08h30 to 11h30) to avoid a possible problem of circadian CORT changes (Breuner et al. Reference Breuner, Wingfield and Romero1999). On the same day we also collected the third primary major covert feather of each nestling and stored it in a dark box at ambient temperature until laboratory analyses of fCORT.
Blood was collected from the brachial vein using a 0.5 x 16 mm needle and heparinized capillary tubes to carefully transfer it into a 1.5 mL tube. Blood was refrigerated and plasma and red blood cells were separated by centrifugation the same day at 13,300 rpm for 5 min. All samples were stored in a -20°C freezer until the end of fieldwork when they were kept in a -80°C freezer until laboratory analyses. Nestlings of the two species were weighed at age of 21 days to the nearest 1 g using a 300 g Pesola spring balance.
Corticosterone assay
Corticosterone concentrations were determined at the Centre d’Études Biologiques de Chizé (France) following the procedure of Lormée et al. (Reference Lormée, Jouventin, Trouve and Chastel2003) for steroid hormones. Briefly, an ethyl ether extraction technique was used to extract CORT from plasma (Lormée et al. Reference Lormée, Jouventin, Trouve and Chastel2003) and a methanol-based extraction technique was used to extract CORT from feathers (Bortolotti et al. Reference Bortolotti, Marchant, Blas and German2008). Plasma and feather CORT extracts were measured using radio-immunoassay with a highly cross-reactive rabbit anti-mouse antibody from Sigma (C8784). Duplicate aliquots of the extracts were incubated overnight at 4°C with 3H-corticosterone and antiserum. The bound and free corticosterone fractions were separated by adding dextran-coated charcoal and after centrifugation, the bound fraction was counted in a liquid scintillation counter. Samples were assayed the same day or were frozen at -20° until analysis. The lowest detectable corticosterone level was 0.28 ng/mL. Intra- and inter-assay coefficients of variation for plasma assays were 6.29% and 15.72%, respectively. For feather assays intra and inter-assay coefficient were 8.95% and 14.05%, respectively.
Parental care
Parental behaviour at each nest was filmed once with video cameras in the first third of the nesting period. This was done in all the non-manipulated nests of the two species in 2011–2015 and 2012–2017 for Rollers and Scops Owls, respectively. As we are dealing with a diurnal and a nocturnal species, recordings were done in the corresponding activity period for each species (through the day for Rollers and after dawn for Scops Owls). For Rollers we used a Sony DCR -SR32 video camera camouflaged and placed at a distance of 5–10 m from the nest hole. For Scops Owls we used infrared cameras (KPC- S500, black and white CCD camera, Essentia Systems Inc.) located inside nest boxes. Recordings lasted between 60 and 90 minutes and then we calculated hourly feeding rate (i.e. the number of visits with prey per hour).
In total we obtained data from 42 Roller and 55 Scops Owl nests located in different habitats. For those nests occupied more than one year we only used data from the first year to avoid pseudoreplication of nests under the same habitat features.
Statistics
Analyses were performed using SAS v.9.4 statistical software (SAS Institute, Cary, NC, USA). Sample sizes may vary amongst analyses because sometimes CORT levels were under the threshold for detection and because, due to logistical problems, not all information could be collected for all nestlings or broods.
We performed General Linear Models (GLM procedure in SAS, Normal distribution, link = identity) with the PCA component scores as dependent variables and species as explanatory factor to analyse distributional patterns of both species in relation to human activities. Moreover, to evaluate intra-specific distribution related to individual quality we performed another General Linear Model (GLM procedure in SAS, Normal distribution, link = identity) for each species in which laying date was the dependent variable and the three PCA component scores the explanatory variables. Laying date is a good predictor of individual quality in birds (Verhulst and Nilsson Reference Verhulst and Nilsson2008, Williams Reference Williams2012). Indeed, laying date predicts reproductive success in both Rollers (Avilés et al. Reference Avilés, Sánchez, Sánchez and Parejo1999, Parejo et al. Reference Parejo, Silva and Avilés2007, Silva et al. Reference Silva, Avilés, Danchin and Parejo2008) and Scops Owls (Hardouin et al. Reference Hardouin, Bretagnolle, Tabel, Bavoux, Burneleau and Reby2009).
Secondly, we ran General Linear Models (GLM procedure in SAS) to test for the effect of human disturbance on average values per nest of stress-induced CORT levels, fCORT levels and weight of Roller and Scops Owl nestlings. We entered the three component scores derived from the PCA on environmental variables for each occupied breeding attempt as explanatory variables. In addition, to control for possible differences in seasonality of perturbations or declining of resources, laying date was included as a further covariate in the models. Finally, brood size was entered as a covariate to take into account inter-nest differences due to sibling competition. Standard model validation graphs (Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009) revealed that model assumptions of homogeneity of variance and normality of residuals were fulfilled.
Finally, we analysed the relationships between feeding rates of each species and habitat features by performing General linear mixed models (MIXED procedure in SAS) in which log-transformed feeding rates are the dependent variables. In all models we entered the three PCA component scores as explanatory variables. We also included laying date and time of the day as covariates. The year was introduced as a random factor to account for the possible effect of variable environmental conditions through the study. Due to low sample size, we disregarded testing interactive effects in the models. Model simplification was performed following backward stepwise elimination of non-significant terms from the initial models.
Results
Distributional responses in relation to human activities
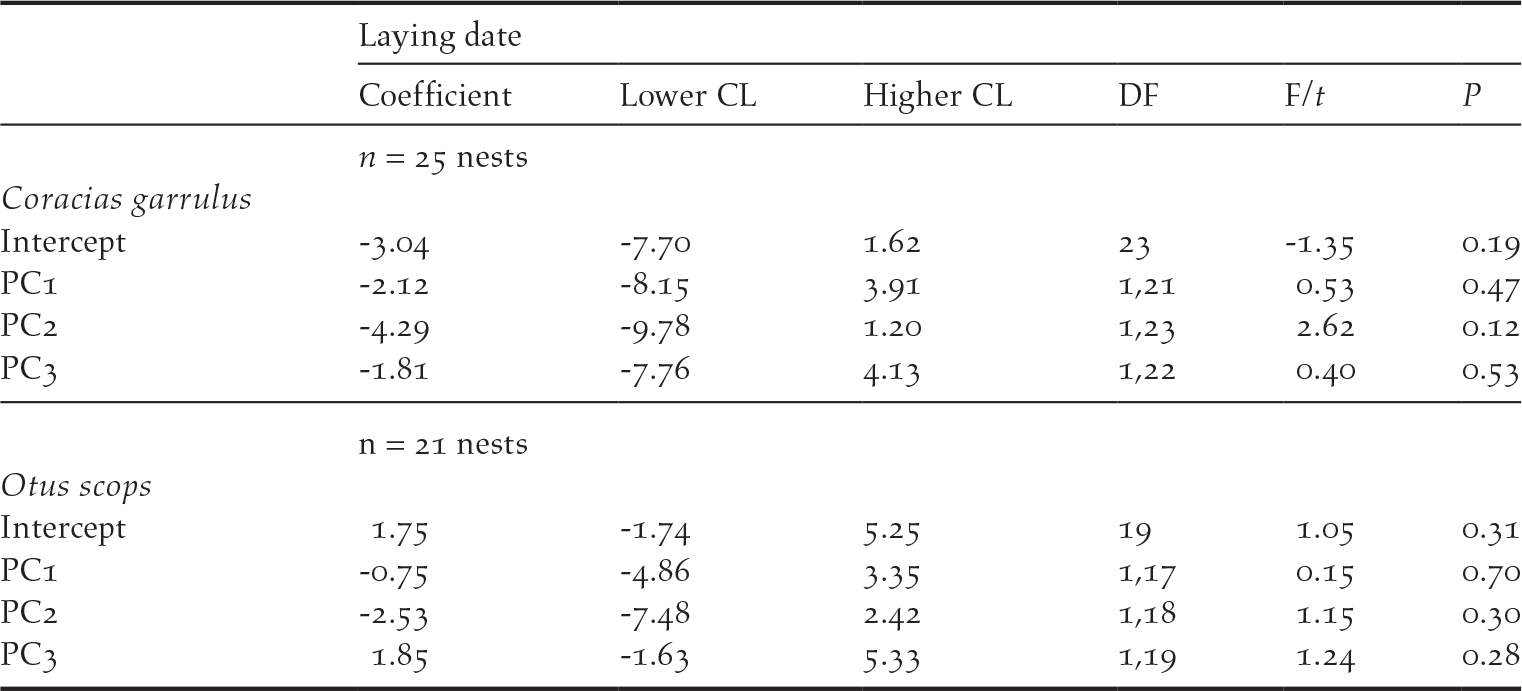
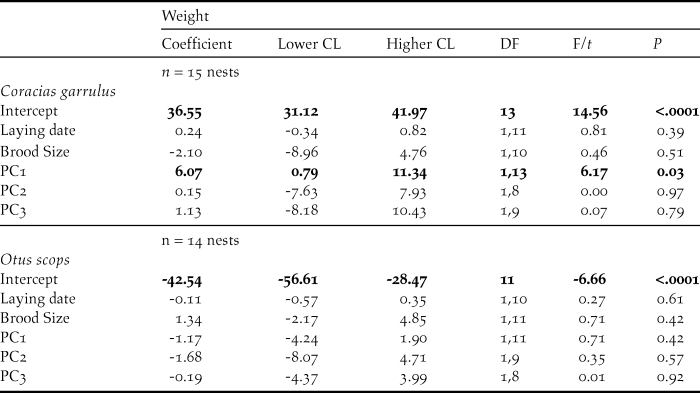
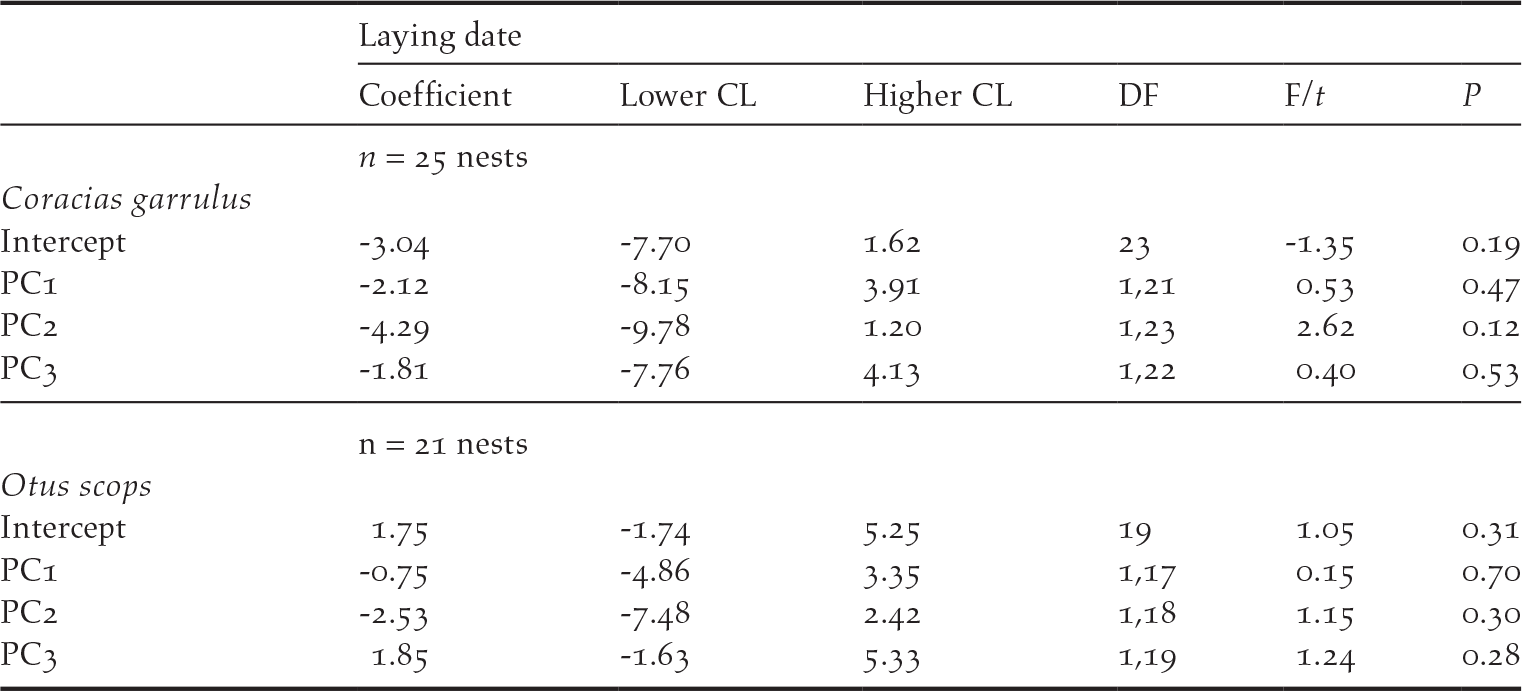
There was no difference between the location of Roller and Scops Owl nests within the habitats in the study area (GLM models, PC1score: F1, 47 = 0.30, P = 0.59; PC2 score: F1, 47 = 0.95, P = 0.33; PC3 score: F1, 47 = 0.15, P = 0.70, n = 49; Figures S1 and S2). In addition, neither Rollers nor Scops Owls seemed to settle down in the different habitats in relation to their quality because their laying dates were not related to the scores of any of the PCA components (Table 2).
Table 2. Results of General Linear Models (Normal distribution, link=identity) testing for the effect of habitat features on laying date of Roller and Scops owl nests as a dependent variable. Non-significant terms were removed following a backward procedure.
Effect of habitat features on nestlings’ corticosterone responses and weight at fledging
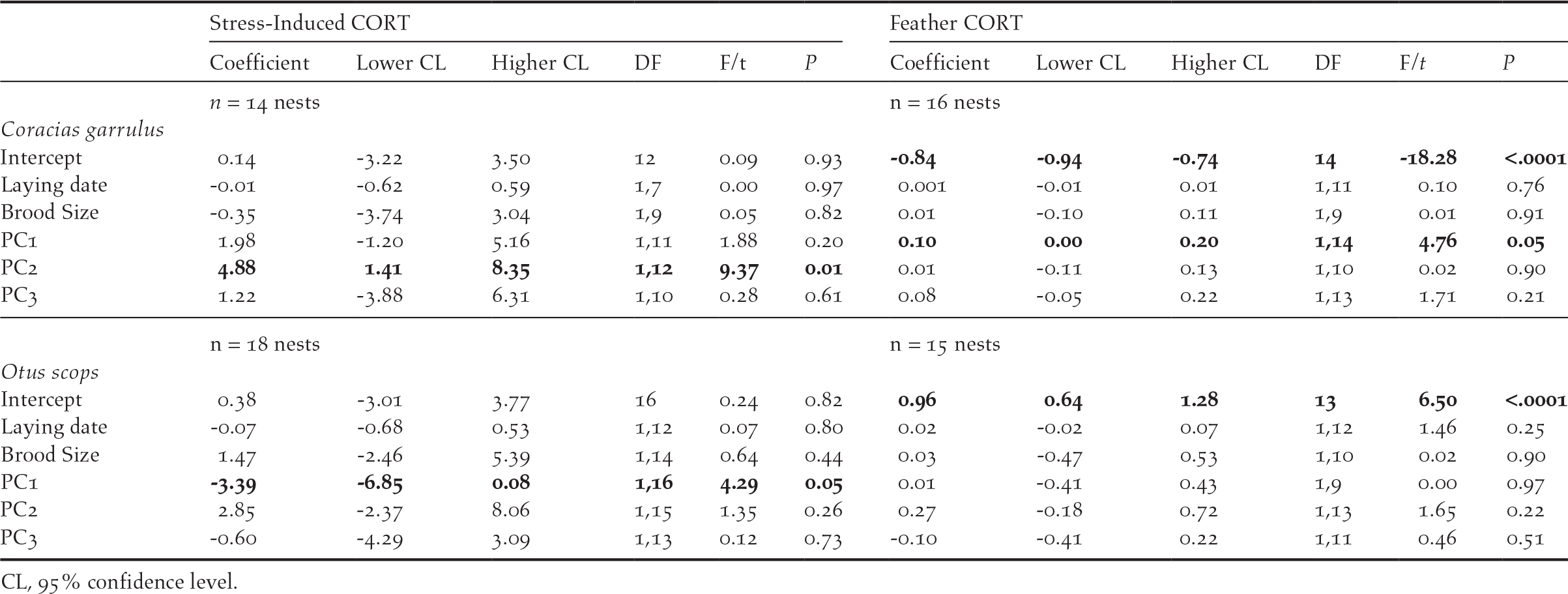
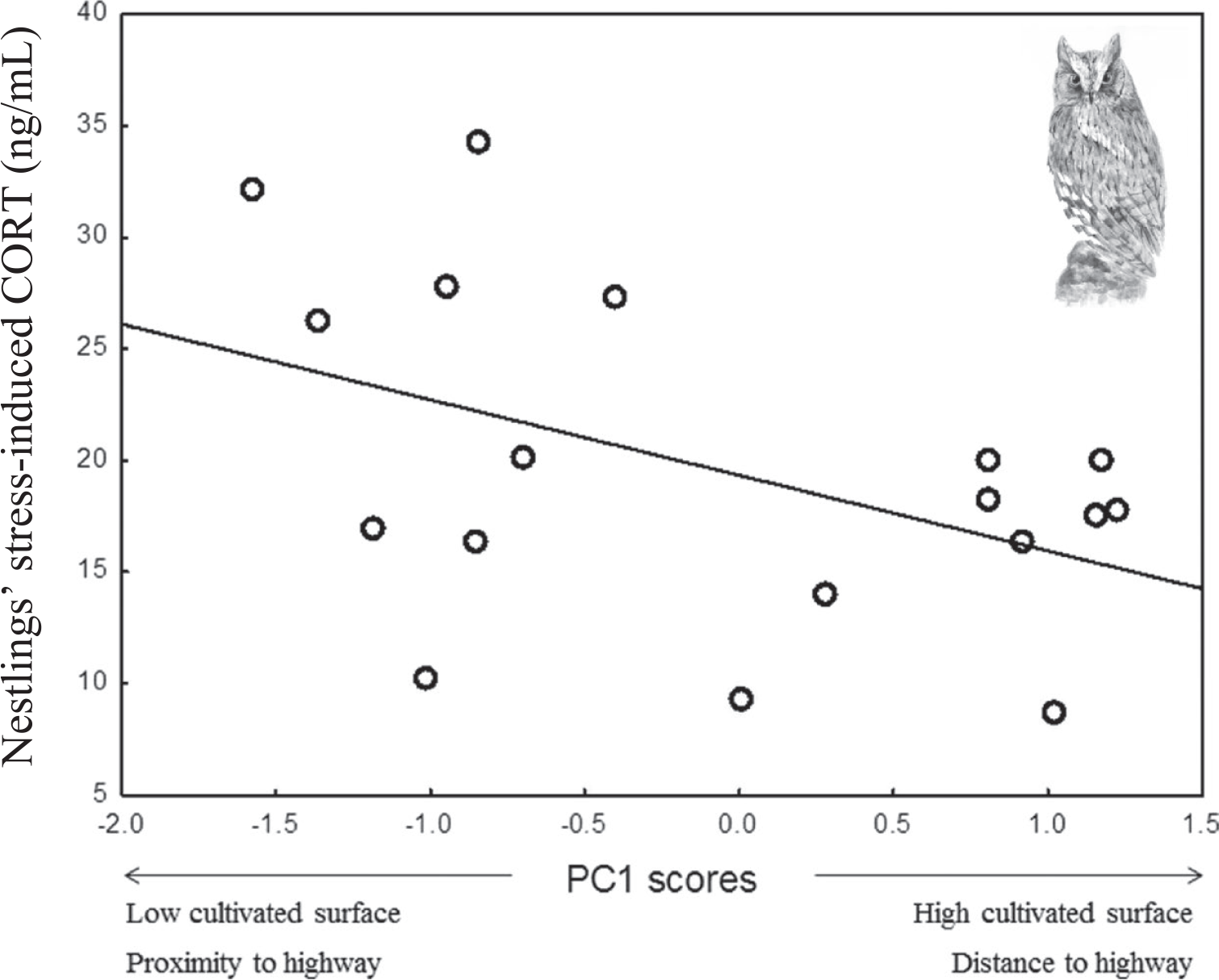
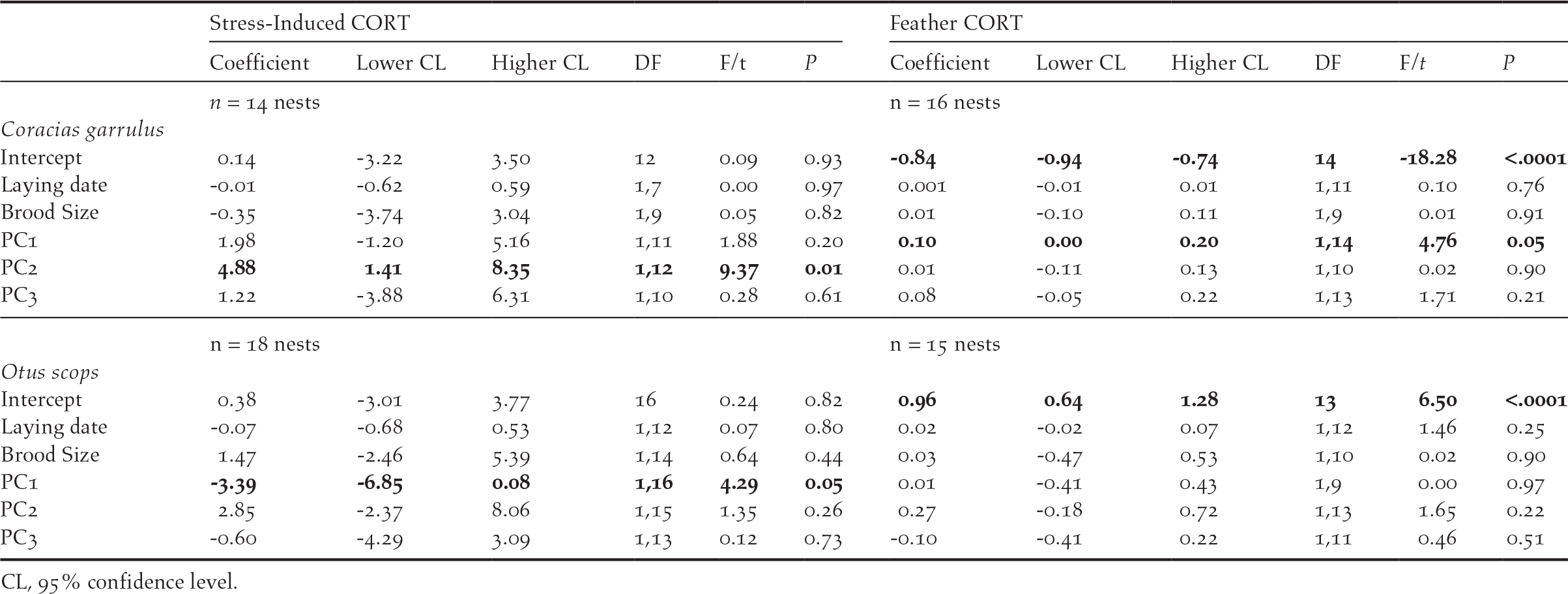
Stress–induced CORT levels varied in both species in relation to different components of habitat features (Table 3). In Rollers, stress-induced CORT levels varied according to PC2 scores (P = 0.01; Table 3), meaning that nestlings raised in nests located in areas with a high percentage of scrublands had higher levels of stress-induced CORT than those raised in nests located in areas with low percentage of scrublands (Figure 1a). On its side, stress-induced CORT levels of Scops Owl chicks were almost significantly associated with PC1 scores (P = 0.05; Table 3): chicks from nests located in areas with high levels of human disturbance induced by infrastructures and low levels of farming activity had higher stress-induced CORT levels that those from nests located in areas with low levels of human disturbance induced by infrastructures and high levels of farming activity (Figure 2).
Table 3. Results of GLMs testing for the effect of habitat features (i.e. PC scores of a PCA on habitat variables) on stress hormone measures (i.e. mean stress-induced CORT and fCORT per nest). Non-significant terms were removed following a backward procedure. Significant terms are highlighted in bold.
CL, 95% confidence level.

Figure 2. Mean stress-induced corticosterone of Scops Owl chicks in relation to PC1 scores of a PCA on habitat features (see table 1).
Concerning fCORT, we found that levels in Roller chicks were almost significantly associated with PC1 scores (P = 0.05; Table 3). Nestlings raised in nests located in areas with intense farming activity and low human disturbance by infrastructures had higher values of fCORT than those raised in nests with low level of farming activity and high human disturbance by infrastructures (Figure. 1b). fCORT levels of Scops Owl chicks were not significantly associated with any of PC scores from the PCA on environmental variables (P > 0.22 in all cases; Table 3).
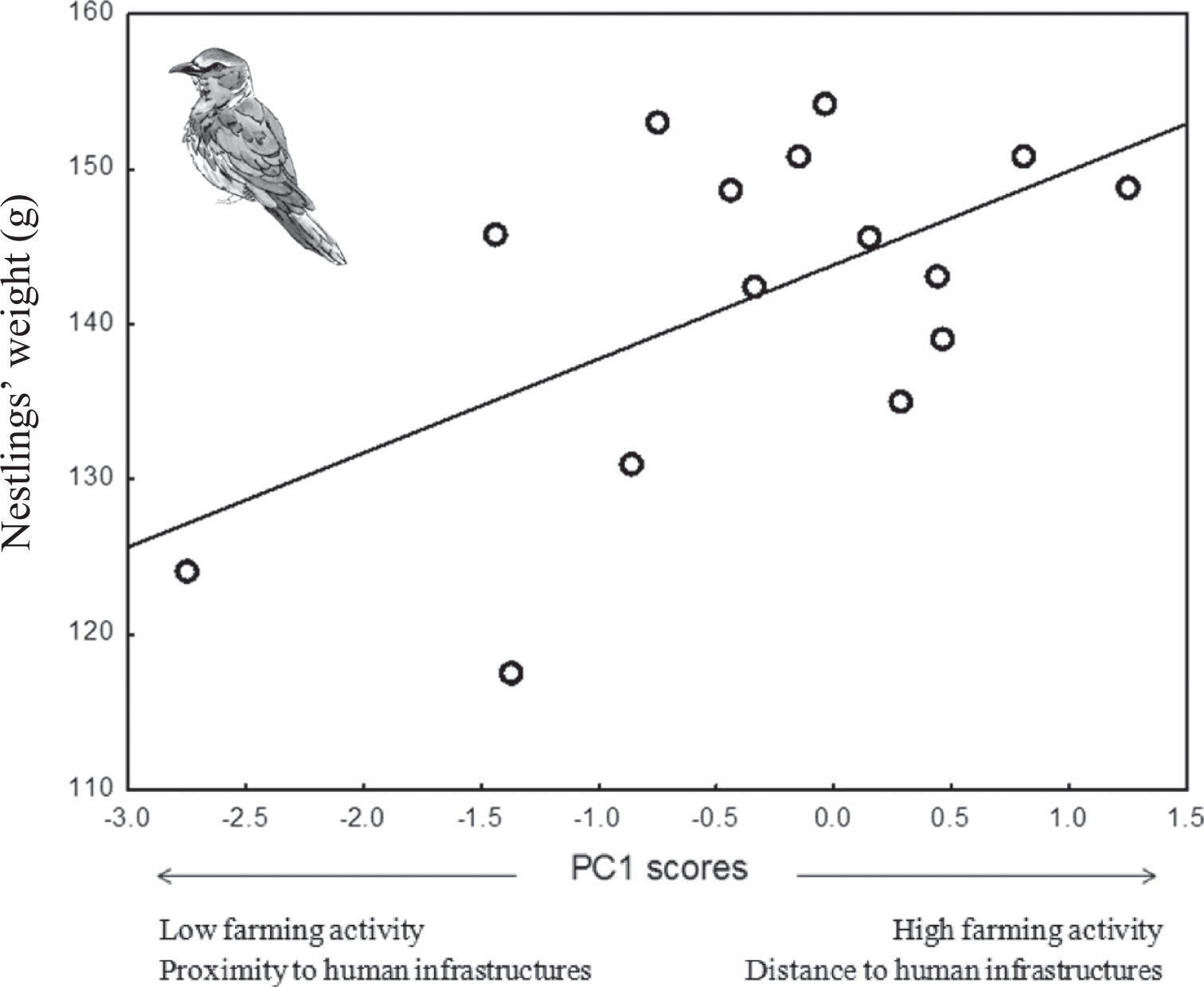
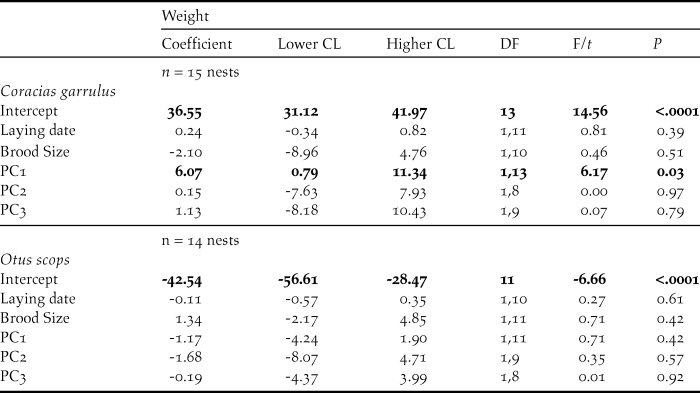
Weight of roller nestlings was positively associated with PC1 scores (P = 0.03; Table 4), meaning that nestlings raised in nests located in areas with a high level of farming activity and low human disturbance by infrastructures were heavier at fledging than those raised in nests with low level of farming activity and high human disturbance by infrastructures (Figure 3). No relationship was found between the mean weight of Scops Owl chicks and PCA components of habitat, laying date or brood size (P > 0.42 in all cases, Table 4).
Table 4. Results of GLMs testing for the effect of habitat features of PCA analysis on weight of Roller and Scops Owl nestlings at 21 days age. Non-significant terms were removed following a backward procedure. Significant terms are highlighted in bold.
CL, 95% confidence level.
Figure 3. Mean weight of Roller nestlings in relation to PC1 scores of a PCA on habitat features (see table 1).
Effect of habitat features on parental care
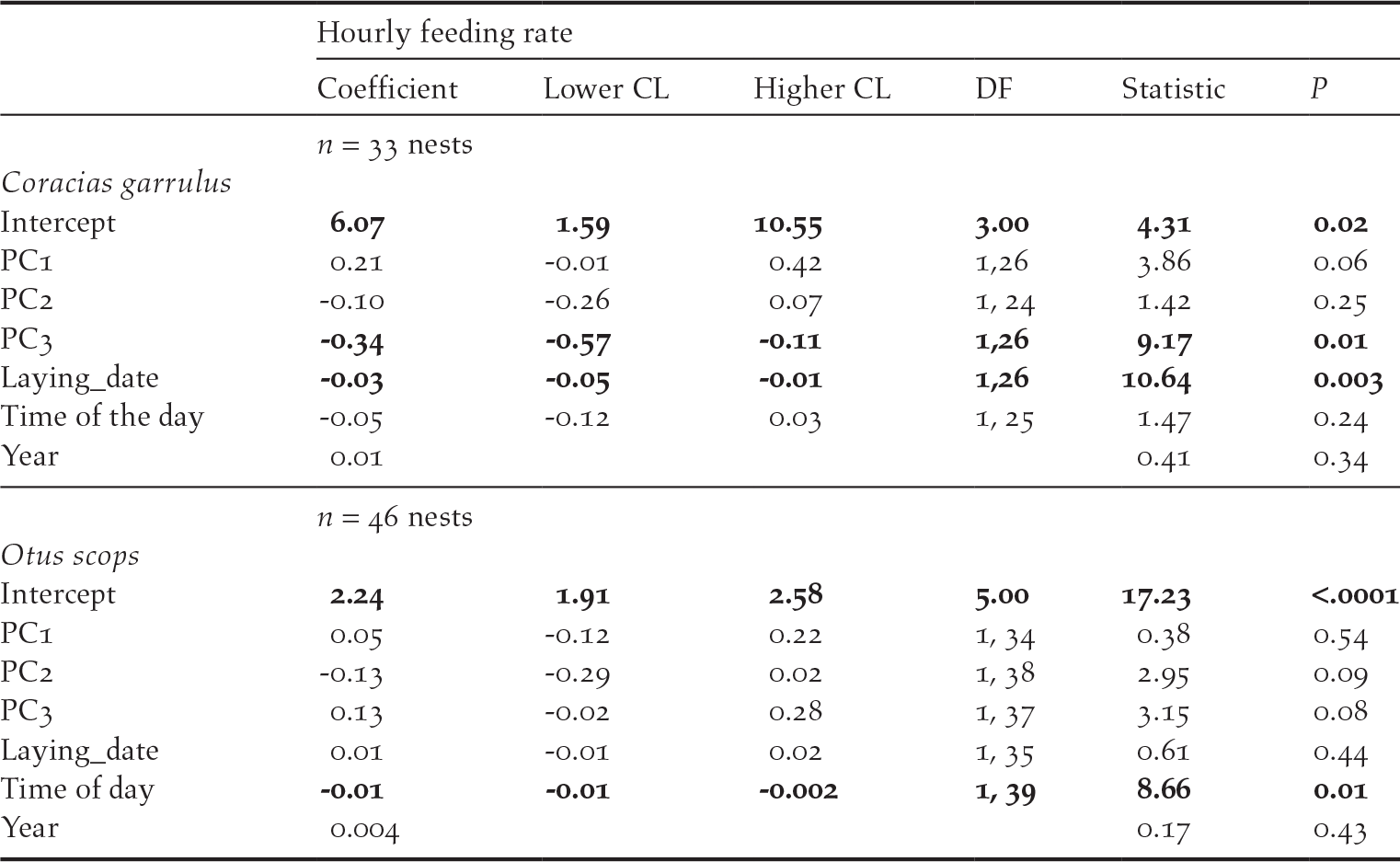
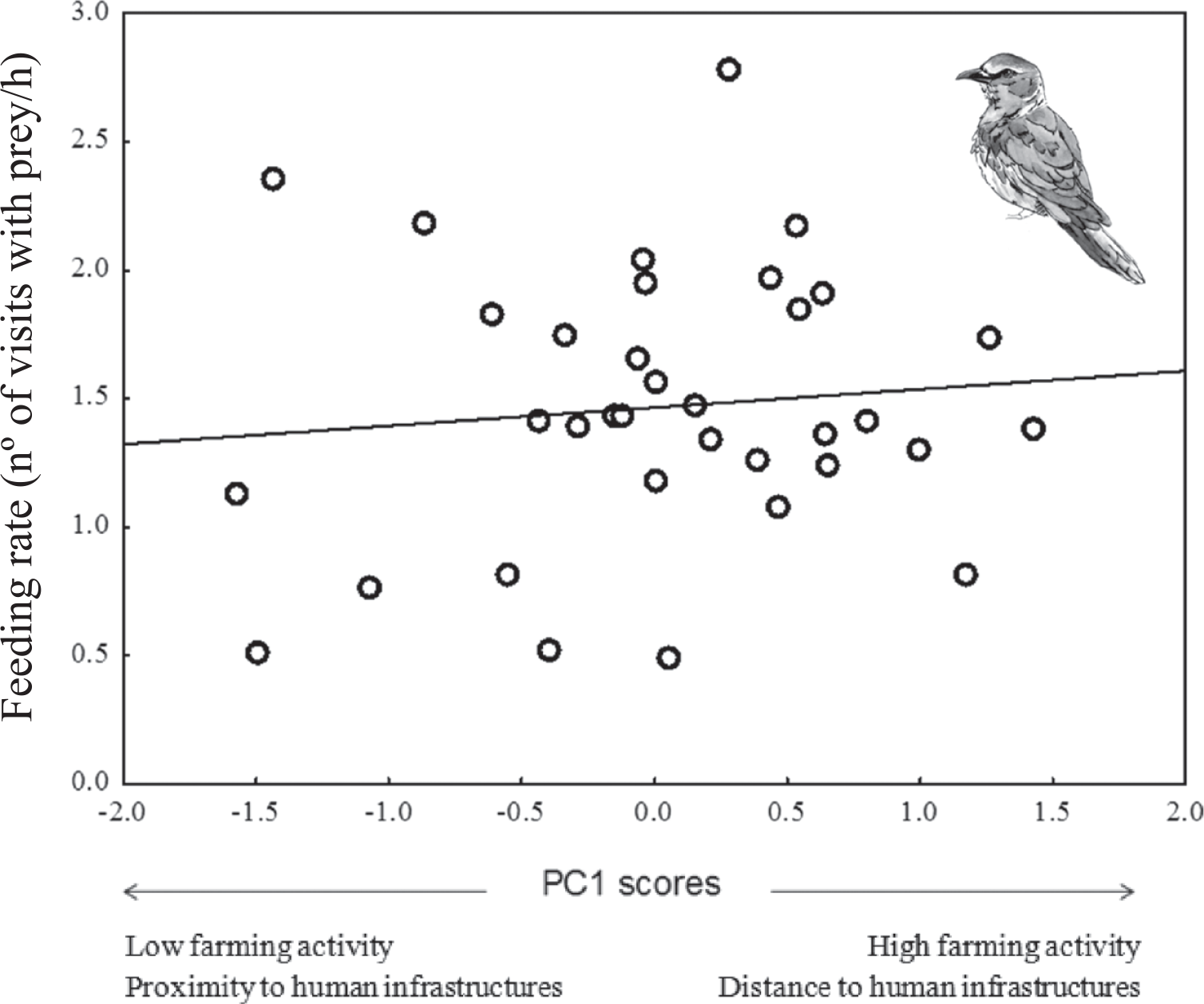
In Rollers, parental behaviour was marginal and positively related to PC1 scores (P = 0.06) and negatively related to PC3 scores (P = 0.01; Table 5): feeding rates tended to be more intense in areas with high farming activity but low human disturbance by infrastructures compared to those in areas with low farming activity and high human disturbance by infrastructures (Figure 4). In addition, feeding rates were higher in less riparian areas and with higher pine cover (Table 5). Parental behaviour of Scops Owls was not related to any of the PC scores from the PCA on environmental variables (P > 0.08 in all cases; Table 5).
Table 5. Results of General Linear Mixed Models (Normal distribution, link=identity) testing for the effect of habitat features on feeding rates of Roller and Scops Owl as dependent variables. Non-significant terms were removed following a backward procedure. Significant terms are highlighted in bold.
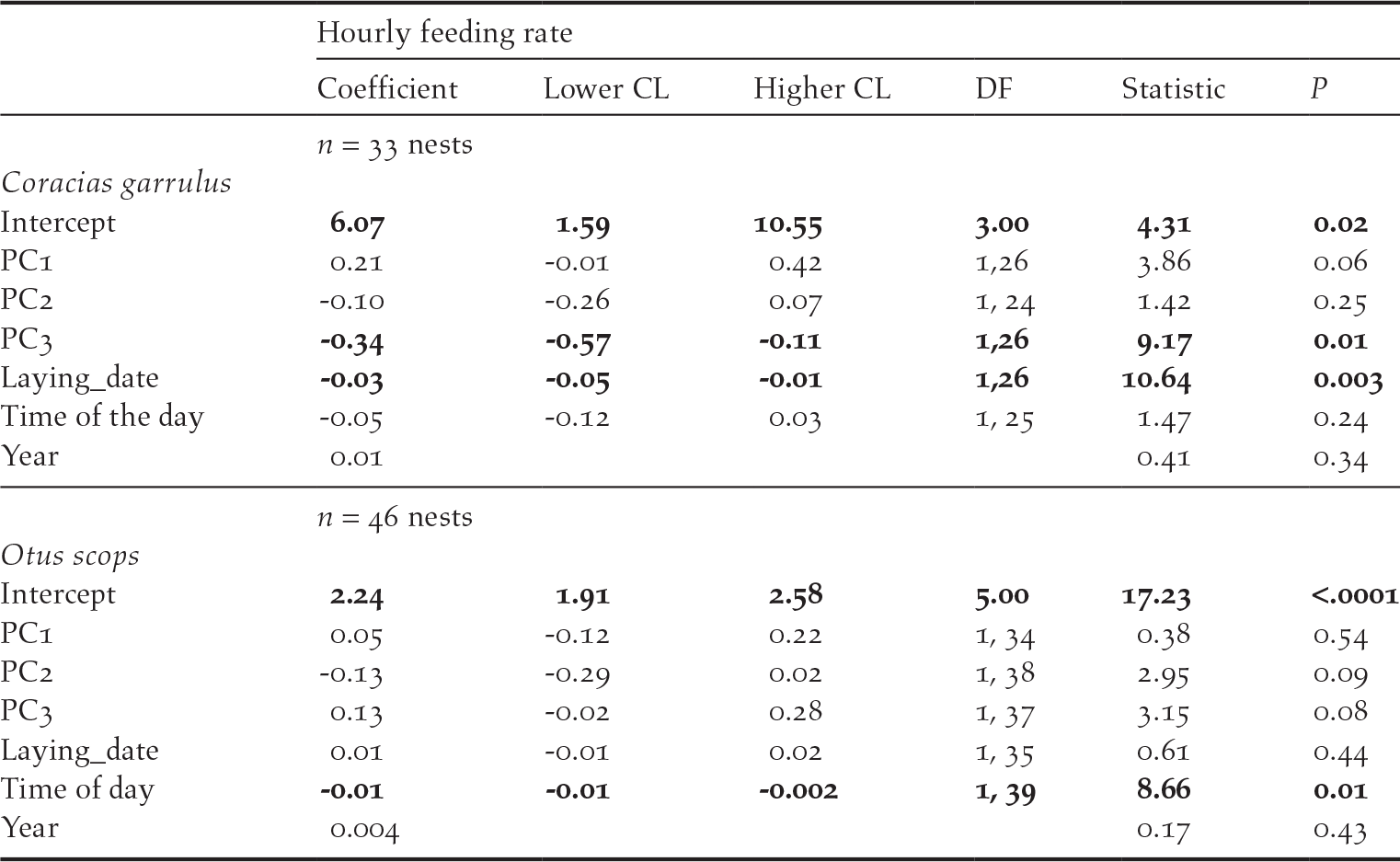

Figure 4. Feeding rate (number of parental visits with prey to the nest per hour) of Roller adults in relation to PC1 scores of PCA on habitat features (see table 1).
Discussion
Rollers and Scops Owls were distributed similarly in relation to habitat features, which would indicate that the two species would be a priori exposed to similar levels of human disturbance. In addition, we did not find support for the idea that best quality individuals of each species avoided nesting in habitats with high level of human disturbance. All this would suggest that Rollers and Scops Owls do not respond to human alteration within our study area by changing their breeding distribution.
We found an association between the nestlings´ physiology of these two farmland birds and habitat features. The found patterns differed between Rollers and Scops Owls. Firstly, roller nestlings from nests in areas with high density of scrublands showed high levels of induced CORT. The explanation to this non-expected result may be the high risk of predation by natural predators in scrublands. Indeed, in our study area, ladder snakes Zamenis scalaris predate on nesting birds (authors’ unpubl. data) and are associated with scrublands from where they can more easily access trees to predate (Pleguezuelos Reference Pleguezuelos, Salvador and Marco2017). Also, garden dormouse Eliomys quercinus, which predate on eggs and nestlings (Gil-Delgado et al. Reference Gil-Delgado, Tamarit, Viñals, Gómez and Vives-Ferrandiz2009), are associated with scrublands. Indeed, dormouse nest-box occupancy in the year of study was positively associated with high percentage of scrublands: mean (±SE) percentage of scrubland: non-occupied boxes = 7.82 % (± 11.04) versus occupied boxes = 12.20 % (± 14.46); ANOVA: F = 5.40, df = 1, 441, P = 0.02. Hence, given that chicks cannot escape from their nest at this developmental stage, high corticosterone levels should be due to parental stress (e.g. Sheriff and Love Reference Sheriff and Love2013). Alternatively, scrublands could be unsuitable for Rollers due to their preference to perch and hunt in open areas (Avilés and Costillo Reference Avilés and Costillo1998, Rodríguez et al. Reference Rodríguez, Avilés and Parejo2011). However, this seems unlikely because feeding rates in Rollers did not differ in relation to density of scrublands. Finally, it could be argued that only good quality parent Rollers avoided areas with scrubland, although this seems improbable because we found no relationships between Rollers’ laying date (i.e. a correlate of quality; Avilés et al. Reference Avilés, Sánchez, Sánchez and Parejo1999) and habitat features in our sample (see above, Table 2).
All the other relationships between nestling physiology and habitat features are concerned with the first PC, which place first the farming activities and human disturbance due to infrastructures. On the one hand, nestling rollers showed high levels of fCORT but were heavier at fledging when being raised in areas with high levels of farming activities. Two explanations to this pattern are possible. Cultivated areas may provide parents with good feeding opportunities (as shown by the higher weight of nestlings) despite the high disturbance produced by farming activities (as shown by the high fCORT levels). Hence, the results may be due to a high abundance of insects or better detectability of prey that farming practices could promote. Accordingly, we found that orthopteran availability during the nestling stage was higher in areas with high farming activity and further from human infrastructures (Appendix S1).
Alternatively, the pattern found may arise if Roller nestlings raised in intensively farmed areas were highly stressed and increased begging (e.g. Kitaysky et al. Reference Kitaysky, Wingfield and Piatt2001, Reference Kitaysky, Kitaiskaia, Piatt and Wingfield2003), which would trigger a higher provisioning effort by adults. This alternative, however, could be discarded because we did not find an increased parental provisioning effort in areas with high density of scrublands where nestling Rollers seemed to be also more stressed.
The two proposed explanation rely on an increase of parental feeding rates in areas with high levels of farming activity as shown in this study. Previous studies have shown that parents of different bird species (Zanette et al. Reference Zanette, White, Allen and Clinchy2011), including the Roller (Expósito-Granados et al. Reference Expósito-Granados, Parejo and Avilés2016), may increase their feeding rates when stressed by natural threats and human activities (e.g. Kross et al. Reference Kross, Tylianakis and Nelson2012, Blas et al. Reference Blas, Abaurrea, D’Amico, Barcellona, Revilla, Román and Carrete2016, but see Fernández and Azkona Reference Fernández and Azkona1993).
On the other hand, for Scops Owls, we found that nestlings raised in highly human-altered areas by infrastructures showed high levels of stress-induced CORT. Roads represent a threat to wildlife (Reijnen and Foppen Reference Reijnen and Foppen1994, Crino et al. Reference Crino, Van Oorschot, Johnson, Malisch and Breuner2011), directly affecting adults and nestlings through traffic noise, light, presence of cars and pollution (Forman et al. Reference Forman, Reineking and Hersperger2002, Dooling and Poppen Reference Dooling and Poppen2007, reviewed in Fahrig and Rytwinski Reference Fahrig and Rytwinski2009). Also, roads could have indirect effects on nestlings by modifying parental behaviour. The highway crossing the study area is one of the most important communication routes in the south of Spain, being highly used both during day and night. Its effects on nestlings could be direct or perhaps induce high levels of stress to parents that are transmitted to their nestlings (Sheriff and Love Reference Sheriff and Love2013, Kidawa et al. Reference Kidawa, Barcikowski and Palme2017). Nevertheless, this parental stress seems not to be translated into changes in body weight of nestlings or in parental behaviour of adults, maybe because the harmful effects of the highway are more due to noise or light than to an effect on prey availability.
Correlative studies like this do not allow causal-effect relationships to be established and cannot exclude the possibility that uncontrolled environmental factors related to habitat may have influenced the physiological traits of nestlings. Experimental studies may help to disentangle how animals cope with human alterations, although they might be questionable when dealing with threatened species.
In conclusion, this study provides evidence of physiological effects of human activities on two birds inhabiting farming areas of the south of Spain. Susceptibility to human disturbance, however, is species-specific (Gomes et al. Reference Gomes, Oostra, Nijman, Cleef and Kappelle2008, Casas et al. Reference Casas, Mougeot, Viñuela and Bretagnolle2009, Samia et al. Reference Samia, Nakagawa, Nomura, Rangel and Blumstein2015, Mgelwa et al. Reference Mgelwa, Abrha, Kabalika, Tamungang and Nigusse2018), and may reflect the role of activity rhythms on susceptibility to daily variation in disturbance due to human activities. Indeed, farming activities are intense during daylight but stop at dusk, while, roads, although busier during the day, are also intensely frequented at nights. Hence, our results would support the idea that Rollers would be more stressed by daily farming activities whereas Scops Owls would be more affected by human disturbance due to infrastructure. Future studies on human alterations and birds should consider the possibility that human disturbance may vary during the day and the fact that activity rhythm of species may buffer or exacerbate its effects.
Conservation recommendations
Most management efforts for conservation of farmland birds are focused on reversing detrimental effects of agricultural intensification (reviewed in Bota et al. Reference Bota, Morales, Mañosa and Camprodon2005). Accordingly, our results show that even when cultivated areas may trigger good apparent performance in some species, it is crucial to get measures of different fitness components (as behavioural and physiological components) to correctly assess the global impact of agriculture on wildlife. Indeed, in our case, despite the fact that cultivated areas seem to be good feeding patches for Rollers, they seem to be also highly stressful for nestlings probably due to the intense human activity. On the other hand, we show that human factors may impact differently on species varying in ecological requirements. This leads us to suggest that management actions, such as nest-box installation plans focused on bird communities, have to take into account all these nuances to favour similarly all targeted species, avoiding the creation of ecological traps for some of them (Rodríguez et al. Reference Rodríguez, Avilés and Parejo2011).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270919000388
Acknowledgements
We thank Olga Corona for helping in NAbs assays at the Estación Experimental de Zonas Áridas (EEZA-CSIC). We thank Charline Parenteau and Colette Trouvé, at the Centre d’Études Biologiques de Chizé (CEBC-CNRS), for corticosterone assays. We thank Juan Rodríguez-Ruiz for his help in fieldwork and environmental data management. This work was supported by the Spanish Ministry of Education and Science/FEDER (Projects CGL2011-27561/BOS and CGL2014-56769-P to D.P. and J.M.A.). D.P. was supported by the Government of Extremadura while writing (contract number TA13002). M.E.G. was supported by the Spanish Ministry of Economy and Competitiveness (grant number BES-2012-051898 and EEBB-I-14-08409).