Introduction
The Scaly-sided Merganser is among the most threatened species of Mergini and has been classified as ‘Endangered’ on the IUCN Red List of Threatened Species since 2002 (BirdLife International 2017). It was listed as a national first-class animal under protection in China, a protected species in the Democratic People’s Republic of Korea (DPRK) and the Republic of Korea (RK) and listed as threatened in Russia (BirdLife International 2001). The small population less than 2000 pairs is likely to be stable after rapid declines in the 1940s–1980s (Solovyeva et al. Reference Solovyeva, Liu, Antonov, Averin, Pronkevich, Shokhrin and Cranswick2014, BirdLife International 2017).
As one of the two specialist mergansers of riverine landscapes, its occurrence may be affected by adiabatic effects and geomorphological complexity as found in other river birds (Buckton and Ormerod Reference Buckton and Ormerod2002). The species prefers to breed on undisturbed rivers in mountains and foothills, with clean rapid waters, gravel or rocky substrates, shingle islands, deep stretches, and shoals (Zhao and Piao Reference Zhao and Piao1998, BirdLife International 2001). The females often nest in holes of old-growth trees among riverine primary forests that provide potential nest cavities in abundance (BirdLife International 2001, 2017). The breeding population is confined to the temperate conifer-broadleaf forest zone in south-east Russia, north-east China, and North Korea (Solovyeva et al. Reference Solovyeva, Liu, Antonov, Averin, Pronkevich, Shokhrin and Cranswick2014). However, it is absent from many of its previously known habitats, such as those of the Wusili River basin and the Great Khingan Ridge, both in China (Solovyeva et al. Reference Solovyeva, Liu, Antonov, Averin, Pronkevich, Shokhrin and Cranswick2014).
Due to the economic value of timber and farmland reclamation, many riverine primary forests in the breeding habitats of Russia, China and North Korea have been greatly altered during recent decades (Liu et al. Reference Liu, Li, Song, Wang, Song, Liu and Piao2010, Wu Reference Wu2007, BirdLife International 2017). Currently, large-scale deforestation in river valleys is forbidden in Russia and China (BirdLife International 2001). The Forest Law has been strengthened since 1999 to improve forest conservation in DPRK (Liu et al. Reference Liu, Li, Song, Wang, Song, Liu and Piao2010). However, some other threats in breeding and wintering grounds, such as hydroelectric dam construction (leading to significant changes to river flow and form and thus affecting the suitability of rivers as feeding areas), dredging (causing a change in river morphology, an increase in turbidity downstream of the activity, and disturbance), pollution (affecting prey availability both directly and indirectly as well as having a direct impact on breeding success), and human disturbance are suspected to continue to contribute to the ‘Endangered’ status of the Scaly-sided Merganser (Shao et al. Reference Shao, Zheng, Tim, Chen, You, Wang and Dai2012, BirdLife International 2017, Solovyeva et al. Reference Solovyeva, Hughes and Cranswick2017, Zeng et al. Reference Zeng, Lu, Li, Guo, Wen and Lei2018.
The Scaly-sided Merganser’s ‘Endangered’ status, and the potentially increased extinction risk associated with human developments has been demonstrated for other threatened taxa (An et al. Reference An, Zhang, Cao, Jia and Wang2018, Li et al. Reference Li, Yang, Zha, Zhang and de Boer2019, Khaliq et al. Reference Khaliq, Aashard, Gill, Chaudhry, Maan, Iqbal, Akbar and Bowler2019). This reflects the necessity for a conservation management programme for this species which to date has not been comprehensive or spatially detailed. Effective and efficient conservation of the breeding Scaly-sided Merganser requires the ability to make decisions over a large range. Though range maps have been delineated for many species, including the Scaly-sided Merganser (Solovyeva et al. Reference Solovyeva, Liu, Antonov, Averin, Pronkevich, Shokhrin and Cranswick2014), the gaps in survey effort have imposed limits on conservation and spatial planning (Merow et al. Reference Merow, Wilson and Jetz2016).
Combining environmental and/or geographic variables with observations of species occurrence, species distribution modelling (SDM) can predict spatial distributions across landscapes over large spatial extents, including inaccessible areas in remote or transboundary zones, and provide strong ecological insights (Phillips et al. Reference Phillips, Anderson and Schapire2006, Elith and Leathwick Reference Elith and Leathwick2009. To zoom in on the elements that need further protection, GAP analysis can be used to determine the extent to which biodiversity elements are represented in existing protected areas (PAs; Pressey et al. Reference Pressey, Humphries, Margules, Vane-Wright and Williams1993). Here, to our knowledge, we created the first SDM for the breeding Scaly-sided Merganser worldwide and carried out a GAP analysis by comparing the results with the pattern of the existing PA network to highlight unprotected or insufficiently protected suitable habitats and put forward suggestions for priority setting in international conservation planning.
Methods
Data preparation
We compiled 112 Scaly-sided Merganser point of occurrence records from our extensive surveys (combining boat and foot surveys to identify the breeders) which were carried out in south-east Russia and north-east China between 2000 and 2020 (Solovyeva et al. Reference Solovyeva, Liu, Antonov, Averin, Pronkevich, Shokhrin and Cranswick2014, Gong et al. unpubl. data), and from a literature review, other publicly accessible databases (including websites of local government and news), and personal communication with experts and birdwatchers (Figure 1; Table S1 in the online supplementary material). To minimize spatial autocorrelation, duplicate presence locations falling within the same cell of a 1-km resolution raster were removed before the SDM analysis. A subset of original presence data was arranged in an appropriate manner for inputting into the SDM.
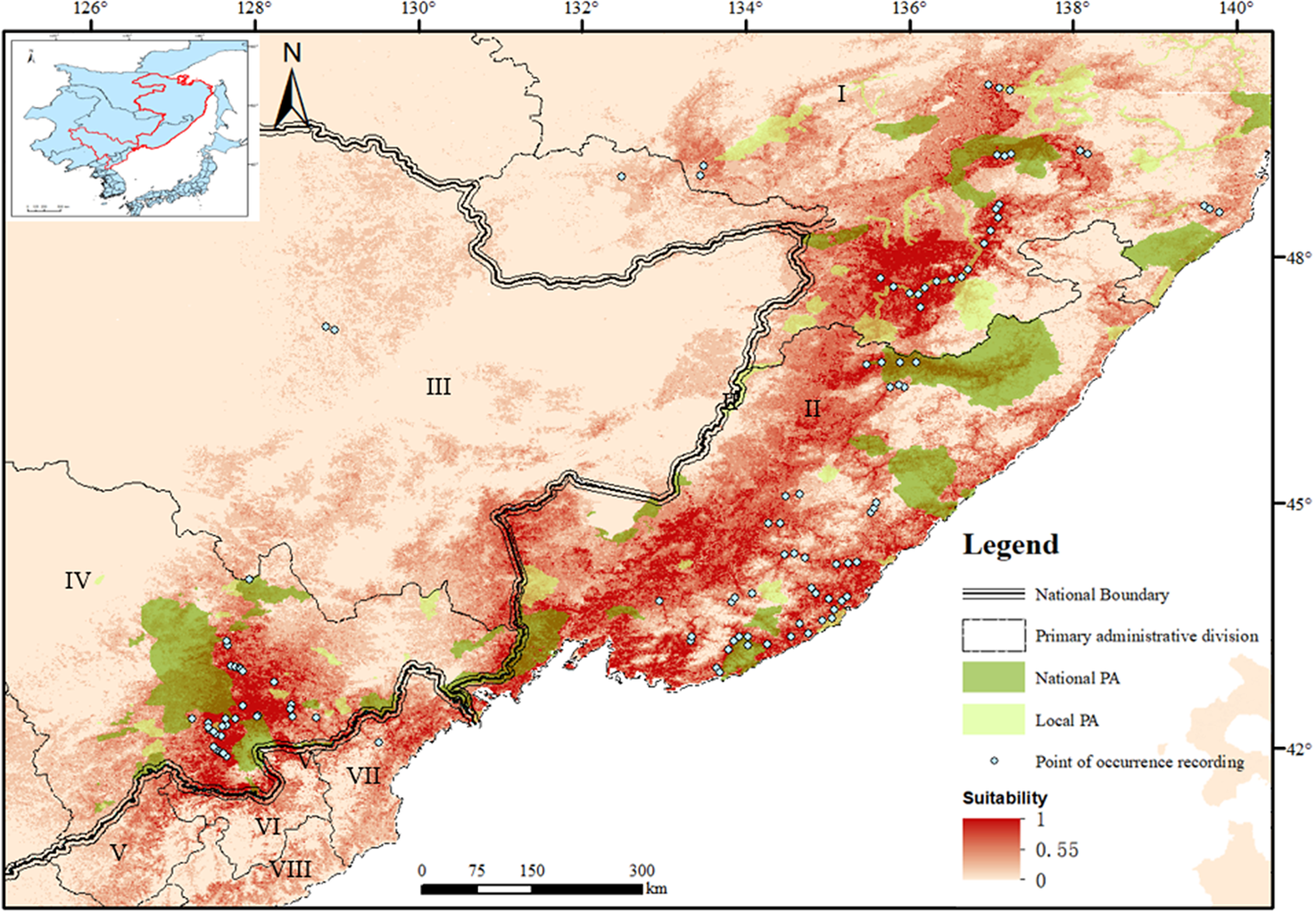
Figure 1. Potential distribution areas and conservation gaps for the breeding Scaly-sided merganser in Northeast Asia. The provincial regions were marked by Roman numbers (I = Khabarovsky Krai; II = Primorsky krai; III = Heilongjiang Province; IV = Jilin Province; V = North Pyongan; VI = Ryanggang; VII = North Hamgyeong; VIII = South Hamgyong).
Environmental variables
Variables used in SDMs should be ecologically relevant to ensure models can offer ecological insight (Elith et al. Reference Elith, Phillips, Hastie, Dudik, Chee and Yates2011). In consideration of the ecological requirements of Scaly-sided Merganser, we collected 26 biotic and abiotic variables, including 19 climatic variables (annual and seasonal temperature/precipitation, etc. from http://www.world clim.org/; Table S2), land cover (including 20 land types, such as broadleaf/coniferous/mixed forest, etc.; http://www.geodata.cn), human influence indicator (as proxies of human settlement, land transformation, accessibility and electrical power infrastructure with values ranging from 0 to 64 which corresponds to no, or maximum, human influence; http://sedac.ciesin.columbia.edu/data/collection/wildareas-v2/sets/browse), three terrain factors (elevation, aspect and slope; DEM, http://www.gscloud.cn), canopy height (generated from the satellite images of the whole globe from MODIS sensor of Terra; https://globalmaps.github.io/) and river density (http://hydrosheds.cr.usgs.gov). The reason why these variables were chosen, and the original resolution, are shown in Table 1. Although the important spatial scale(s) of the habitat selection remain unexplored, habitat characteristics within a 1 km-section were found to affect the occurrence of Scaly-sided Merganser (Zeng et al. Reference Zeng, Zhang, Sun, Duo, Wen and Lei2015a, Reference Zeng, Shi, When, Chen, Duo and Lei2015b) and of closely related species (Gregory et al. Reference Gregory, Carter and Baillie2010, Kajtoch et al. Reference Kajtoch, Zmihorski and Piestrzynska-Kajtoch2014). Therefore, the scale of 1 km was applied to match the pixel size of environmental variables with the spatial resolution of the species records in this study (Feng et al. Reference Feng, Park, Walker, Peterson, Merow and Papeş2019).
Table 1. Biotic and abiotic predictors in the SDM.

To avoid biasing results, we screened variables for collinearity using the Variance Inflation Factor (VIF) in R with package “raster” and “usdm” (Naimi et al. Reference Naimi, Hamm, Groen, Skidmore and Toxopeus2014). The remaining variables (13 predictors with vif < 10; Elith et al. Reference Elith, Burgman and Regan2002, Worthington et al. Reference Worthington, Zhang, Logue, Mittelstet and Brewer2016; Table 1) were converted to 1-km resolution layers for further analysis. The spatial processes were performed using Spatial Analyst tools and Raster Processing in ArcGIS10.2.
Species distribution model
The distribution of breeding Scaly-sided Merganser was predicted using MaxEnt version 3.3.3k (Philips et al. Reference Phillips, Anderson and Schapire2006). The base models were run using the default settings within MaxEnt and model fit was assessed based on the Area Under the Curve (AUC) statistic after 10-fold cross validation (Worthington et al. Reference Worthington, Zhang, Logue, Mittelstet and Brewer2016). A value of AUC close to one indicated good model fit (Elith et al. Reference Elith, Phillips, Hastie, Dudik, Chee and Yates2011). A jackknife analysis was applied to estimate consistency in variable importance for model building. During this process, a series of models were generated to screen the most informative model by in turn excluding each variable and modelling with the remaining variables. Then, a model was also created for each variable to assess which one had the most information that was not present in the others, i.e. the most uncorrelated variable. The relative importance of each environmental variable to the SDM was estimated using the percentage contribution and permutation importance averaged over 10 replicates. Response curves were used to investigate how the predictors effected the SDM prediction.
Gap analysis
To examine the extent of the currently protected breeding habitat and to show gaps in the existing network, we produced a GAP analysis of the suitable habitat contained within each PA and the total percentage of the species’ habitat in all PAs by overlaying the international PA-network dataset on top of the SDM in ArcGIS 10.2. To further understand the extent to which the habitats were protected by the existing network, the classification (local vs. national PA; https://panda.maps.arcgis.com) and how much suitable habitat contained by each PA within the target area was determined worldwide and in provincial administrative regions across countries. To calculate the size of highly suitable habitats, the areas of each grid (1 km2) representing the most suitable habitats with occurrence probability 0.55 (Zeng et al. Reference Zeng, Zhang, Sun, Duo, Wen and Lei2015a) in the SDM analysis were summed in ArcGIS 10.2 with Spatial Analyst tools.
Results
The species distribution model
The total area of highly suitable habitat estimated by the model for the Scaly-sided Merganser was 43,125 km2, located in south-east Russia (31,801 km2, 74% of the global total, mainly in the Sikhote-Alin Range), north-east China (9,772 km2, 23%, concentrated in the Changbai Range) and north-east DPR Korea (1,552 km2, 3%, in the Paektu Massif; Figure 1).
The SDM predicted potential suitable habitat for the Scaly-sided Merganser with high success rates (AUC training = 0.988, AUC test = 0.983; Figure S1). The contribution of environmental predictors was variable, ranging from 0.2 to 32.3%. The percentage contribution of each predictor is shown in Table 2, and the results of the jackknife test of variable importance are shown in Figure S2. Land cover was the most important variable in the occurrence of breeding Scaly-sided Merganser, with a model contribution of 32.3%. Temperature seasonality, precipitation of wettest month and coldest quarter, and human influence were of lesser importance. Terrain factors (aspect, slope, and elevation), canopy height, and isothermality contributed the least to the model. The response curve of the highest influential variable, land cover, is presented in Figure 2. The four variables with lesser importance are shown in Figure S3. It was evident that the non-linear relationships between the occurrence probability of the breeding Scaly-sided Merganser and the environmental variables were strong. The most suitable breeding habitat was broad-leaved deciduous forest, distributed in six provincial areas (Primorsky Krai, Khabarovsky Krai, Jilin province, North Hamgyong, Ryanggang and South Ryanggang).
Table 2. Percentage contribution of each predictor in the SDM of the breeding Scaly-sided Merganser


Figure 2. Response of Scaly-sided Merganser to land use type (demonstrating the mean response of 10 replicate MaxEnt runs and +/- one standard deviation). 1 = Broadleaf Evergreen Forest; 2 = Broadleaf Deciduous Forest; 3 = Needleleaf Evergreen Forest; 4 = Needleleaf Deciduous Forest; 5 = Mixed Forest; 6 = Tree Open; 7 = Shrub; 8 = Herbaceous; 9 = Herbaceous with Sparse Tree/Shrub; 10 = Sparse vegetation; 11 = Cropland; 12 = Paddyfield; 13 = Cropland/Other Vegetation Mosaic; 14 = Mangrove; 15 = Wetland; 16 = Bare area, consolidated (gravel, rock); 17 = Bare area, unconsolidated (sand); 18 = Urban; 19 = Snow/Ice; 20 = Water bodies.
Gap analysis
There are 28 protected areas containing highly suitable habitats (Table S3), 19 in Russia (nine local and 10 national PAs) and nine in China (five local and four national). No PA was found in the DPRK. The gap analysis revealed that 18% (7,755 km2) of the global highly suitable breeding habitats lay within the existing PAs, leaving 82% (34,569 km2) unprotected. The gaps were distributed among six provincial administrative regions in East Asia, the largest in Russia (17,908 km2 in Primorsky Krai; 8,116 km2 in Khabarovsky Krai), less in China (7,794 km2 in Jilin province), and smallest in DPR Korea (750 km2 in North Hamgyong; 684 km2 in Ryanggang; 80 km2 in North Pyongan; 38 km2 in South Ryanggang; Figure 1). The last reflected the small area of highly suitable habitats in this range state.
Discussion
Potential highly suitable habitat
Our dataset, based on 112 occurrence records for native populations, updates the distribution maps of breeding Scaly-sided Merganser and improves the IUCN assessment (Figure S4). The predictive map shared a 56.38% overlap with the range delineated from surveys and added some potential new distribution areas in part of Jilin province (some areas around Dunhua-city and Baishan-city) which were confirmed with evidence of occurrence of the Scaly-sided Merganser in the field survey in 2018–2020; Gong et al. unpubl. data). However, the suitability was intermediate in the northern part of the eastern slope of Sikhote-Alin Range (predicted by experts as the area most densely populated by the Scaly-sided Merganser; Solovyeva et al. Reference Solovyeva, Liu, Antonov, Averin, Pronkevich, Shokhrin and Cranswick2014). As this site is highly localized, it only results in few occurrence points, which may lead to underestimation of highly suitable areas (Kramer-Schadt et al. Reference Kramer-Schadt, Niedballa, Pilgrim, Schröder, Lindenborn, Reinfelder, Stillfried, Heckmann, Scharf and Augeri2013). Despite this limitation, the SDM could be used as a reference point for conservation planning.
Environmental predictors
Response curves represent the quantitative relationship between environmental predictors and the logistic probability of occurrence, and they help to understand the ecological niche of the species (Yi et al. Reference Yi, Cheng, Yang and Zhang2016). Forest-cover (broad-leaved deciduous forest in particular) exerted the strongest influence on the probability of presence; the variable was different from the SDM predicting potential habitat in winter, mainly influenced by the minimum temperature of the coldest month and the mean temperature of the coldest quarter (Zeng et al. Reference Zeng, Zhang, Sun, Duo, Wen and Lei2015a). The Scaly-sided Merganser is not capable of excavating its own nesting cavities and relies on those already created in trees (Sánchez et al. Reference Sánchez, Cuervo and Moreno2007). This explains the preference for broad-leaved deciduous forest, due to the higher of cavities than in coniferous forest (Newton Reference Newton1998, van Balen et al. Reference van Balen, Booy, Franeker and Osieck1982). Canopy height contributed little to the landscape-scale model, though the suitable canopy height (15–20m) is similar to that recorded in field investigations (Yi et al. Reference Yi, Yang, Chen, Li, Hao and Zhao2008). As old-growth broad-leaved trees, especially those preferred by the Scaly-sided Merganser, often had a broken top, canopy height may not always be a reliable indicator of potential nest site. Alternatively, canopy height may be a preferred element at a smaller scale, which could potentially be affected by a trade-off between concealment and view of the surroundings (Gotmark et al. Reference Gotmark, Blomqvist, Johansson and Bergkvist1995).
Temperature seasonality, precipitation of wettest month and coldest quarter, and human disturbance made a lesser contribution. Temperature seasonality is a measure of temperature change over the course of the year (Ma and Sun Reference Ma and Sun2018), reflecting the difference between maximum and minimum temperatures. Temperatures well below freezing were observed during the early breeding season (late March to early April) but were unlikely to affect waterfowl or their eggs, as egg-laying begins in the second half of April and most clutches are laid by early May (BirdLife International 2001). Summer heat can be compensated for by using the cold water of fast-flowing rivers and by resting in the shade of trees. Breeding range of the Scaly-sided Merganser significantly overlaps the northern range limits of Korean pine Pinus koraiensis, Amur linden Tilia amurensis, Mongolian oak Quercus mongolica, Manchurian fir Abies holophylla, Manchurian ash Fraxinus mandshurica and other representatives of Manchurian fauna such as Amur tiger Panthera tigris altaica and yellow-throated marten Martes flavigula, indicating the overall influence of climate (Krestov Reference Krestov, Kolbek and Srutek2003, Miquelle and Pikunov Reference Miquelle, Pikunov and Newwell2003, Chutipong et al. Reference Chutipong, Duckworth, Timmins, Choudhury, Abramov, Roberton, Long, Rahman, Hearn, Dinets and Willcox2016).
Rainfall is the predominant and ultimate source of the land surface water budget (Fekete et al. Reference Fekete, Vorosmarty, Roads and Willmott2004). The high-altitude mountain rivers are mainly derived from atmospheric water replenishment and mountain snowmelt (Woo et al. Reference Woo, Thorne and Brown2019). Precipitation of the wettest month and coldest quarter may be among the most important climate variables in maintaining water levels, which could be essential for effective foraging of fish-hunting duck and providing refuge against predators (Cerón and Ferreiro Reference Cerón and Ferreiro2017) and could additionally support growth of the forest with potential nest sites (Lu et al. Reference Lu, Zhang, Fang and Zheng2016). However, the nesting and incubation success of the Scaly-sided Merganser may be less influenced by the variation in water level than other ground-nesting waterbirds (e.g. Hake et al. Reference Hake, Dahlgren, Ahlund, Lindberg and Eriksson2005, Carneiro et al. Reference Carneiro, Correia, Goncalves, Brito, Luis and Alves2016). Human disturbance is often negatively related to species occurrence (Zhang et al. Reference Zhang, Fox, Cao, Jia, Lu, Prins and de Boer2019), and the Scaly-sided Merganser is not an exception. In proximity to human developments, it could be at risk of threats such as deforestation, reclamation, and hydro-electric dam construction (Li et al. Reference Li, Yang, Zha, Zhang and de Boer2019, Khaliq et al. Reference Khaliq, Aashard, Gill, Chaudhry, Maan, Iqbal, Akbar and Bowler2019), which could result in changing more habitats from suitable to unsuitable.
Highly suitable habitats, proposed by the SDM for south-west Primorsky Krai (such as Anuchinsky, Yakovlevskiy and Shkotovsky Districts), are actually areas destroyed by intensive agriculture and industry, which lack old-grown trees and fish resources in the rivers, supporting a single pair of the Scaly-sided Merganser according to our field surveys (Solovyeva et al. unpubl. data), though these districts support human densities of only 100–500 people/km2. Similar to the ecological parameters, human populations often differ among geographic scales and populated areas in Russia are not comparable to those in China (Johnson Reference Johnson1979). However, many Scaly-sided Mergansers live close to human developments (roads and/or residential houses) in the Changbai Mountains of Jilin province, China. Those birds may colonize suboptimal habitats because of low availability of highly suitable habitats or be lured to less suitable patches as cues used by individuals to assess site quality not affected by human influence (Doligez and Boulinier Reference Doligez, Boulinier, Jorgensen and Fath2009). Therefore, potentially suitable habitats close to or overlapping with human influence (Figure S5), should be given a priority in protection and restoration planning.
Conservation GAP
As a cornerstone of conservation, designation of PAs plays an important role in safeguarding species and ecosystems globally (Watson et al. Reference Watson, Dudley, Segan and Hockings2014). The GAP analysis showed that about 1/6 of the highly suitable breeding habitats were covered by PAs. However, it is also important to prevent downgrading, downsizing, and degazettement of protected areas (PADDD; Mascia and Pailler Reference Mascia and Pailler2011) events in national PAs and strengthen management in local PAs which may be less effective than national PAs, through the absence of active management of human activities and the demand for local resources. For example, local PAs in Russia are usually designated only “on paper” which means they do not have any equipment and most of the highly suitable habitat is unprotected by any PA. To further protect this species,the PA-network coverage needs to be increased, but local reserves should also be effectively evaluated and managed (Kleijn et al. Reference Kleijn, Cherkaoui, Goedhart, Jasper, Lammertsma and Fuller2014, Amano et al. Reference Amano, Szekely, Sandel, Nagy, Mundkur, Langendoen, Blanco, Soykan and Sutherland2018).
Decisions should be made on which elements, such as the conservation GAP area, need attention first, because resources available for conservation efforts are always scarce and need to be invested in strategic ways to ensure that conservation efforts make the greatest contribution to preserving endangered species (Pressey et al. Reference Pressey, Humphries, Margules, Vane-Wright and Williams1993). The conservation GAP for on-ground implementation within the Sikhote-Alin mountain range (in Primorsky Krai and Khabarovsky Krai) should be prioritized, as this unique mountain area contains most of the highly suitable breeding habitat supporting 85% of the global breeding population (Solovyeva et al. Reference Solovyeva, Liu, Antonov, Averin, Pronkevich, Shokhrin and Cranswick2014). Most of the remaining highly suitable breeding habitat and breeding population (14%) is found in the Changbai Mountains, straddling China (Jilin province) and the DPR Korea (Ryanggang). Philopatry and site fidelity in waterfowl are often female-biased (Rohwer and Anderson Reference Rohwer, Anderson and Johnston1988). Smaller breeding enclaves such as Changbai could more likely be threatened and therefore need attention, because when these populations decline, only males— due to strong philopatry of females—can be efficiently supplemented by immigration from larger populations (Hefti-Gautschi et al. Reference Hefti-Gautschi, Pfunder, Jenni, Keller and Ellegren2009). Under the rapid pace of development in China (e.g. Songjianghe, a town within the Changbai Mountain area, is projected to increase from 10-20,000 to 100,000 people with plans for a highway and rail link), the risk of dam construction, dredging and disturbance are likely to increase. The vulnerable status of irreplaceable breeding habitat in the Changbai Mountains should be considered another conservation priority. As small and subdivided populations have often greatly decreased in size in the recent past, some potentially suitable patches, such as those in Kur-Urmi rivers in Khabarovsky Krai, Hamgyong and Ryanggang in the DPRK, may be scarcely occupied, but nevertheless need to be preserved (e.g. by managing habitat in terms of meta-reserves) to allow individuals to move in the future (Doligez and Boulinier Reference Doligez, Boulinier, Jorgensen and Fath2009). Setting a goal of 40% of highly suitable habitats to be protected, we suggest an additional 10,414 km2 of PA in Russia and 6,836 km2 in China. Among the priority areas to be protected in Russia we suggest Pavlovka and Zhuravlevka river catchments in Chuguevsky District of Primorsky Krai (c.3,600 km2) and enlarging the existing Udygeiskaya Legenda NP by adding the upper reaches of the Iman River. In China, to minimize the impacts of human activities on the Scaly-sided Merganser, prioritizing conservation action and land management or small PA establishment along the Fuer, Songjianghe, and Manjiang rivers (i.e. key habitats overlaid with human developments) may be essential. The presence of breeding Scaly-sided Merganser along the modelled highly suitable habitats in DPRK was not confirmed; we suggest that surveys for the Scaly-sided Merganser along the rivers Tumen, Yalu, and Puktae Chion should be the first step in this country.
This study has attempted to model habitat suitability and identify the conservation GAP area, providing insights on conservation priority-setting for the Scaly-sided Merganser. The distribution of highly suitable habitats and the extent to which they are protected should be viewed as a reference point for establishment of conservation areas or implementing conservation actions for the breeding Scaly-sided Merganser in north-east Asia. The map of habitat suitability and the conservation GAP should be subject to continual evaluation and implementation under the International Single Species Action Plan (Solovyeva et al. Reference Solovyeva, Hughes and Cranswick2017). The map should be refined when up-to-date data become available. In addition, it is remarkable that the current network of protected areas may become unsuitable in future as a result of poleward shifts in temperature (Leroy et al. Reference Leroy, Ballard, Dubos, Colliot, Vasseur, Courtial, Bakkenes, Canard and Ysnel2014). Future studies should account for ICCP climatic scenarios to assess ongoing gaps between current and future protected areas.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0959270921000137.
Acknowledgements
We thank the reviewers for suggestions that greatly improved this manuscript. Sincere thanks for great support by the Wildlife Conservation Society of Jilin province, World Wide Fund for Nature (Northeast Region of China), Fundamental Research Funds for the Central Universities, China (2412018QD015), the "13th Five-Year" Science and Technology Research and Planning Project of Jilin Provincial Department of Education (JJKH20201176KJ), and the Wildfowl and Wetlands Trust, UK as well as data support from National Earth System Science Data Center and National Science and Technology Infrastructure of China (http://www.geodata.cn). We thank Peiqi Liu, Longguo Piao, Qing Zeng for support in data collection.






