Introduction
Wildlife tourism can have mixed effects on nature conservation. When properly conducted, it can generate resources to fund both conservation and research, engage local communities through economic incentives, and encourage governments to manage nature better (Buckley Reference Buckley2010, Ribeiro et al. Reference Ribeiro, Soares Filho, Costa, Bachi, de Oliveira, Bilotta and Queiroz2018). Conversely, the literature is full of examples where tourism practices have negative outcomes for wildlife, including through baiting and capture (D’Cruze et al. Reference D’Cruze, Machado, Matthews, Balaskas, Carder, Richardson and Vieto2017). As nature conservation is mostly an unprofitable activity (Strand et al. Reference Strand, Soares-Filho, Costa, Oliveira, Ribeiro, Pires, Oliveira, Rajão, May, Hoff, van der, Siikamäki, Motta and Toman2018), tourism, especially conservation tourism (sensu Buckley Reference Buckley2010), is one of the few profitable activities that can generate funding for conservation (Kirkby et al. Reference Kirkby, Giudice-Granados, Day, Turner, Velarde-Andrade, Dueñas-Dueñas, Lara-Rivas and Yu2010, Reference Kirkby, Giudice, Day, Turner, Soares-Filho, Oliveira-Rodrigues and Yu2011, Vianna et al. Reference Vianna, Meekan, Rogers, Kragt, Alin and Zimmerhackel2018, García-Jiménez et al. Reference García-Jiménez, Morales-Reyes, Pérez-García and Margalida2021). Therefore, even if initially imperfect, tourism for conservation should be improved and refined rather than prohibited (SEMA 2018, Muntifering et al. Reference Muntifering, Linklater, Naidoo, !Uri‐≠Khob, Preez, Beytell, Jacobs and Knight2019).
Wildlife tourism is an industry that creates a million trips worldwide per year (UNWTO 2015). In the Amazon Forest, South America, however, it is still restricted to relatively few locations. This 6,300,000 km region (Goulding et al. Reference Goulding, Barthem and Ferreira2003) has some world-class wildlife attractions (Burger and Gochfeld Reference Burger and Gochfeld2003, Lees et al. Reference Lees, Zimmer, Marantz, Whittaker, Davis and Whitney2013, Vidal Reference Vidal2018). Collectively, locations connected with global tourism markets represent less than 0.01% of the Amazon region, which points to a clear need for expansion. The Amazon Forest is being incinerated at its southern and eastern margins to provide land for meat and grain production. This region is called the Arc of Deforestation (Fearnside and Figueiredo Reference Fearnside and Figueiredo2015), and is virtually terra incognita regarding biodiversity, with new vertebrate species, including even new primates, being described every year (Boubli et al. Reference Boubli, Byrne, da Silva, Silva-Júnior, Araújo, Bertuol, Silva, Gonçalves, Melo, Farias, Schneider, Hrbek, Rylands, A.Mittermeier, E. Silva, Rossik, Carneiro, Sampaio, Farias, M. Alencar, D.Nash, Canale and Hrbek2019, Costa-Araújo et al. Reference Costa-Araújo, Melo, Canale, Hernández-Rangel, Messias, Rossi, Silva, Silva, Nash, Boubli, Farias and Hrbek2019). In Brazil, substantial reductions in conservation funding (Magnusson et al. Reference Magnusson, Grelle, Marques, Rocha, Dias, Vale, Tomas, Cerqueira, Collevatti, Pillar, Malabarba, Lins-e-Silva, Neckel-Oliveira, Martinelli, Akama, Rodrigues, Silveira, Scariot and Pillar2018) increase the need for the private sector to take a larger role in conservation funding in the Amazon Forest. The region harbours several charismatic species that have potential to become important ecotourist attractions, one important species being the Harpy Eagle Harpia harpyja (Figure 1).

Figure 1. A Harpy Eagle female arriving at nest with a woolly monkey Lagothrix cana as prey for her fledgling.
The Harpy Eagle is the world’s largest eagle. They form long-term pair bonds, nesting in the same tree for decades and producing clutches that take 53 days to hatch (Watson et al. Reference Watson, McClure, Vargas and Jenny2016). Harpy Eagles produce one dispersing juvenile every 30–36 months. The eaglets fledge at 5–7 months, but parent Harpy Eagles continue to bring food to these dependent juveniles until they reach 30–36 months of age, at which point the offspring disperse (Muñiz-López et al. Reference Muñiz-López, Limiñana, Cortés and Urios2016, Urios et al. Reference Urios, Muñiz-López and Vidal-Mateo2017). Birders describe Harpy Eagles as the most prized species to spot (Pivatto et al. Reference Pivatto, Sabino, Favero and Michels2007).
During the 19th century, Harpy Eagle distribution extended south to northern Argentina and north to southern Mexico. The historical distribution has suffered a 40% reduction, and today their core habitat and last stronghold is the Amazon Forest (Miranda et al. Reference Miranda, Menezes, Farias, Munn and Peres2019). In the Atlantic Forest, their distribution is restricted to two populations—one in northern Argentina and another in north-eastern Brazil—with fewer than 10 known nests in each (Srbek-Araujo and Chiarello Reference Srbek-Araujo and Chiarello2006, Anfuso et al. Reference Anfuso, Suarez and Chebez2008, Sánchez-Lalinde et al. Reference Sánchez-Lalinde, Silveira, Vélez-García, Cornélio and Alvarez2011). Central American populations of Harpy Eagles appear to be in somewhat better shape, probably reaching a total of a few hundred nests (Vargas-González and Vargas Reference Vargas-González and Vargas2011, Watson et al. Reference Watson, McClure, Vargas and Jenny2016).
With the expansion of the Arc of Deforestation—and the economy of ranching in that region—new roads and airports have made the Arc accessible from the rest of Brazil (ZSEE 2008, Carrero et al. Reference Carrero, Fearnside, do Valle and de Souza Alves2020). An extensive network of roads for logging and trails used for Brazil nut Bertholletia excelsa extraction provide relatively easy access to a number of Harpy Eagle nests (Cavalcante et al. Reference Cavalcante, Tuyama and Mourthe2019, Miranda et al. Reference Miranda, Menezes, Farias, Munn and Peres2019). These roads and trails make Harpy Eagles relatively visible in a highly accessible landscape. The same state-sponsored migrants who created the Arc of Deforestation (Schneider and Peres Reference Schneider and Peres2015) can provide important assistance in finding and providing access to Harpy Eagle nests. Harpy Eagle tourism can help to generate concrete financial value for habitat conservation, as has happened with other predators (Macdonald et al. Reference Macdonald, Gallagherb, Barnettd, Brunnschweilere, Shiffmanf and Hammerschlaga2017, Tortato et al. Reference Tortato, Izzo, Hoogesteijn and Peres2017). Although practical protocols have been developed for better practices of wildlife tourism (Haskell et al. Reference Haskell, McGowan, Westling, Méndez-Jiménez, Rohner, Collins, Rosero-Caicedo, Salmond, Monadjem, Marshall and Pierce2015), few have addressed Amazonian wildlife. Creating evidence-based visitation schedules and tailoring them to offer higher viewing probabilities can help jumpstart the region’s potential.
Our present study was designed to fine-tune relationships in the conservation-tourism alliance, aiming to provide the best opportunities to view nesting Harpy Eagles in the shortest time, with the Harpy Eagles remaining unharmed by human presence. Consequently, we calculated how many nests are required to guarantee at least one with a nestling, during which adults are most visible. Further, we used camera trap data to describe the circadian activity pattern of Harpy Eagles (adults and eaglets) and to characterise the daily activity patterns. We also calculated the number of days that a tourist needs to wait at a nest in order to spot an adult eagle. Finally, we describe rates of reutilization of nests by eagles exposed to tourism. These factors can improve the outcomes of tourists visiting nests and decrease the time tourists need to spend near Harpy Eagle nests. By offering better-tailored schedules to tourists and using a policy-oriented management strategy, we aim to establish this apex predator as a tool for conservation of the Amazon Forest.
Methods
Study area
The Arc of Deforestation is the region of the Amazon Forest comprising the southern, south-eastern, and eastern regions of the Amazon Basin. We work in the northern part of Mato Grosso State, Brazil (Figure 2). The climate is generally humid and hot, with mean temperatures of 24ºC, 80% humidity (Vourlitis et al. Reference Vourlitis, Filho, Hayashi, Nogueira, Caseiro and Campelo2002), and annual rainfall averaging 2,300 mm (Noronha et al. Reference Noronha, Lima, Velasquez, Almeida, Barros and Rodrigues2015). The region includes primary and secondary, open, ombrophilous Amazon Forest (Siqueira et al. Reference Siqueira, Ricaurte, Borges, Nunes and Wantzen2018, Veloso et al. Reference Veloso, Rangel Filho and Lima1991). The succession of anthropogenic land use in the region starts with selective logging, followed by forest incineration and planting of pasture for cattle (Junior and Lima Reference Junior and Lima2018, Eri et al. Reference Eri, Junior, Lima, Júnior, Oliveira-Júnior, Teodoro, Capristo-Silva, Caione and Peres2020).

Figure 2. Distribution of Harpy Eagle nests monitored in the present study throughout Mato Grosso state, Brazil (insert). Nests sampled using camera traps for this study are represented by light circles.
Nest finding
We offered a reward representing ~US$100 (BRL500), about 50% of the minimum monthly wage in Brazil, for each active Harpy Eagle nest that was communicated to us. This reward was widely publicised in posters and pamphlets that we disseminated in the study area among key groups of rural workers, particularly Brazil nut collectors. Most of the local population, including the nut collectors, are migrants or descendants of migrants from other parts of Brazil (Schneider and Peres Reference Schneider and Peres2015), and none of them hunt canopy vertebrates (Michalski and Peres Reference Michalski and Peres2005, Trinca and Ferrari Reference Trinca and Ferrari2007, Barbosa Reference Barbosa2012). This release from hunting pressure results in abundant, readily easily-seen canopy vertebrates that are attractive for tourism (Oliveira et al. Reference Oliveira, São Bernardo, Melo, Santos-Filho, Peres and Canale2019). The payment of nest rewards allowed us to rapidly discover Harpy Eagle nest locations.
Considering that finding enough active nests is the main challenge to developing viable Harpy Eagle tourism, we calculated how many are necessary to have at least one in nestling phase for most of the time. We made the assumption of aseasonal reproduction based on published observations of egg-laying occurring in 10 months of the year (Watson et al. Reference Watson, McClure, Vargas and Jenny2016). There is, however, limited evidence from captive individuals that Harpy Eagles are seasonal breeders (Blank et al. Reference Blank, Oliveira, Cubas, Morae, Moreira and Pereira2020), but that possible seasonality appears to be very modest, and all the data are from the southern limit of the species’ distribution. For the purposes of the present analysis, we assume that this species is an aseasonal breeder, though we encourage researchers to collect more data to test further this assumption. This will ensure that tourism operators are always able to present a nest with certain Harpy Eagle detection throughout the year. The nestling phase—when adults can be seen all the time—lasts for at least five months, and each successful nesting cycle lasts a minimum of 30 months (Urios et al. Reference Urios, Muñiz-López and Vidal-Mateo2017). The nestling phase thus represents only 16.6% of the nesting cycle. We therefore used the binomial distribution to calculate how many nests are required to be monitored to always have at least one in the nestling phase:
In this case, “p” is the probability of a nest being active (1/6 or 16.6%) and n is the number of nests. We then calculated the probability of having at least one active nest for 90%, 95% and 99% of the time.
Tourism model
Harpy Eagle tourism originated as a cooperative venture between a conservation and research project and a private tour company, SouthWild. The company specialises in wildlife photography ecotourism that supports conservation action. SouthWild team install mobile observation towers, with a maximum capacity of 12 persons, near Harpy Eagle nests. Tower construction at a nest starts 15 or more days post-hatching and lasts 48–72 hours depending on tower size (25–35 m) and model.
Our initiative required that SouthWild pay ~US$20 (BRL100) per tourist per day to the landowner of the forest where the Harpy Eagle nest was located. In exchange, each landowner signed a legal contract stipulating that they 1) would not damage or disturb the nest tree or the surrounding vegetation; 2) would not clear-cut any tracts of forest within a 1-km radius of the nest; c) would not hunt or allow hunting on the property; d) would not enlarge pastures by burning forest; e) would not carry out any legal or illegal logging within a 1-km radius of the nest. After careful confirmation that the parent birds were tolerant of human presence, SouthWild erected the viewing tower at 25–40 m from the nest tree. The vast majority of Harpy Eagle individuals in our study site have been habituated through the innocuous presence of Brazil-nut collectors at each nest tree base. This being stated, we recommend readers to learn about the degree of tolerance at their study site before following any of our recommendations. The tower was always tall enough to permit eye-level viewing of the nest and also ensured a green background to the nest. Local inhabitants earned money from the project by transporting and building the towers, as well as by trail-cleaning, driving, cooking for tourists and staff, and other associated logistical services. In order to qualify for the operation, tourists needed to stay on the tower from sunrise until sunset hours for one, two, or three complete days, which thereby guaranteed viewing of at least one adult bird or fledged eaglet. If any guest had not seen an adult bird after the waiting period, they would receive a 100% refund of all jetport-to-jetport ground services.
Climbing, nest access and camera trapping protocols
To instal the camera traps (several models from Bushnell, Kansas, USA) at nests, we used the best practices of accepted, published, raptor-specific rope climbing protocols (Pagel and Thorstrom Reference Pagel, Thorstrom and Bird2007, Rosenfield et al. Reference Rosenfield, Grier, Fyfe and Bird2007). We used an arborist slingshot to shoot a monofilament line over a branch near the nest, and then used this line to pull up a 4 mm line, which then pulled an 11 mm climbing rope so that an experienced rope climber could reach the vicinity of the nest. We then fastened two or three camera traps on branches at 0.5–2m from each nest, choosing camera angles that facilitated prey identification. We hammered between two and four 15–20-cm-long nails into a chosen branch and used flexible, 1.65-mm diameter malleable wire to attach the camera trap to the nails. We set the camera traps to take one still photograph every 10 min. Some cameras reset configurations, taking photos every few seconds. These data were used to calculate time of adult stay at nests. At nests where we installed more than two camera traps, one was set to video mode.
Nest access protocol for climbing was to cause minimal disturbance to the eagles while maximising the safety of the climber. We only installed cameras after the nestling was at least 15 days old. We avoided climbing nests during the first days after hatching because the chick depends on the adults for thermoregulation and can suffer from excessive heat or excessive cold resulting from direct sun or rain, respectively (Collopy Reference Collopy1984, Ellis and Schimitt Reference Ellis and Schimitt2017). Adult Harpy Eagles, particularly the females, can be extremely aggressive during the first days after hatching, so going into nests should be avoided at this stage (Seymour et al. Reference Seymour, Hatherley, Contreras, Aldred and Beeley2010). Taking these factors into consideration, we only climbed and installed cameras in the nest during periods that were safe for the nestling.
Statistical analyses
The circadian activity pattern of fledgling eagles and adults delivering prey were analysed using data from the camera traps. We did not include nestling Harpy Eagle data (5–7 months old) because adults usually observe nestling eaglets at close range (or are inside the nest) and therefore are easy to observe and photograph. We used the circular Kernel method for analyses of activity data (Ridout and Linkie Reference Ridout and Linkie2009). The 95% isoline was utilised to describe the complete activity pattern for the nesting eagles, and the 50% isoline to represent the core activity range. The bandwidth parameter used was five, as recommended by Oliveira-Santos et al. (Reference Oliveira-Santos, Zucco and Agostinelli2013). A bootstrap of 10,000 samples with the original sample size, with replacement, as recommended by Ridout and Linkie (Reference Ridout and Linkie2009) was performed to calculate the confidence interval of the measures of presence duration. Records were considered independent if they occurred at an interval of more than 20 min. This criterion was used to achieve a fine temporal resolution for circadian patterns, without oversampling moments where individuals (especially adults) triggered the camera repeated times during one quick visit.
To estimate the estimated time until detection of a Harpy Eagle, we ran an analysis in two steps. First, we used generalised linear mixed models (GLMM) to estimate probability of a camera detecting fledglings and adults as a function of days passed from the start of sampling (i.e. the deployment of the camera traps), since eagles visit their nest less often as a breeding cycle nears its end. Analyses were run using the binomial family and a logit link function (Ashe et al. Reference Ashe, Noren and Williams2010). We know that cameras may fail to detect adults when they visit the nest (from records of prey delivery without a visible adult), so we corrected the estimated detection probabilities using the false omission rate (FOR) of adults, by applying Bayes’ theorem:
Where
![]() $ P\left( Detection| Presence\right) $
is the complement of the false omission rate (1 - FOR),
$ P\left( Detection| Presence\right) $
is the complement of the false omission rate (1 - FOR),
![]() $ P\left( Presence| Detection\right) $
is 1 (since there is no false omission errors),
$ P\left( Presence| Detection\right) $
is 1 (since there is no false omission errors),
![]() $ P(Detection) $
is the estimated probability by the GLMM, and
$ P(Detection) $
is the estimated probability by the GLMM, and
![]() $ P(Presence) $
is the focal value, the probability of a Harpy Eagle actually visiting the nest. The second step consisted of the estimation of time until detection by tourists for both age classes using a bootstrap analysis. We ran 10,000 simulations where we sampled a) the date since nest detection in which a nest was visited by a tourist, b) the estimated detection probability for that date onwards (as predicted from the GLMM, accounting for uncertainty). We then simulated Bernoulli trials over each day starting from the sampled date at a) and recorded the numbers of days sampled until first successful detection.
$ P(Presence) $
is the focal value, the probability of a Harpy Eagle actually visiting the nest. The second step consisted of the estimation of time until detection by tourists for both age classes using a bootstrap analysis. We ran 10,000 simulations where we sampled a) the date since nest detection in which a nest was visited by a tourist, b) the estimated detection probability for that date onwards (as predicted from the GLMM, accounting for uncertainty). We then simulated Bernoulli trials over each day starting from the sampled date at a) and recorded the numbers of days sampled until first successful detection.
We split the nest visitation schedules by adults (from which males and females are separated by talon size) and eaglets into two main categories: 1) early nesting, composed of recently fledged birds (from ~6 to 12 months of age) and 2) late nesting, composed of late fledglings from 12 to 20 months of age. Older fledglings sporadically visit nests too seldom to be useful for tourism, and during that stage of the nesting cycle, parent birds offer food at increasing distances from the nest to stimulate dispersion of the juveniles (Muñiz-López et al. Reference Muñiz-López, Limiñana, Cortés and Urios2016). The analyses were performed using the coding environment R version 4.0.2 (R Core Team 2020), and are available at https://github.com/KenupCF/HarpyEagleTourism.
Results
We found 35 different nests in four years. Considering a 16.6% chance of a nest having a nestling, we estimate that 13, 17 and 26 nests are required to have at least one nest with nestling on 90%, 95% and 99% of the days. The camera trap sampling resulted in 21,554 photographs and videos of 32 Harpy Eagles (21 adults and 10 eaglets) from 11 different nests. Three further nests sampled were excluded because forest fragmentation created food stress resulting in reduced visitation rates by adults. From the identified records, 3,650 independent records of adult and fledgling Harpy Eagles were obtained (Table 1).
Table 1. List of nests monitored for the present study with respective geographical coordinates and number of independent photographs (>20 min, see methods for details) used in the analyses of the study.
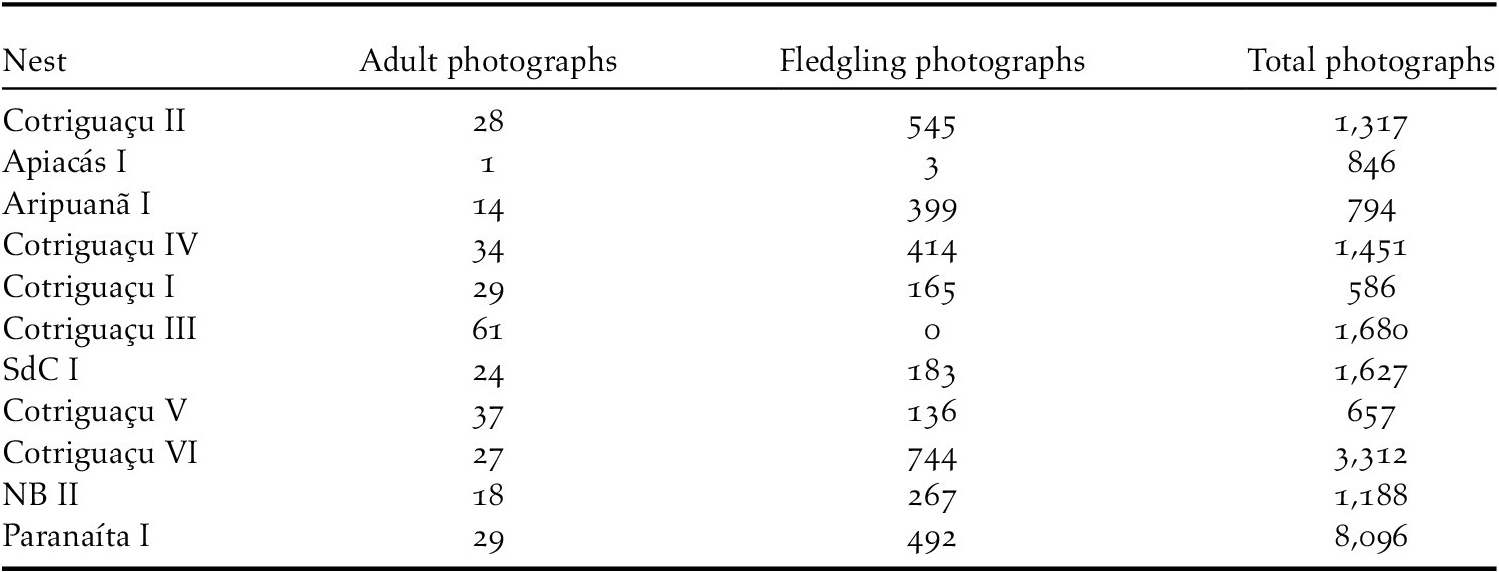
The circadian pattern of Harpy Eagle adults nest visits was diurnal, and the mean of active hours core duration (50% isoline) was 6 h 20 min for young and 7 h 20 min for adults. Adults visited the nests predominantly from the morning to the middle of the day, from 08h20 to 10h35 and again from 11h15 to 16h20. The core activity of fledglings was diurnal, and they were predominantly active from 08h30 to 14h00 and again 14h20 to 15h00. Fledgling Harpy Eagles differed from adults mainly by: 1) having less-pronounced activity peaks; 2) the second peak was more pronounced than the first; and 3) core activity was 12.75% longer (Figure 3).
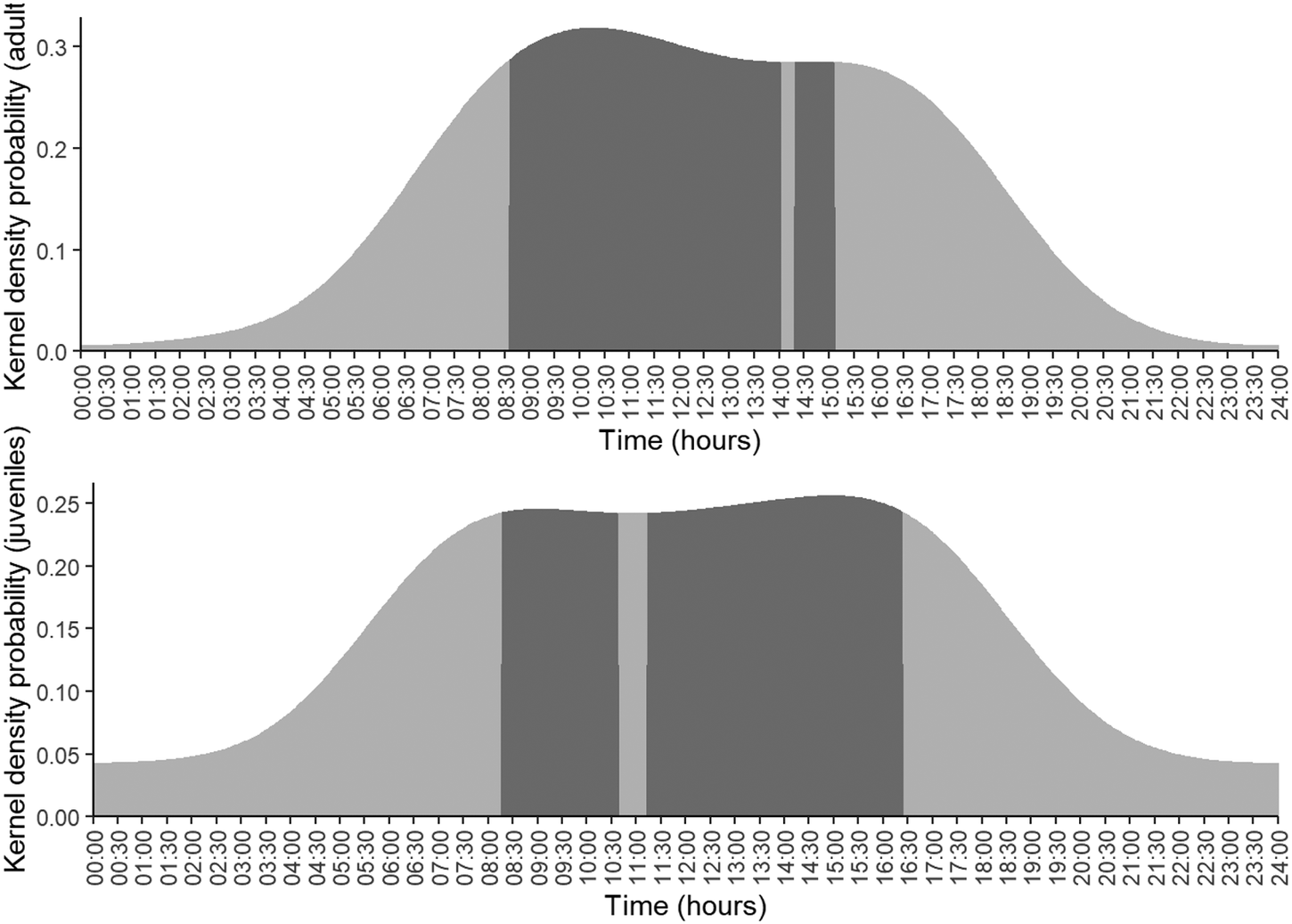
Figure 3. Harpy Eagle circadian patterns of nest visits for adults and fledged juveniles. (Dark grey shows the core activity 50% isoline). Core activity lasted 6.5 hours for adults, peaking at 10h00, and 7.45 hours for fledged eagles, peaking at 15h00.
Each visit from an adult to its nest lasted on average 4.7 minutes. The required observation time for a tourist to sight a Harpy Eagle varied with age of the young bird and nest phase. At early nesting (meaning young eaglets from fledging at 5–7 to 12 months of age), it averaged two days for fledgling individuals and 4.16 days for adults. For a 95% probability of sighting of an adult-sized bird (either fledged bird or a parent bird), tourists must stay a minimum of five days for fledglings and 12 days for adults. At late nesting (12 to 20 months), the observation time required averaged 2.5 days for fledgling eagles and 5.2 days for adults. For a 95% sighting rate, tourists must stay seven and 15 days for fledglings and adults, respectively. Further details on the odds can be seen in the Table 2 and Figure 4. Table S1 in the online supplementary material contains summary statistics of the detection models.
Table 2. Number of days necessary for first Harpy Eagle sighting at nests from platforms or towers. Values refer to expected percentage of tourists seeing an eagle on different bootstrapping scenarios. Last column is the average number of days until first detection. Early nesting refers to the nest cycle from 5–7 to 12 months old, and late nesting to birds 12–20 months of age.
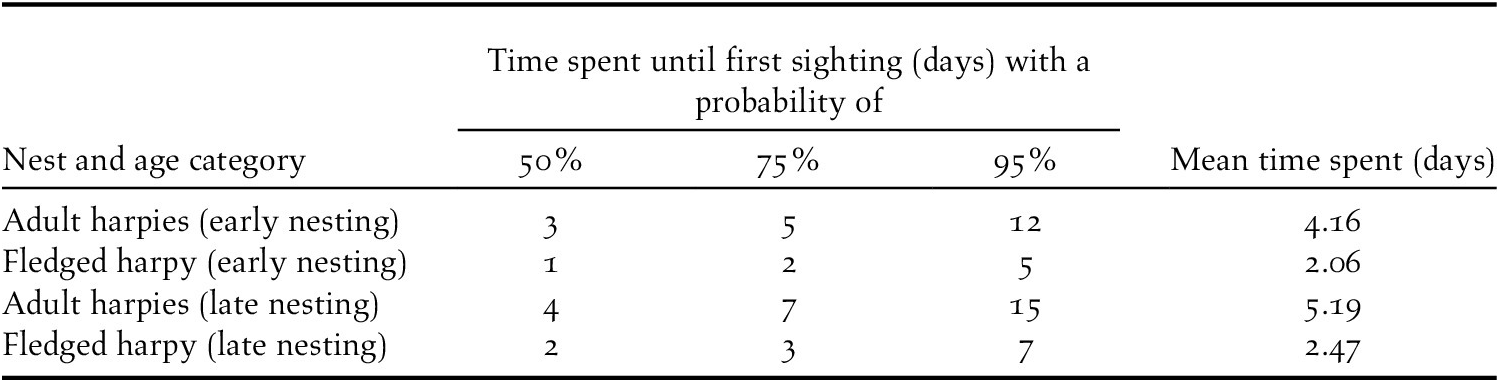
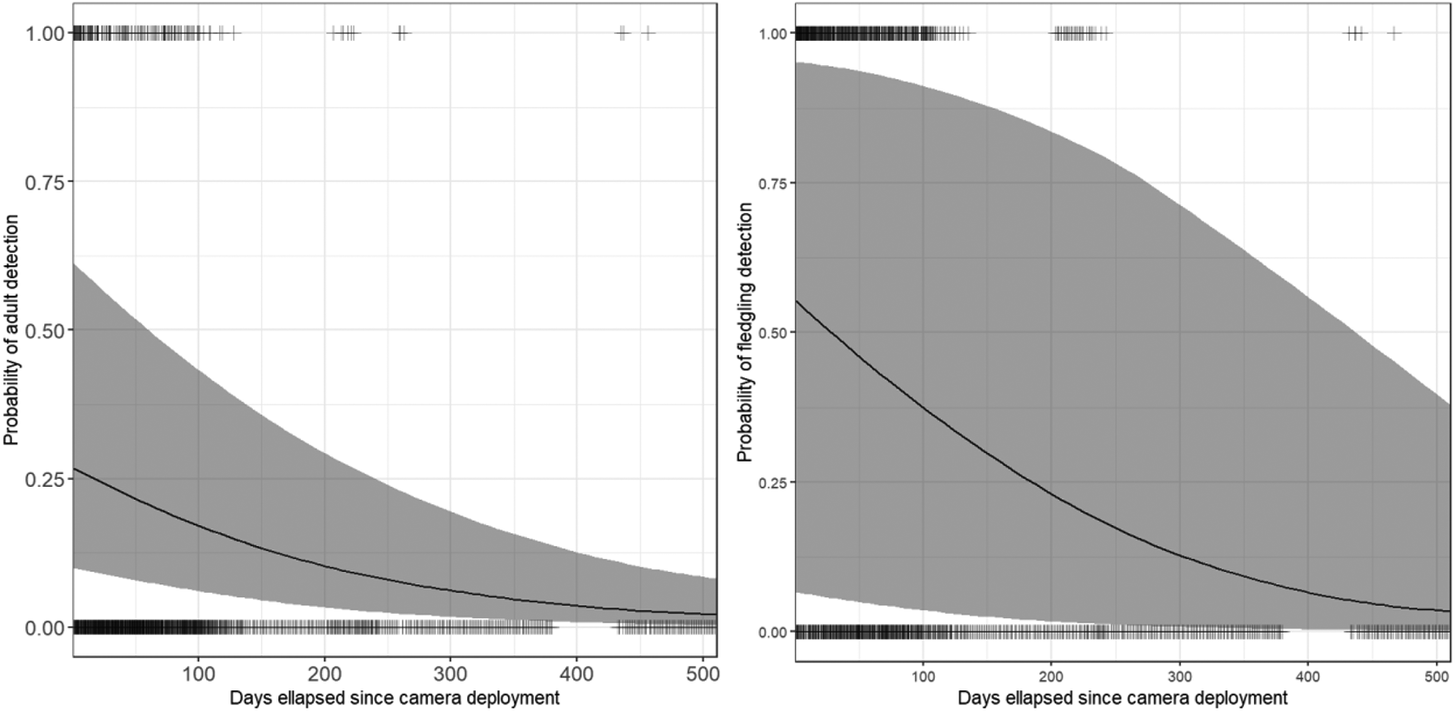
Figure 4. Predictions of probability of sighting an adult (left) and a fledgling (right) Harpy Eagle, according to generalised linear mixed model analyses and corrected for false omissions. The uncertainty for fledgling detection probability occurred because we accounted for random variation between nests, as expected because camera trapping started at different stages in eaglet development at the different nests.
As evidence of incidental habituation, 95.4% of fledged Harpy Eaglets remained in the nest tree or approached the climber during camera installation or removal (n = 44). The two occasions where the fledgling Harpy Eagles fled when we climbed the tree were from a nest in an indigenous reserve, where the tribesmen actively hunt Harpy Eagles, and a logging site where a tall tree next to the nest tree had dealt a major, glancing blow to the nest tree while being felled by loggers. All nests in which we installed observation towers had Harpy Eagle pairs re-nesting on them at usual times (n = 7). Neither adults nor juveniles showed typical stress behaviours towards people, like head-bobbing, facial disc expansion and swooping towards people during tourism or tower building activities.
Discussion
Creating methods to manage the intricacies between tourism and Harpy Eagle conservation should be based on evidence, and those approaches should be evaluated though field tests such as presented in this study. Here we showed that when Harpy Eagles could be found predictably at their nests, allowing tourists to enjoy reliable viewing. Besides making the first concrete analysis of adult and fledgling eaglet activity on nests, we describe the circadian patterns that allow us to identify the timing of eagle behaviours that tourists and media professionals desire—namely adults flying to the nest with prey. Finally, we offer tourism managers and stakeholders a tool to predict how to estimate length of tourist stays to have high chances of seeing Harpy Eagles. Ill-conceived or poorly implemented wildlife-viewing practices that displace or harm wildlife can create further threats to the species, but our results can avoid those issues, thereby positively-effecting Harpy Eagle conservation.
South America’s two long-running Harpy Eagle research projects (one in Venezuela and the other in Brazil) have found around ~120 nests in 20–30 years of work (pers. obs.). This represents fewer than five nests per year, whereas by relying on the assistance of local people, we found 35 nests in four years (>8 nests/year). Harpy Eagle nests generally are extremely hard to locate, and finding even one is highly noteworthy even for ornithologists (Pereira and Salzo Reference Pereira and Salzo2006, Ubaid et al. Reference Ubaid, Ferreira and Antas2011, Rotenberg et al. Reference Rotenberg, Marlin, Pop and Garcia2012). Therefore, relying on locals who work in the eagle’s habitat was a highly cost-effective method for finding nests, and one that was capable of generating high-quality data reported in the only previous study that relied on a relatively large number of nests (Vargas-González and Vargas Reference Vargas-González and Vargas2011). Furthermore, it quickly provides a meaningful way of reaching nest numbers that allow reasonable chances of having at least one nestling available for year-round photo-tourism. The minimum number of 13 nests to have nestlings for 90% of days can be reached in 1.5–2 years. Degraded landscapes bear the advantage of allowing nests to be discovered even faster, besides bearing more infrastructure (Macedo et al. Reference Macedo, Salvador, Moschen and Monjeau2018).
Fledgling Harpy Eagles, being approximately adult-sized with grey-white plumage are generally the most sought-after bird after adults themselves (pers. obs.). The detection of Harpy Eagles was estimated by camera traps only, and therefore represents a shortcoming regarding undetected adult and fledged eagle visits to the tree, but not to the nest itself. While our methods are statistically sound, our resulting requirements for staying at a nest and guaranteeing a sighting represent a conservative approximation of the real requirements. Therefore, the number of days required for 50, 75 or 95% probability of sighting must be interpreted with caution. On the other hand, the average length of days required to see an eagle are consistent with what we observed on a day-to-day basis during our nest visits, and therefore represent robust estimates.
Regarding tourism, the seminal paper by Reynolds and Braithwaite (Reference Reynolds and Braithwaite2001) stated that wildlife attractions must be: (1) predictable in activity or location; (2) approachable; (3) readily viewable (open habitats); (4) tolerant of human intrusion; (5) possess elements of rarity or local super abundance; and (6) have a diurnal activity pattern. Fortunately, Harpy Eagles match all these traits, except for the fact that they inhabit the canopy (item 3), a problem that we overcome by using custom-designed, purpose-built observation towers and platforms. Predictability of nesting sites (item 1) is also a subject of concern, because 16.6% of nesting pairs of eagles also have alternative nests (Vargas-González and Vargas Reference Vargas-González and Vargas2011), which means that in some years, they may be using the other, undiscovered nest tree. Our work contributes directly to the understanding of items 1 and 6. Those discoveries are of prime interest regarding Harpy Eagles as a wildlife attraction and the management of tourism that fits nest visitation schedules of eagles.
Responsible and controlled schedules for tourists viewing nests are particularly important for sensitive species with multi-decadal life cycles, low breeding potential, and high tourism value (Ashe et al. Reference Ashe, Noren and Williams2010, Haskell et al. Reference Haskell, McGowan, Westling, Méndez-Jiménez, Rohner, Collins, Rosero-Caicedo, Salmond, Monadjem, Marshall and Pierce2015, Tortato et al. Reference Tortato, Izzo, Hoogesteijn and Peres2017). For Harpy Eagles, our data showed that both adults and eaglets were mainly active during the early and late hours of the day, with a higher peak in activity during the morning (10h15–10h45) for adults and late afternoon (15h15–15h45) for fledgling, adult-sized eaglets. Nest visits by tourists can be planned to allow the incorporation of other wildlife attractions such as viewing toucans, macaws, or primates during times of low viewing chances for Harpy Eagles. Our present study provides evidence of ideal times for nest visits by tourists when an optimal photographic experience would be most likely.
One clear pattern emerging from our analyses is that later parts of the nesting cycle offered relatively low potential for tourism. Tourism activities therefore should then be focused on the first 12 months post-hatching, especially 0–5 months when the odds of viewing adults with the chick are extremely high. Harpy Eagle tourism trips are currently commercialised as 2–4 day excursions for the ~4,780 guests that annually visit the Pantanal for jaguars (Tortato et al. Reference Tortato, Izzo, Hoogesteijn and Peres2017). Longer packages do not fit in the current model—tourists visiting Harpy Eagles normally had already spent 5–7 watching jaguars in Pantanal—so tourism must focus on early phases of the Harpy Eagle breeding cycle, when eagles can be seen in shorter periods. It is therefore imperative to have agreements with the dozens of landowners so that visitation can alternate between many nests, thus increasing the chances that at least a few of the nests will be in the right phase for successful Harpy Eagle sightings and tourism development.
Poor tourism practices that could cause disruption or abandonment of nests would threaten both the eagles and the tourism business models, thus requiring yet more nests to be found. The operation of responsible, sustainable, profitable Harpy Eagle nest photo-tourism is a laborious and expensive process, as towers must be moved, and new agreements reached with landowners. Besides threatening wildlife, poor management decisions would ultimately affect the attractions they were built on, compromising the sustainability of the business model (Haskell et al. Reference Haskell, McGowan, Westling, Méndez-Jiménez, Rohner, Collins, Rosero-Caicedo, Salmond, Monadjem, Marshall and Pierce2015). Furthermore, having Harpy Eagle nests under a relatively constant watch could open the door to several avenues of conservation actions in the face of fairly heavy deforestation. For example, supplementary food could be offered to Harpy Eagles that are under food stress in severely fragmented landscapes (Miranda et al. Reference Miranda, Peres, Carvalho-Rocha, Miguel, Lormand, Huizinga, Munn, Semedo, Ferreira, Pinho, Piacentini, Marini and Downs2021). Eaglets that fall from nests while learning to fly (the main cause of natural mortality for fledglings; Muñiz-López Reference Muñiz-López2017) can be returned to their nests. Fledgling Harpy Eagles that are stranded in isolated forest patches and thus unable to disperse could be translocated to other, larger forests, thus preventing the parents from killing the young that need to disperse (Muñiz-López Reference Muñiz-López2017) and genetic diversity loss (Banhos et al. Reference Banhos, Hrbek, Sanaiotti and Farias2016).
We highlight that the same protocols used to install the camera traps should be applied to tourism, and those terms addressed in contracts and permits, which demanded that up to 15 days of age no disturbance occurs, and we strongly recommend readers interested in using camera traps to check Miranda et al. (Reference Miranda, Peres, Carvalho-Rocha, Miguel, Lormand, Huizinga, Munn, Semedo, Ferreira, Pinho, Piacentini, Marini and Downs2021) to learn about their limitations. We consider the tourism model presented here to be a work in progress, since we may have missed some impact to the eagles, undocumented possible solutions, or proposed some approaches that would not work in other study sites. Continuous updates of the model we propose, with subsequent improvements as new information comes to researcher’s attention, will provide a better basis for future work. We encourage investigations of: (1) breeding seasonality, (2) feeding frequency with and without tourism, (3) critical levels for tower distance, (4) physiological responses to tourism, and (5) possible tourism business models. We invite our readers and colleagues to improve the model we propose by providing evidence for any oversights or possible misinterpretations done by us, as new published information becomes available.
Habituation can be defined as the process of reducing an animal’s instinct of escape in the presence of humans (Geffroy et al. Reference Geffroy, Samia, Bessa and Blumstein2015). In a tourism context, habituation is encouraged or desired to improve guest experience and to reduce animal displacement and stress (Higham and Shelton Reference Higham and Shelton2011). In our study region, 72% of the Harpy Eagle nests were in Brazil nut trees Bertholletia excelsa (n = 35; E.B.P. Miranda unpubl. data). In the system described here, the presence of nut collectors working below nest trees for many years before the inception of the project, coincidentally made Harpy Eagles accustomed to people, therefore no formal, elaborate habituation was required. Nevertheless, we encourage researchers to collect data to test the degree of habituation of the eagles within their study site to objectively test this assumption and prevent stress to the eagles; tourism has shown to reduce nest occupancy—without demographic consequences—in other species (Kaisanlahti-Jokimaki et al. Reference Kaisanlahti-Jokimaki, Jokimaki, Huhta, Ukkola, Helle and Ollila2008).
On two occasions, one of which occurred before the inception of this project, camera traps were installed during the nest building process. In both cases the Harpy Eagles abandoned the nest. Simultaneously, an old logging road passing 150 m from one nest was reopened. In the other case the female Harpy Eagle was reportedly killed (she was never seen again) by members of a nearby community (pers. comm.). Although we cannot definitively attribute these two cases of abandonment to installing camera traps during the nest-building phase, we nevertheless recommend that future researchers avoid disturbing nests before incubation is concluded. Other than the cases reported, no Harpy Eagles abandoned the nests, and several have already re-nested at the same nest sites during the present study. Since the information presented here rely in a small sample size, and other species show tolerance regarding camera installing during the pre-laying phase (Margalida et al. Reference Margalida, Ecolan, Boudet, Bertran, Martinez and Heredia2006), we believe that the subject deserves formal testing in the future.
By creating functional systemic and economic links between our conservation project, ecotourism investors, and stakeholders (including local people), we structured the system so that the ecotourism would gain momentum and spread in the region through similar initiatives, protecting more nests and more forest. As evidence of this momentum, five new nests were communicated to us after our publicity on nest finding was terminated in February (due to the pandemic), even without any recent advertising. This represents a successful case of conservation marketing (Wright et al. Reference Wright, Veríssimo, Pilfold, Parsons, Ventre, Cousins, Jefferson, Koldewey, Llewellyn and McKinley2015). Without continued management to promote tourism and other conservation strategies in the Arc of Deforestation, the Harpy Eagles will continue to face substantial distribution range loss or local extinction that made them almost disappear from the Atlantic Forest (Srbek-Araujo and Chiarello Reference Srbek-Araujo and Chiarello2006, Suscke et al. Reference Suscke, Verderane, de Oliveira, Delval, Fernández-Bolaños and Izar2017).
Here we show in what parts of the nesting cycle can be visited safely, and for how long—for the benefit of both tourists and Harpy Eagles. To succeed in the goal of Harpy Eagle conservation, evidence-based management actions must be in place. Tourism can be one of those actions and may be the only one that can generate funding to fuel other conservation activities. The appearance of the Arc of Deforestation is a double-edged weapon for Harpy Eagles. It created a fragmented landscape with high levels of habitat loss, while also creating a landscape where Harpy Eagles are, for the first time, easily accessible thanks to a wide network of roads and airports. Approached under a policy-oriented strategy, Harpy Eagles will become a tool for preventing further degradation of the Amazon Forest.
Acknowledgements
We greatly appreciate the generous financial support of the following donors: the SouthWild.com Conservation Travel System, Rainforest Biodiversity Group, Idea Wild, The Mamont Scholars Program of the Explorer’s Club Exploration Fund, Cleveland Metroparks Zoo, and the Rufford Small Grants Foundation (18743-1, 23022-2 and 31091-B). CTD is grateful to the University of KwaZulu-Natal and the National Research Foundation (ZA) for funding. Logistical support was given by the Peugeot-ONF Carbon Sink Reforestation Project, based at the São Nicolau Farm in Cotriguaçu, Mato Grosso, Brazil. This project is a Peugeot initiative to fulfil some of the Kyoto Protocol directions and is run by the ONF-Brasil enterprise. We also thank the fieldwork help given by Dr Alexander Blanco, Gilberto Araujo, Lucas Buttura and Roberto Stofel. Finally, we thank ICMBio for authorising the project activities under the permit SISBIO 58533.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S095927092100040X.








