Introduction
Human activities such as habitat destruction and alteration, introduction of invasive species, overfishing and pollution are major threats to marine ecosystems, changing species abundance and distribution, and the structure, function and resilience of ecosystems (Doherty et al. Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016, Xu et al. Reference Xu, Liu, Wu, Sun, Zhao and Chen2016, Millán Reference Millán2018). Biodiversity loss is particularly high for insular ecosystems because island populations tend to be small, present a high degree of endemism due to geographical isolation and consequently low level of resilience to introduced invasive species and the impact of climate change (e.g. Manne et al. Reference Manne, Brooks and Pimm1999, Brooke et al. Reference Brooke, Bonnaud, Dilley, Flint, Holmes, Jones, Provost, Rocamora, Surman and Buxton2017). The marked decline in many seabird populations is an indicator of long-term and large-scale changes in insular, coastal, and offshore marine ecosystems (Paleczny et al. Reference Paleczny, Hammill, Karpouzi and Pauly2015). Pelagic seabird species are the group of birds showing the largest decline worldwide, with by-catch and predation by invasive species the most harmful threats at-sea and on-land, respectively (Anderson et al. Reference Anderson, Small, Croxall, Dunn, Sullivan, Yates and Black2011, Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012, Dias et al. Reference Dias, Martina, Pearmaina, Burfielda, Small, Richard and Croxall2019).
At their breeding sites many seabird populations may face high levels of egg, chick and adult predation by introduced invasive species such as rats Rattus spp. mice Mus musculus, and cats Felis catus (Jones et al. Reference Jones, Tershy, Zavaleta, Croll, Keitt, Finkelstein and Howald2008, Sarmento et al. Reference Sarmento, Brito, Ladle, Leal and Efe2014, Jones et al. Reference Jones, Risi, Cleeland and Ryan2019). Other introduced mammals such as rabbits Oryctolagus cuniculus and goats Capra hircus may also cause the loss of seabird breeding habitats (Jones et al. Reference Jones, Tershy, Zavaleta, Croll, Keitt, Finkelstein and Howald2008). Globally, predation by introduced mammals is the leading cause of decline in eight species of Procellariidae, classified as ‘Critically Endangered’ by IUCN on islands around the world: Fiji, Reunion, Jamaica, Chatham, Galapagos, Balearics and Melanesia (Le Corre Reference Le Corre2008). Similar cases of predation by introduced mammals are reported in the north-central islands of Chile (Simeone et al. Reference Simeone, Luna-Jorquera, Bernal, Garthe, Sepúlveda, Villablanca and Ellenberg2003), the Galapagos Archipelago (Riofrío-Lazo and Páez-Rosas Reference Riofrío-Lazo and Páez-Rosas2015), Guadalupe island, the Pacific Ocean and Socorro island (Nogales et al. Reference Nogales, Vidal, Medina, Bonnaud, Tershy, Campbell and Zavaleta2013). Other inland threats, such as human capture and trampling of nests (e.g. Cima and Laje Branca islets), the destruction of shore areas to build human infrastructure (hotels, ports), habitat fragmentation and light pollution also affect seabird populations (Hazevoet Reference Hazevoet1995, Ratcliffe et al. Reference Ratcliffe, Zino, Oliveira, Vasconcelos, Hazevoet, Neves, Monteiro and Zino2000, Paleczny et al. Reference Paleczny, Hammill, Karpouzi and Pauly2015). Seabird populations may also be threatened in their coastal and pelagic foraging areas, including entanglement in fishing gear, overfishing, climate change and/or marine pollution (Furness and Taske Reference Furness and Taske2000).
In the North Atlantic there was a huge reduction in seabird populations following the European colonization of the Azores (Monteiro et al. Reference Monteiro, Ramos and Furness1996), Madeira, Canary, and Cabo Verde (Vasconcelos et al. Reference Vasconcelos, Rocha, Afonso, Almeida, Lopes, Loura and Ferreira2015, Saavedra et al. Reference Saavedra, Santos, Valcarce, Freitas, Silva and Pipa2018) archipelagos. Currently, introduction of exotic mammals is one of the main factors explaining the distribution and abundance of small Procellariiformes in these archipelagos, such as Bulwer’s Petrel Bulweria bulwerii and Madeiran Storm-petrel Hydrobates castro, which breed only on islets or steep inaccessible cliffs on main islands that are free from rats (Monteiro et al. Reference Monteiro, Ramos, Pereira, Monteiro, Feio, Bearhop and Graz1999). In relation to the subtropical region of Cabo Verde, information on the status and distribution of seabird species is scarce, unavailable, or dispersed in grey literature. The main work carried out to date in Cabo Verde reports some information on the occurrence, breeding phenology, and threats to seabird species (Hazevoet Reference Hazevoet1994). Recently, one species, Cape Verde Petrel Pterodroma feae, was characterized in terms of population size and threats, though only in a single breeding site (Militão et al. Reference Militão, Dinis, Zang, Calabuig, Stefan and González-Solís2017). However, there is still a great lack of information on the status, distribution, abundance, and threats to breeding seabird populations throughout the archipelago.
In the Azores, a historical review of seabird distribution together with survey and censuses directed at the various species made it possible to infer a strong reduction in seabird populations in the archipelago following colonization of the islands by humans (Monteiro et al. Reference Monteiro, Ramos and Furness1996, Reference Monteiro, Ramos, Pereira, Monteiro, Feio, Bearhop and Graz1999). Similarly, Cabo Verde seabirds have been exploited as a food resource for centuries, leading to a decline in their numbers (Murphy Reference Murphy1924, Hazevoet Reference Hazevoet1994, Reference Hazevoet, Leyens and Lobin1996). Preliminary observations made in Cabo Verde by Rendall and Pile (Reference Rendall and Pile2007), Oliveira et al. (Reference Oliveira, Oliveira, Melo, Melo and Geraldes2013) and Vasconcelos et al. (Reference Vasconcelos, Rocha, Afonso, Almeida, Lopes, Loura and Ferreira2015) show that as with species nesting in the northernmost archipelagos (Azores, Canary and Madeira), smaller seabird species are largely confined to islets without exotic predators such as rats and cats. Larger seabird species should have a wider distribution in the archipelago, similar to what occurs in the Azores and Madeira (Romano et al. Reference Romano, Fagundes, Zino and Biscoito2010), where Cory’s Shearwater Calonectris borealis, a medium-size seabird, breeds along the cliffs of most of the islands (Monteiro et al. Reference Monteiro, Ramos and Furness1996).
Three orders of seabirds are found in Cabo Verde: Procelariiformes, Suliformes and Phaethontiformes. There are six species of Procelariiformes: Bulwer’s Petrel Bulweria bulwerii, White-faced Storm-petrel Pelagodroma marina aedesorum, Cape Verde Shearwater Calonectris edwardsii, Cape Verde Storm-petrel Hydrobates jabejabe, Cape Verde Petrel Pterodroma feae and Boyd's Shearwater Puffinus lherminieri boydi, the last four species/subspecies being endemic to the Cabo Verde archipelago (del Hoyo et al. Reference del Hoyo, Elliott, Sargatal, Christie and Kirwan2014). Within the Suliformes, there are two species, Magnificent Frigatebird Fregata magnificens and Brown Booby Sula leucogaster, and one species of Phaethontiform, the Red-billed Tropicbird Phaethon aethereus (del Hoyo et al. Reference del Hoyo, Elliott, Sargatal, Christie and Kirwan2014). Based on bibliographic records, unpublished information and field work, this study compiled all historical and current information on the seabird species breeding in Cabo Verde archipelago, in order to: (1) map the historical and current distribution of all breeding seabird species for the whole archipelago, (2) provide a measure of relative abundance for the procellariform species using their nocturnal calls frequency in some islands and islets, and (3) describe the main breeding habitat characteristics and threats to these species. Overall, we expect: (1) main islands to currently contain a lower number of seabird species than in the past, and possess a lower diversity of seabird taxa than islets; (2) a higher relative abundance of each seabird species on inaccessible islets and more remote areas of the main islands, and (3) the majority of seabird species to occur in areas far from human settlements and in more elevated and steep areas, where accessibility to introduced invasive species and/ or human harvesting should be lower. Overall, this study compiled essential knowledge on the occurrence, relative abundance, and threats for Cabo Verde seabird populations, and provides a strong framework for applied conservation measures that should be implemented at the level of the archipelago.
Methods
Study area
This research was conducted between January 2017 and June 2019 in the Cabo Verde archipelago, located about 385 km off West Africa (Figure 1). Cabo Verde is one of the five Atlantic archipelagos that make up Macaronesia, which also includes the Azores, Madeira, Selvagens, and Canary Islands (Freitas et al. Reference Freitas, Romeiras, Silva, Cordeiro, Madeira, González, Wirtz, Falcón, Brito, Floeter, Afonso and Porteiro2019). The archipelago is formed by 10 islands and several islets, with a total land area of 4,033 km2, divided in relation to the trade winds into Southern (locally known as the Sotavento group) and Northern islands (locally known as the Barlavento group; see Figure 1 for toponymic details).The eastern islands are geologically older and more eroded (Sal, Boavista, and Maio) than the mountainous and newer western islands (Ramalho et al. Reference Ramalho, Helffrich, Schmidt and Vance2010). All islands are of volcanic origin, but only Fogo Island has an active volcano (Dionis et al. Reference Dionis, Pérez, Hernández, Melián, Rodríguez, Padrón, Sumino, Barrancos, Padilla, Fernandes, Bandomo, Silva, Pereira, Semedo and Cabal2015). The archipelago's topography ranges from plains to high mountains, reaching 2,829 m at the summit of the active volcano on Fogo Island. The elevation, slope and orientation of the mountains influence the amount of precipitation each island receives. The landscape is eroded and rugged, with vegetation mainly in inland valleys (Riva-Martínez et al. Reference Rivas-Martínez, Lousã, Costa and Duarte2017).
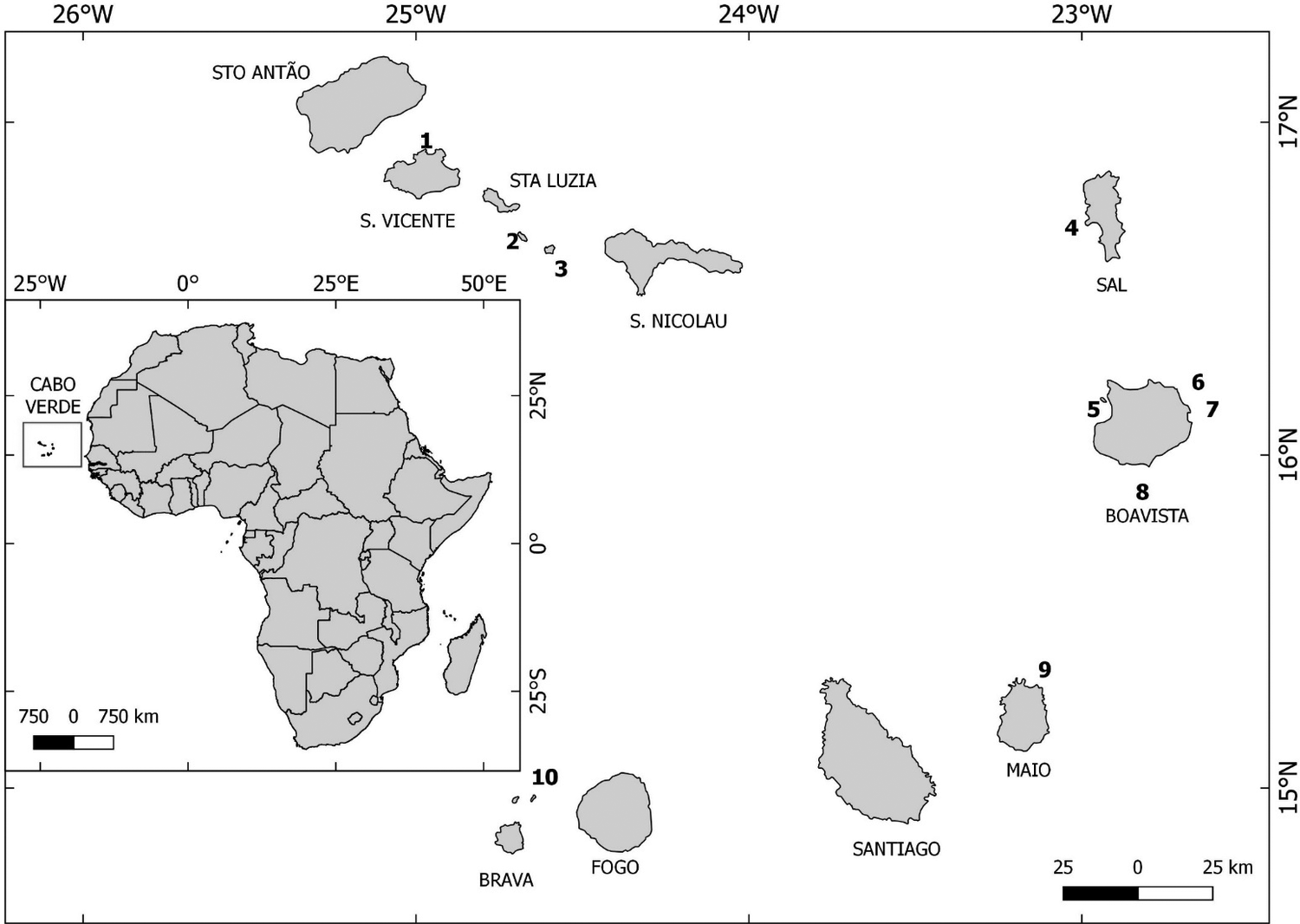
Figure 1. Map of the Cabo Verde archipelago with numbers representing the Islets of (1) Pássaros (São Vicente), (2) Branco, (3) Raso, (4) Rabo do Junco (Sal), (5) Sal Rei (Boavista), (6) Passáros (Boavista), (7) Baluarte (Boavista), (8) Curral Velho (Boavista), (9) Laje Branca (Maio), (10) Rombo (Grande, Cima, Sapado and Luís Carneiro).
Past and present distribution of seabirds in the Cabo Verde archipelago
To assess the past distribution of seabirds in the archipelago we compiled information from historical expeditions, museums, grey and scientific literature until 1995 (Table S1 in the online Supplementary Material). To assess present distribution, nocturnal and diurnal surveys, together with some mist-netting, were carried out to identify the presence / absence of species in potential breeding sites. For nocturnal species with active calls in flight (Cape Verde Petrel, Cape Verde Shearwater, Boyd's Shearwater and Cape Verde Storm-petrel), the recognition was made through their calls for Santo Antão, São Vicente, Santa Luzia, São Nicolau, Santiago and Fogo Islands, and for Raso and Branco Islets. For the species that do not call in flight, surveys were made to locate nests of: a) Bulwer’s Petrel in the Raso, Branco, and Rombo Islets at night, and b) White-faced Storm-petrel during the day in Branco, Laje Branca (Maio), Rombo, and Pássaros (Boavista) Islets. In the Cabo Verde archipelago, Phaethontiformes and Suliformes occur in the rugged coastal areas, canyons and rocky platforms of the islands and islets. Therefore, the occurrence and censuses of Red-billed Tropicbird and Brown Booby colonies were made by walking along the coast and cliffs, and by boat along the coast of Santo Antão, São Vicente, São Nicolau, Santiago, Fogo and Brava Islands, and Rabo de Junco, Curral Velho, Baluarte, Pássaros, Rombo, Branco and Raso Islets. For Sal and Boavista Islands the censuses were carried out by walking along the coast only.
Abundance of all seabirds in the Northern Islands (Barlavento group) and of Cape Verde Petrel in the whole archipelago
To identify possible breeding sites of the different procellariiform species with active calls in flight, and to assess the relative abundance of their populations, counts of nocturnal calls were made during the winter (January to April) and summer (May to December) breeding seasons of 2017–2019, at predetermined sites (Figure S1). In the Northern Islands (Santo Antão, São Vicente, Santa Luzia, Branco, Raso and São Nicolau) the following species were censused: Cape Verde Petrel, Cape Verde Storm-petrel, Cape Verde Shearwater and Boyd’s Shearwater. In the Southern Islands (Santiago and Fogo) only Cape Verde Petrel was censused. Ideally, prospections and census work should have been done similarly in all islands, but this was not possible because it demands a high number of trained fieldworkers. Prospecting locations were determined based on bibliographic records, unpublished data, and geospatial analysis of areas with potential for seabird occurrence in the archipelago, including islets, remote mountain areas, coastal cliffs and canyons. Prior to census, a site reconnaissance visit was carried out to locate and mark point-counts, and to identify the presence of birds (traces of bird droppings on rocks and nests). Information was also obtained from people living near the sampling area, including species identification (through photos and calls) and the possible threats they face at the site.
Nocturnal call counts were performed from 19h00 to 24h00, except for some cases in which they lasted until around 01h00, due to the absence of moonlight. The counts were directed only at species that visit breeding sites at night, characterized by performing calls in flight, as well as in the nest during breeding. Surveys were conducted during new moon or when the moon was not visible in the sky, because this is when most seabird species call in flight as they approach the nest (Buxton and Jones Reference Buxton and Jones2011) or when they come to land to perform courtship call flights (Cape Verde Petrel). At each sampling point, three censuses were performed, lasting five minutes each and with a five-minute interval between censuses. During each five-minute period, all the calls emitted by each seabird species were counted, and for the seabirds that vocalized in the five-minute intervals only their presence was recorded. Surveys were conducted by the same experienced team of fieldworkers.
Characterization of seabird habitats
All nocturnal survey sites were characterized according to their topography and land cover. Altimetric data from the Shuttle Radar Topography Mission (SRTM) 30 were downloaded in grid format from the US Geological Survey (https://earthexplorer.usgs.gov) and used to generate a 30 m Digital Elevation Model (DEM) translating into (1) elevation. From these data we calculated (2) the percentage of slope. Additionally, land use was characterised using data extracted from OpenStreetMap (OSM) (http://www.geofabrik.de) and we calculated the Euclidean distance from each land use cluster such as the distance to (3) coastline, (4) forests, (5) roads and (6) human settlements. Pixel values in a radius of 250 m around the sampling point were selected for this analysis.
Data analysis
All records published between 1783 and 1995, the year of publication of Birds of Cape Verde (Hazevoet Reference Hazevoet1995), representing an interval of 212 years were considered historical records. We did not include the previous work by Boessneckt and Kinzelbach (Reference Boessneckt and Kinzelbach1993) about the 8th century seabird sub-fossils found on Sal Island, as this study refers to a specific period and only to this island. Records after 1995 were considered as current distribution, because most records were obtained during this study or during pilot surveys that were carried out up to seven years previously.
We georeferenced all seabird calls at point counts with a portable GPS and later mapped the distribution and relative abundance of seabird populations for each island and islet. A chi-square test of equality was used to assess whether the cumulative number of nesting species identified in the archipelago differed among 1969 (reported by Naurois Reference Naurois1969), 1995 (reported by Hazevoet Reference Hazevoet1995) and 2017–2019 by our surveys; significant differences would be expected if local extinction rates increased recently). A student t-test was used to evaluate if the current number of nesting species differs between islands and islets of the archipelago. We also correlated island size with the number of species on islands (logarithmically transformed data).
Data from the call censuses were used to assess the relative abundance of each seabird species for the islands of Santo Antão, São Vicente, Santa Luzia, São Nicolau, Santiago and Fogo, and for Branco and Raso Islets; the average of the three calls was used for each sampling point. Kruskal-Wallis tests were used to compare median number of calls 5 min-1: a) Cape Verde Shearwater, Boyd’s Shearwater and Cape Verde Storm-petrel among Santo Antão, São Vicente, Santa Luzia, São Nicolau and Raso; b) Cape Verde Petrel among the islands of Santo Antão, São Nicolau, Santiago and Fogo. When significant differences were found with the Kruskal-Wallis test, a post-hoc test was used to assess pairwise differences between islands and islets. The Branco Islet was not used in these analyses because there were only two sampling points due to the inaccessibility of this islet.
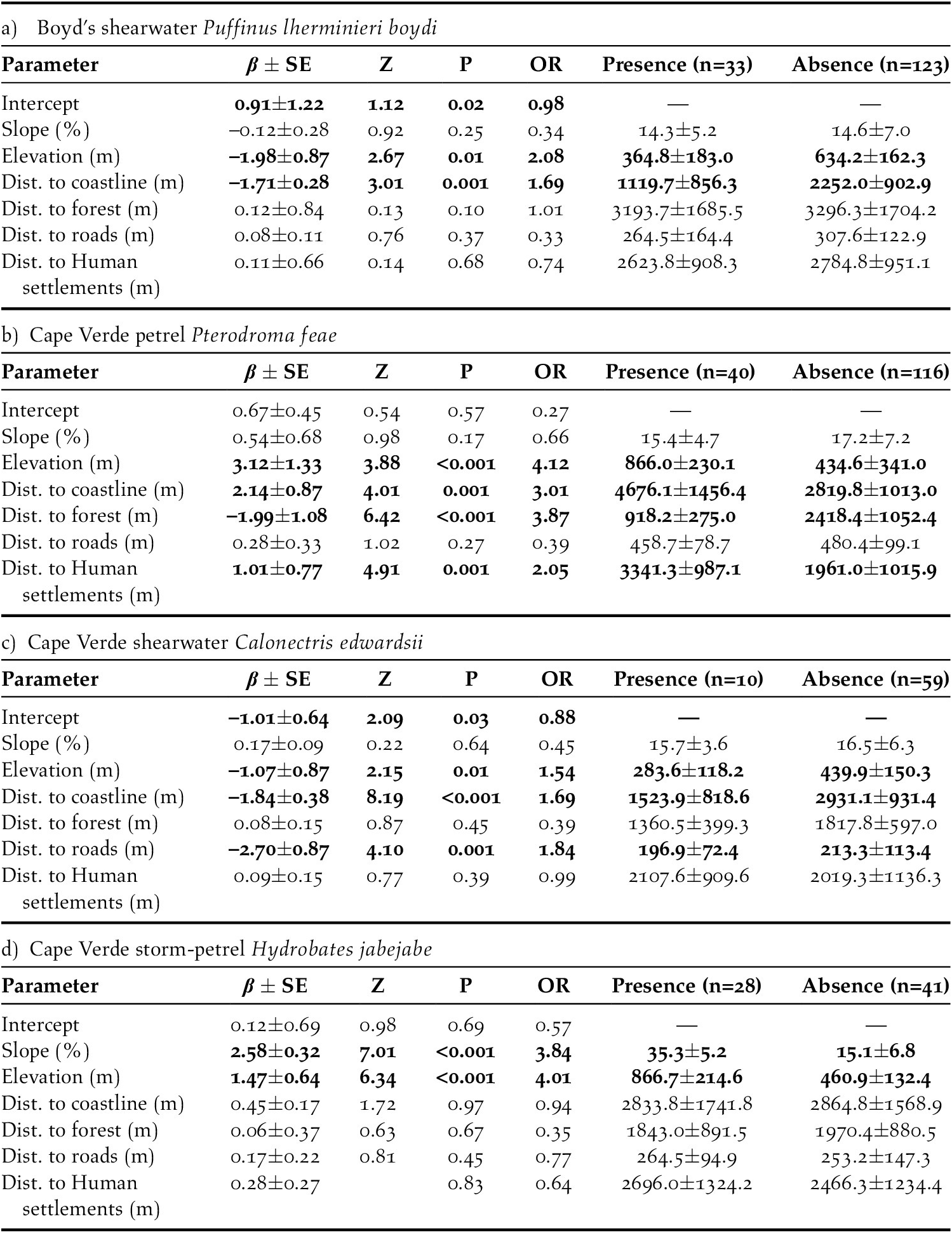
The influence of habitat characteristics on the presence (1 = call) and absence (0 = no call) of (1) Boyd’s Shearwater, (2) Cape Verde Petrel , (3), Cape Verde Shearwater and (4) Cape Verde Storm-petrel was tested with generalised linear models with binomial distribution, fitting the influence of (1) slope (%), (2) elevation (m), distance to (3) coastline (m), (4) forests (m), (5) roads (m) and (6) human settlements, according to the formula: ‘fit’ = glm (seabird presence ∼ slope + elevation + dist. to coastline + dist. to forest + dist. to roads + dist. to human settlements, family = binomial (link = “logit”)). The odds ratio was calculated as ‘exp’ (coef (mylogit)). Models were run only for data collected on Santo Antão and São Nicolau Islands, where prospections and nocturnal call surveys had a better coverage (i.e. a sufficient number of presences/ absences to allow statistical modelling). Regression models (GLMs) were run on the R platform (R Core Team 2019) using functions within the MASS package (Venables and Ripley Reference Venables and Ripley2002).
Results
Past and present seabird distribution in the Cabo Verde archipelago
The earliest reference to seabirds in the Cabo Verde archipelago was the discovery of the Boyd's Shearwater, White-faced Storm-petrel, Cape Verde Shearwater, Brown Booby and Magnificent Frigatebird sub-fossils dating from the 8th century on Sal Island (Boessneckt and Kinzelbach Reference Boessneckt and Kinzelbach1993). Several researchers who visited the archipelago over the last three centuries (Table S2), obtained formal historical records on Cabo Verde seabirds. Regarding the expeditions to Cabo Verde archipelago, the oldest records refer to the period when the naturalist João da Silva Feijó lived in the archipelago (Expedition from 1783 to 1796). Due to the poor support he received from the Portuguese authorities (Roque and Torrão Reference Roque and Torrão2013), the samples were not shipped to Portugal and most records were lost. Data from the National Museum of Natural History of Paris refer to expeditions to Cabo Verde from 1883 to 1970; Boyd’s Shearwater, Cape Verde Storm-petrel and Bulwer’s Petrel specimens were referenced and collected from Raso and Rombo Islets, similarly to accounts by Bourne (Reference Bourne1955) and Hazevoet (Reference Hazevoet1995).
Bulwer’s Petrel was not reported by Alexander and Fea during their expeditions in 1897/1898 and was firstly mentioned by Correia during his first expedition to the Raso Islet in May, and to Cima Islet in June/July (Murphy Reference Murphy1924) (Figures 2A and 2B). Presently, the species occurs in Branco, Raso, Rabo de Junco and Rombo Islets (Figures 2C and 2D).
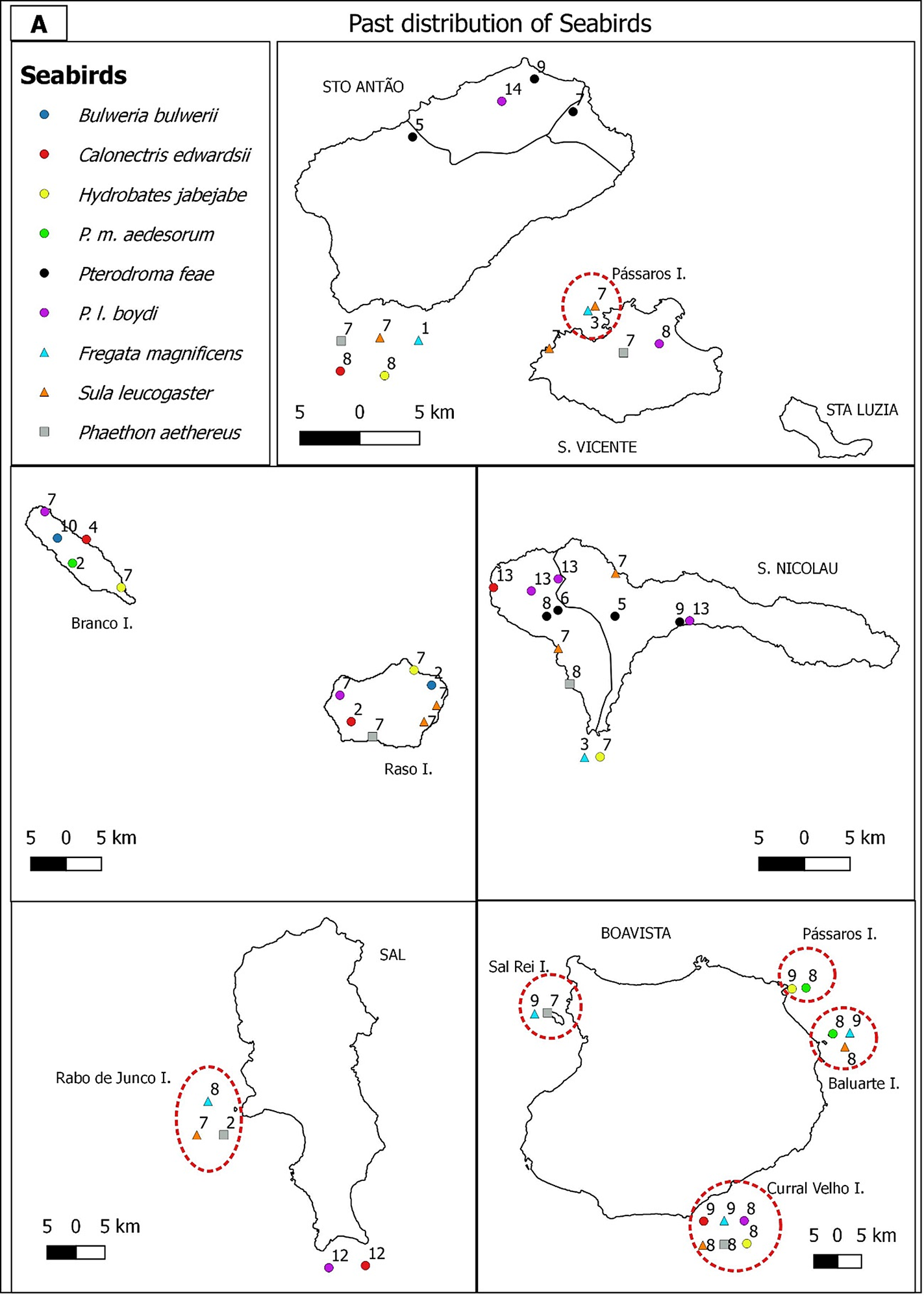
Figure 2A. Past (A, B) and current (C, D) distribution of seabirds in the Barlavento (A, C) and Sotavento (B, D) island groups of the Cabo Verde archipelago. The numbers refer to researchers who identified the species on each site: (1) MacGillivray (Reference MacGillivray and Hazevoet1852); (2) Bolle (Reference Bolle1856) (3) Keulemans (Reference Keulemans1866); (4) Milne-Edwards (Reference Milne-Edwards1883); (5) Salvadori (Reference Salvadori1899); (6) Bocage (Reference Bocage1902); (7) Murphy (Reference Murphy1924); (8) Bourne (Reference Bourne1955); (9) Naurois (Reference Naurois1969); (10) Naurois (Reference Naurois1970); (11) Ledant (Reference Ledant and Hazevoet1988); (12) Boessneckt and Kinzelbach (Reference Boessneckt and Kinzelbach1993); (13) Hazevoet (Reference Hazevoet1994); (14) Hazevoet (Reference Hazevoet1995). Circles represent the Procellariformes, triangles the Suliformes and squares the Phaethontiformes. Symbols laid outside the islands indicate presence but without an exact location. Islets surrounded with a dashed line.

Figure 2B. Continued

Figure 2C. Continued
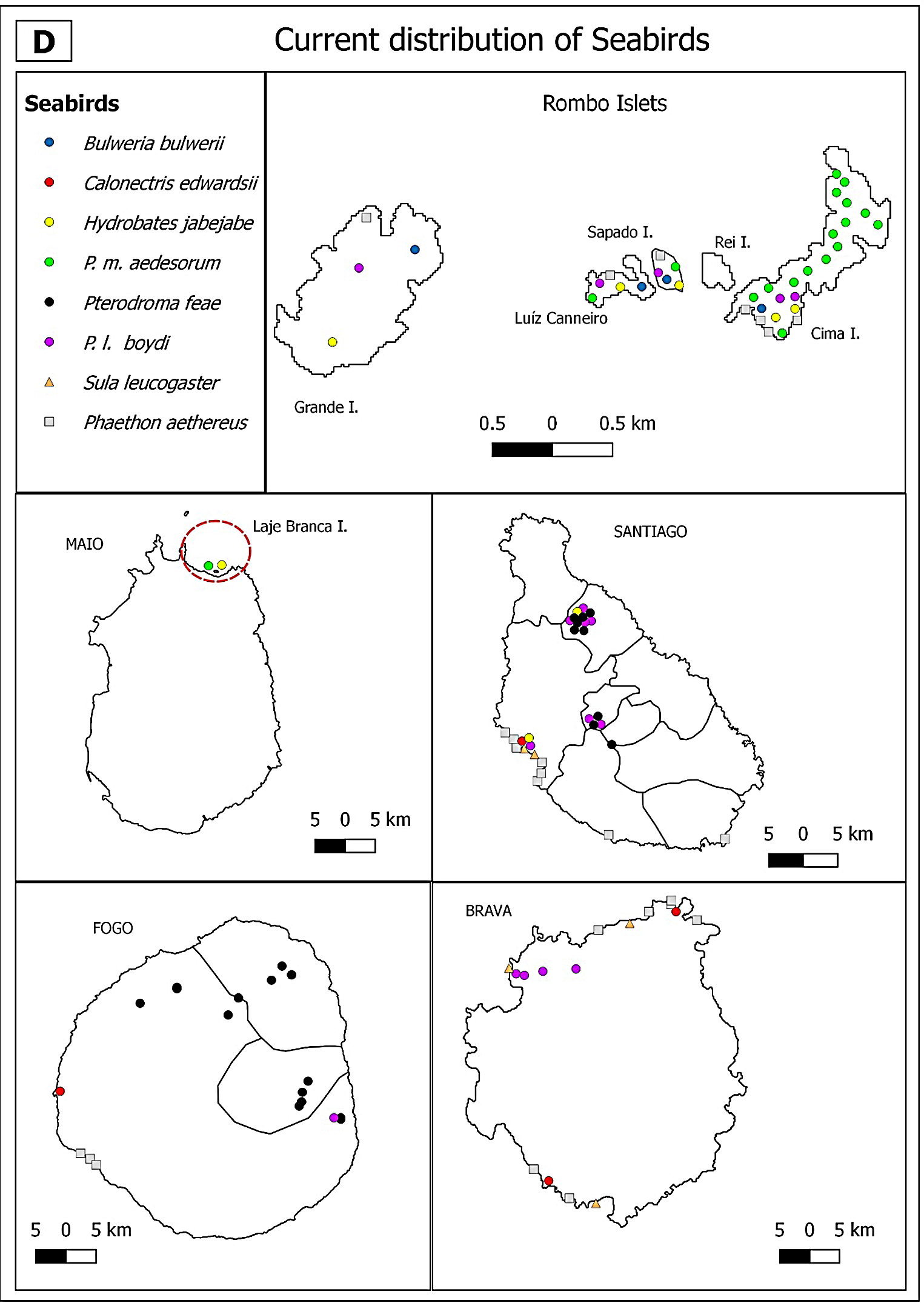
Figure 2D. Continued
First records of Cape Verde Shearwaters were reported for the Branco Islet by Milne-Edwards (Reference Milne-Edwards1883). Hazevoet (Reference Hazevoet1995) reported the occurrence of this species throughout the archipelago, except for Rombo Islets, Santa Luzia, Maio and São Vicente Islands (Figures 2A and 2B). In 1990 Hazevoet confirmed two colonies with breeding individuals in eastern São Nicolau Island (Ponta da Tapadinha and Fundo de Dagu) (Hazevoet Reference Hazevoet1995). However, our surveys conducted in 2017–2018 detected this species in São Vicente Island (Monte Verde) but not in São Nicolau Island (Figure 2C). In Boavista and Sal Islands one breeding location was confirmed in their nearby islets (Curral Velho and Rabo de Junco, respectively) by Naurois (Reference Naurois1969) and Hazevoet (Reference Hazevoet1994, Reference Hazevoet1995) (Figure 2A) and by our surveys (Figure 2C). In the middle of the 20th century the largest breeding colonies for the archipelago were on Raso and Branco Islets, and smaller breeding colonies on Brava and Santo Antão Islands (Naurois Reference Naurois1969) (Figures 2A and 2B). This was confirmed by our present surveys (Figures 2C and 2D). The species was also reported to occur on Fogo and Santiago (Baía do Inferno) Islands, though without confirmation of breeding.
There are previous records of Cape Verde Storm-petrels throughout the archipelago, except for Santa Luzia Island (Murphy Reference Murphy1924, Frade Reference Frade1976, Hazevoet Reference Hazevoet1994) (Figures 2A and 2B). Our surveys recorded Cape Verde Storm-petrel in Santo Antão, Santa Luzia, São Nicolau, Maio and Santiago Islands, and on Raso, Branco, Rabo de Junco (Sal), Curral Velho (Boavista) and Rombo Islets (Figures 2C and 2D). In Baía de Inferno (Santiago) several individuals were heard and in Laje Branca Islet (Maio) several birds were captured with mist-nets.
The White-faced Storm-petrel was recorded on Rombo (Cima), Branco, Pássaros (Boavista) and Laje Branca (Maio) Islets by Bourne (Reference Bourne1955) and Hazevoet (Reference Hazevoet1995) (Figures 2A and 2B). On Santa Luzia Island one specimen was collected by Murphy (Reference Murphy1924). Presently, nests were located on Branco, Pássaros (Boavista), Laje Branca (Maio) and Cima Islets (Figures 2C and 2D). This species may also occur on Sapado and Luiz Carneiro Islets, but more surveys are needed to confirm its presence.
The reproduction of Cape Verde Petrel on Santo Antão and São Nicolau Islands was confirmed by Leonardo Fea (in Salvadori Reference Salvadori1899) during the American expedition of "The Blossom" (in Murphy Reference Murphy1924). On Fogo and Santiago Islands, the presence of this species was already known through the records of inhabitants and Fea (Bourne Reference Bourne1955) (Figures 2A and 2B). Naurois (Reference Naurois1969) confirmed the breeding areas of this species and determined the altitude at which they occurred on São Nicolau (600 m) Fogo (2,200 m) and Santiago (400–800 m) islands. Our surveys indicate that currently this species occurs at different elevations: in Santo Antão (905–1,465 m), São Nicolau (603–708 m), Fogo (439–2,222 m) and Santiago (356–581 m) islands (Figures 2C and 2D).
Boyd’s Shearwater was reported as abundant on Raso, Branco, and Cima Islets by Correia (cited by Murphy Reference Murphy1924). On Santiago Island, the species was first recorded by Leonard Fea (in Salvadori Reference Salvadori1899) and confirmed by Naurois (Reference Naurois1969). On Curral Velho Islet (Boavista), the species was confirmed by Naurois in March 1968. This species was described as having a wider distribution at the archipelago, except for Maio and Luzia Islands (Naurois Reference Naurois1969) (Figures 2A and 2B). Presently, Boyd's Shearwater occurs in Santo Antão, São Nicolau, Sal (Furna and Cadjetinha), Santiago, Fogo and Brava Islands, Branco, Raso, Rabo de Junco (Sal) and Rombo Islets group (Figures 2C and 2D).
There are records of Brown Booby in 1786 for Fogo Island (Roque and Torrão Reference Roque and Torrão2013), and Bourne (Reference Bourne1955) and Naurois (Reference Naurois1969) refer to this species for Brava, Santiago, Maio and Boavista Islands, and Rombo and Raso Islets but considered it absent on Santa Luzia Island (Figures 2A and 2B). Hazevoet (Reference Hazevoet1995) refers to the possibility of reproduction in Santo Antão, São Vicente, Sal and Fogo Islands but did not provide details. During the 20th century several authors refer to the decline in the number of individuals on the Rombo Islets due to human predation (Hazevoet Reference Hazevoet1995). During our surveys Brown Booby was identified on Santiago (Baía do Inferno) and Brava islands, and Raso, Baluarte and Curral Velho Islets (Figs. 2C and 2D). On São Nicolau Island local fisherman mentioned a colony of Brown Booby, but this was not confirmed. Its current absence was also confirmed on Cima and Grande Islets (Rombo Islets group).
The earliest historical records of Red-billed Tropicbird are on Santiago Island (Keulemans Reference Keulemans1866). In the 20th century this species was reported on Branco, Raso, Rabo de Junco and Rombo (Cima and Grande) Islets, and for all Islands except Maio and Santa Luzia (Frade Reference Frade1976, Hazevoet Reference Hazevoet1995) (Figs. 2A and 2B). Our surveys identified Red-billed Tropicbird on Santo Antão, São Vicente, São Nicolau, Sal, Boavista, Santiago, Fogo and Brava Islands, and on the islets of Raso, Rabo de Junco (Sal), Curral Velho (Boavista) and all Rombo Islets. On the Island of Fogo, Red-billed Tropicbird colonies were identified in some cliffs south of São Filipe Island and on Pena Islet, also nearby São Filipe (Projecto Vitó NGO pers. comm.), but the population size was not estimated due to the difficult access. Several Red-billed Tropicbird populations were confirmed on Boavista Island, on Ponta do Sal, Ponta Rincão, Varandinha, Morro Negro and Ponta do Roque (López-Suárez Reference López-Suárez2012) and on Sal Island, in Serra Negra, Furna, Cadjetinha and Monte Leão. We also confirmed 50 individuals on Santo Antão Island between Tarrafal and Monte Trigo, in three groups (10, 20, 20), 35 individuals on a cliff in São Vicente Island between Baía do Mindelo and Farol de São Pedro, and less than 10 individuals in the southern part of São Nicolau Island. On Brava Island we confirmed four colonies, but in just one of those we could count 15 individuals in flight (Figures 2C and 2D).
Although the Magnificent Frigatebird is currently extinct in the Cabo Verde archipelago, in the past it occurred on Pássaros Islet (São Vicente), Santo Antão, São Nicolau (Keulemans Reference Keulemans1866) on Rabo de Junco Islet (Murphy Reference Murphy1924), Curral Velho and Baluarte Islets (Boavista) and Maio(Naurios Reference Naurois1969) (Figures 2A and 2B). The last successful breeding of the species in the archipelago occurred in 1998 (López-Suárez et al. Reference López-Suárez, Varo Cruz, Hazevoet and López Jurado2005). The species made several breeding attempts in the following years, with unhatched eggs. Two females and one male were sighted in 2015 and just two females the following year, the last record of the species in the archipelago (Pedrin López-Suárez pers. comm.).
Overall, Hazevoet (Reference Hazevoet1995) recorded a cumulative number of 52 breeding species on islands and islets of the archipelago (one species per Island/ Islet), and our study recorded a number of 57 species (considering the Rombo Islet as a sampling unit, similarly to the records of Naurois and Hazevoet). However, if we add each islet of the Rombo group separately (Cima, Grande, Luiz Carneiro, and Sapado) the cumulative number increases to 71 breeding species. We found no difference in the cumulative number of breeding seabird species per island and islet of Cabo Verde among 1969 (n = 55 species), 1995 (n = 52 species) and 2019 (n = 57 species; χ22 = 2.08; P = 0.83). We also found no significant difference between the number of seabird species currently occurring on islands (10 islands and an average of 2.9 species per island) and islets (13 islands and an average of 3.3 species per Islet; t 21 = -0.46; P = 0.64). There was no correlation between island size and number of species (r = 0.58; P = 0.07, n = 10). The two most mountainous islands of the archipelago, Santiago, and Santo Antão, possessed the largest number of seabird species (six and five, respectively), followed by São Nicolau, Fogo and Brava with four species on each island (Table 1). Cape Verde Petrel is the only species absent from islets.
Table 1. Current number of seabird species per Island/Islet in the Cape Verde archipelago. BB – Bulwer’s Petrel Bulweria bulwerii; CE – Cape Verde shearwater Calonectris edwardsii; HJ – Cape Verde storm-petrel Hydrobates jabejabe, PM – White-faced storm-petrel Pelagodroma marina aedesorum; PF – Cape Verde petrel Pterodroma feae; PB – Boyd´s shearwater Puffinus lherminieri boydi; SL – Brown booby Sula leucogaster and PA – Red-billed tropicbird Phaethon aethereus.

Relative abundance of Cape Verde Shearwater, Cape Verde Storm-petrel, Boyd's Shearwater and Cape Verde Petrel
Call surveys on the islands of the Barlavento group revealed that Cape Verde Shearwaters were relatively more abundant on Raso Islet followed by São Vicente, and Santo Antão Islands (mean nº of calls 5 min-1 = 14, 3 and 1, respectively; Figure 3A). There was an almost significant difference in median number of calls 5 min-1 among Raso Islet, Santo Antão, São Vicente, Santa Luzia and São Nicolau Islands (Kruskal-Wallis: H4 = 8.82; P = 0.066; n = 92).

Figure 3. Map of the relative abundance of (A) Cape Verde shearwater Calonectris edwardsii (CE), (B) Boyd’s shearwater Puffinus lherminieri boydi (PB) and (C) Cape Verde-storm petrels Hydrobates jabejabe (HJ) in the Santo Antão, São Vicente Islands and Branco and Raso Islets.
The largest density of Boyd's Shearwater was present on Raso, followed by Santo Antão and São Nicolau (mean nº of calls 5 min-1 = 19, 3 and 1, Figure 3B). The Kruskal-Wallis test showed a significant difference in the median number of calls 5 min-1 by Boyd's Shearwater among Santo Antão, São Vicente, São Nicolau, Santa Luzia Islands and Raso Islet (K-W: H4 = 39.5; P <0.001; n = 185). Post-hoc test showed that the relative abundance of Boyd's shearwater was higher on Raso Islet than on other islands (P < 0.001). Cape Verde Storm-petrel occurred in largest densities on Raso Islet, followed by São Nicolau, Santo Antão, and Santa Luzia Islands (mean nº of calls 5 min-1 = 72, 25, 10, 1, respectively; Figure 3C). The Kruskal-Wallis test showed a significant difference in the median number of calls 5 min-1 by Cape Verde Storm-petrel among Raso Islet, Santo Antão, São Vicente, São Nicolau and Santa Luzia Islands (K-W: H4 = 17.8; P = 0.001; n = 92). Post-hocs revealed that relative abundance of Cape Verde Storm-petrel was higher on Raso Islet than on Santo Antão, São Vicente and São Nicolau Islands (P < 0.01). Population relative density of Cape Verde Petrel was higher on Santo Antão, followed by São Nicolau, Fogo and Santiago Islands (mean nº of calls 5 min-1 = 22, 21, 18, 16, respectively; Figures 4A and 4B). The Kruskal-Wallis test showed a significant difference in the median number of calls 5 min-1 by Cape Verde Petrel among Santo Antão, São Nicolau, Santiago and Fogo Islands (K-W: H3 = 27.1 P < 0.001; n = 190), but the post-hoc test was unable to identify differences among islands.
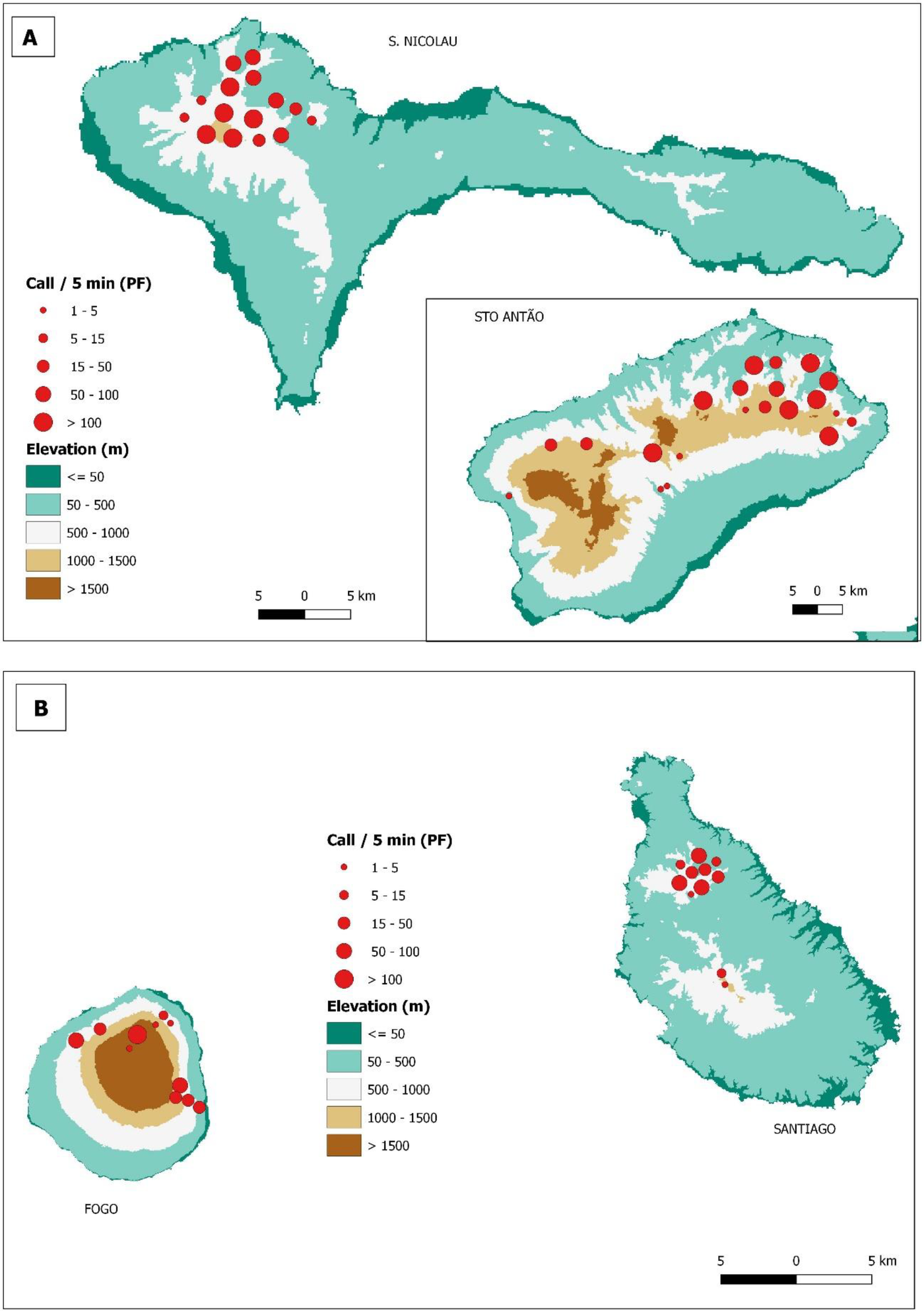
Figure 4. Map of the relative abundance of Cape Verde petrel Pterodroma feae (PF) in (A) Santo Antão and São Nicolau and (B) Santiago and Fogo Islands.
Habitat characteristics determining the seabird presence in Santo Antão and São Nicolau
As elevation and distance to coast decreased, Boyd’s Shearwater was 2.08 and 1.69 times more likely to occur, respectively. Cape Verde Petrel was 4.12, 3.01, and 2.05 times more likely to occur with increasing elevation, distance to coastline and distance to human settlements, respectively. Plus, this species was 3.87 times more likely to occur with decreasing distance to forest areas. Cape Verde shearwaters was 1.54, 1.69 and 1.84 times more likely to occur as elevation, distance to coastline and distance to roads decreases, respectively. As elevation and slope increased, Cape Verde Storm-petrel was 3.84 and 4.01 times more likely to occur, respectively (Table 2).
Table 2. General linear models (GLM) with binomial distribution, fitting the influence of habitat characteristics on the presence (1) or absence (0) of (a) Boyd’s shearwater Puffinus lherminieri boydi, (b) Cape Verde petrel Pterodroma feae, (c) Cape Verde shearwater Calonectris edwardsii and (d) Cape Verde-storm petrel Hydrobates jabejabe on Santo Antão and São Nicolau Islands. Mean values ± SD. OR, odds ratio. Significant differences (P < 0.05) marked in bold.
Discussion
Seabird distribution and abundance
Currently, the Cabo Verde archipelago holds populations of all seabird species referred to two centuries ago except the Magnificent Frigatebird, which has not been recorded in the Cabo Verde archipelago since February 2014 (López-Suárez et al. Reference López-Suárez, Hazevoet and Palma2012, Hazevoet Reference Hazevoet2014), and the compilation of our data points to its extinction in the archipelago. The initial decline of the Magnificent Frigatebird population in Cabo Verde was likely triggered by human persecution (Hazevoet Reference Hazevoet1994, Reference Hazevoet1995, López-Suárez et al. Reference López-Suárez, Varo Cruz, Hazevoet and López Jurado2005).
Our study reports a slightly higher number of seabird species for islets than for islands: the main seabird populations are located on Raso, Branco, Rabo de Junco and Rombo Islets. Branco and Raso are particularly important for seabird populations given the orographic characteristics of the islets, strong marine currents and large swells (Lopes et al. Reference Lopes, Ferreira, Rocha, Vasconcelos, Rocha, Afonso, Almeida, Lopes, Loura and Ferreira2015), and the absence of large freshwater springs, which prevented colonisation by humans (Gomes et al. Reference Gomes, Nevesa, Kenova, Campuzanoa and Pinto2015). The difficulty of landing on Branco and Raso kept these islets under less anthropogenic pressure and without exotic mammals such as rats and cats, unlike the nearby Santa Luzia Island, whose extensive beach areas allowed easier human landings and the expansion of cats and rats (Oliveira et al. Reference Oliveira, Oliveira, Melo, Melo and Geraldes2013). Vocalization data from the Barlavento group suggest that, as expected, populations are more abundant on Raso Islet (and possibly on Branco Islet, but there were insufficient data) than on the nearby islands of São Nicolau, São Vicente, and Santo Antão. Therefore, the lower anthropogenic pressure combined with the absence of mammalian predators on the islets, should be the most important factors to explain these results. Overall, introduced mammalian predators directly influence the abundance of seabird populations, may lead to breeding habitat modification and loss, and cause local extinction of species on islands and islets around the world (Nogales et al. Reference Nogales, Vidal, Medina, Bonnaud, Tershy, Campbell and Zavaleta2013, Xu et al. Reference Xu, Liu, Wu, Sun, Zhao and Chen2016). Seabird populations are present on all but one island of the Cabo Verde archipelago, Maio Island. A possible reason for this could be the impact of cows, goats, and sheep grazing freely on Maio Island since 1490 (Santos and Semedo Reference Santos and Semedo2006) causing habitat degradation. The other reason is that Maio Island presents a soft relief and large flat areas, similarly to the islands of Sal and Boavista (INE 2017). However, in addition to flat areas, both Sal and Boavista have more elevated and rocky coastal cliffs, which helps to explain why these islands also hold breeding populations of Red-billed Tropicbird and Boyd’s Shearwater.
As expected, larger species had a wider distribution along the archipelago, but small procellariformes such as the Cape Verde Storm-petrel occur on islands with rugged relief, mainly in mountainous sites and steep cliffs, where introduced cats and rats are less likely to be present. They also occur only on rat- and cat-free islets, except Santa Luzia Island (where mice and cats are present) and Rombo Islet (mice are present). Similar results were obtained elsewhere, notably in the Azores, with populations of Band-rumped Storm-petrel Hydrobates castro and Audubon's Shearwater Puffinus baroli present in rugged and inaccessible locations on several inhabited islands such as Flores and São Miguel (Monteiro et al. Reference Monteiro, Ramos, Pereira, Monteiro, Feio, Bearhop and Graz1999). The Cape Verde Petrel is confined to the mountainous islands of Santo Antão, São Nicolau, Santiago, and Fogo, as reported by Hazevoet (Reference Hazevoet1995) and Ratcliffe et al. (Reference Ratcliffe, Zino, Oliveira, Vasconcelos, Hazevoet, Neves, Monteiro and Zino2000). The White-faced Storm-petrel and Bulwer’s Petrel are the only species restricted to islets. The breeding distribution of White-faced Storm-petrel is limited by the availability of nesting habitat because it needs sand to build its nest (Tavares and Ratão Reference Tavares and Ratão2017). We could not evaluate the distribution of Bulwer’s Petrel effectively as it does not call in flight when arriving at the breeding sites (Monteiro et al. Reference Monteiro, Ramos, Pereira, Monteiro, Feio, Bearhop and Graz1999).
Currently, Brown Booby is absent in Santo Antão, São Vicente and São Nicolau islands and on Cima Islet but in the past, it occurred there in large numbers. For instance, Murphy (Reference Murphy1924) refers to them occurring in large numbers on Santa Luzia Island and thousands of individuals on Rombo Islets. Numbers were so large that the guano was used by the inhabitants of Brava in their plantations as well as exported to Lisbon and South America. We also did not detect Cape Verde Shearwater on São Nicolau Island, Cape Verde Storm-petrel on Pássaros Islet (Boavista) and Boyd’s Shearwater on Curral Velho Islet. The reasons for the apparent local extinction of these species in these islands and islets could be human capture and breeding habitat modification (Hazevoet Reference Hazevoet1995). Again, one century ago Murphy (Reference Murphy1924) refers to Cima as holding “thousands of Brown Booby on this island, although the fishermen slaughter great numbers for food” and to Raso Islet as being an islet where “great numbers of maritime birds come to breed, and the human visitors often kill more birds than fish”.
Habitat characteristics determining seabird occurrence on islands
As expected, Boyd’s Shearwater and Cape Verde Shearwaters were distributed on low elevation areas and closer to the coastline. These are some of the species more easily recognized by local people, especially on islands where relative densities were higher, like Santo Antão. Such habitat characteristics also drive the selection of nests of other burrowing breeders, such as the Sooty Shearwater Ardenna grisea from the South Pacific (Clark et al. Reference Clark, Matthiopoulos, Bonnet-Lebrun, Campioni, Catry, Marengo, Poncet and Wakefield2019). Moreover, in the Canary Islands, the related Macaronesian Shearwater Puffinus lherminieri baroli and Cory’s Shearwater Calonectris borealis are rescued in higher numbers, when compared to other seabirds, in areas close to the coastline and human settlements, after fledglings (mostly) are attracted by artificial lights (Rodriguéz et al. Reference Rodríguez, Rodríguez and Lucas2012). The lower relative abundances of both species when compared to Cape Verde Storm-petrel might be partially explained by a higher level of historical human predation of larger species occurring closer to human settlements/ activities. Cape Verde Petrel and Cape Verde Storm petrels were only detected at high elevation and remote areas (far from the coastline and roads). Higher elevation and remoter areas are also the characteristic habitat driving the occurrence of other gadfly petrels, like the endangered Trindade Petrel Pterodroma arminjoniana (Krüger et al. Reference Krüger, Paiva, Petry, Montene and Ramos2018) and mid-size petrels, like the South Georgia Diving-petrel Pelecanoides georgicus (Fisher et al. 2017). Overall, seabird populations were mostly confined to specific and restricted habitats, giving strength to the idea that historical and current human-based interference (e.g. capture, disturbance and introduction of alien predators) might have forced them to those remoter and mostly inaccessible areas (e.g. Probst et al. Reference Probst, Corre and Thébaud2000, Rayner et al. Reference Rayner, Hauber and Clout2007).
Threats to seabirds in Cabo Verde
The introduction of alien mammal species, such as cats, rats, mice and even dogs to the Cabo Verde Islands devastated historically large seabird populations. This has been reported for Santa Luzia (Naurois Reference Naurois1969, Oliveira et al. Reference Oliveira, Oliveira, Melo, Melo and Geraldes2013) and for Fogo Islands (Ratcliffe et al. Reference Ratcliffe, Zino, Oliveira, Vasconcelos, Hazevoet, Neves, Monteiro and Zino2000, Militão et al. Reference Militão, Dinis, Zang, Calabuig, Stefan and González-Solís2017) and Grande Islet (Murphy Reference Murphy1924). Habitat loss and modification due to anthropogenic actions was particularly important for Pássaros (São Vicente) and Sal Réi (Sal) Islets. Pássaros Islet harboured Magnificent Frigatebird and Brown Booby colonies in the 19th century (Keulemans Reference Keulemans1866), but after its use for military purposes and construction of the lighthouse (Hazevoet Reference Hazevoet1995), both seabird colonies were extirpated. In Sal Réi Islet the construction of the lighthouse may also explain the absence of seabirds. Historically, Santa Luzia Island had large colonies of several seabird species, but for two centuries only Cape Verde Storm-petrel still breeds there (Bourne Reference Bourne1955, Hazevoet Reference Hazevoet1995). It appears that the strong presence of mammal domestic herds during the 19th and until mid-20th centuries (Melo et al. Reference Melo, Melo, Cabral, Loura, Vasconcelos, Rocha, Afonso, Almeida, Lopes, Loura and Ferreira2015) led to habitat destruction on this 35-km2 island: in 1880 the presence of 100 cows, 600 goats, 350 sheep, 13 donkeys and 24 other domestic animals were reported here (Pina Reference Pina2010). On the other hand, the recent record of Cape Verde Storm-petrel in the steep cliff areas of Santa Luzia (Oliveira et al. Reference Oliveira, Oliveira, Melo, Melo and Geraldes2013) after two centuries without recording seabirds on this island, indicates that remnant seabird populations may persist in steep and remote locations. Similarly, Grande Islet, the largest of the Rombo Islets, housed large colonies of seabirds in the past, as indicated by the thick layers of guano (Murphy Reference Murphy1924). The presence of goats altered the natural habitat of this Islet. Presently, the loss of breeding habitat could be a major reason for the absence of breeding seabird populations on Pássaros Islet (São Vicente Island) and on Sal Rei Islet (Sal Island). Presently, on the most populated islands, seabirds are mainly found in areas almost inaccessible to humans such as Baía of Inferno (Santiago), Monte Pico of Antonia (Santiago), Monte Santinha (São Nicolau), and Bordeira (Fogo).
Droughts and famines have persisted in the Cabo Verde archipelago since its colonization, and several extreme events took place throughout the 17th, 18th and 19th centuries which decimated many domestic animals and people (Caniato Reference Caniato2006). Hunting of wild species, including seabirds, resulted from the island's low level of natural resources, cyclical drought and famine events that plagued the Cabo Verde archipelago (Naurois Reference Naurois1969). For the decline of seabird populations in the archipelago, predation of eggs and birds by the inhabitants was relevant, as reported by Murphy (Reference Murphy1924) and Hazevoet (Reference Hazevoet1995). During the two weeks that Murphy (Reference Murphy1924) remained on Raso Islet he noted the killing of 3,000 Cape Verde Shearwaters by fishermen. The slaughter of thousands of seabirds on this islet during the reproductive period was common until 2006/07, mainly by fishermen from Santo Antão, São Vicente, and São Nicolau Islands (Rendall and Pile Reference Rendall and Pile2007), when the NGO Biosfera began protection and monitoring campaigns (Melo Reference Melo2011). BirdLife International (2019) reports that populations of Pelecaniformes and Suliformes on the Rombo Islets has declined dramatically over the past 100 years due to excessive human predation. Murphy (Reference Murphy1924) refers to thousands of individuals inhabiting the islets in 1922, and in the early 1950s there were still many hundreds. Between 1986 and 1990 there were only 50 pairs of Brown Boobies and 5–10 pairs of Red-billed Tropicbirds (Hazevoet Reference Hazevoet1995) and currently there is no record of Brown Booby. On Cima Islet, we confirmed the capture of Red-billed Tropicbirds by humans and we found tools to capture other seabird species (e.g. Cape Verde Shearwater) in Curral Velho in 2019. Despite recent conservation efforts, there are still some recent reports of human predation and vandalism of Red-billed Tropicbird on Sal and Boavista Islands, of Cape Verde Petrel on Fogo and Santo Antão Islands (Ratcliffe et al. Reference Ratcliffe, Zino, Oliveira, Vasconcelos, Hazevoet, Neves, Monteiro and Zino2000), and of Cape Verde Shearwater and Brown Booby (Tosco Reference Tosco, Castro and Anderson2000, López-Suárez Reference López-Suárez2012).
During this study we recorded the predation of Cape Verde Shearwater chicks on Raso Islet and White-faced Storm-petrel on Cima Islet by ghost crab Ocypode cursor. Seabird predation by ghost crabs was historically reported by Murphy (Reference Murphy1924), who reports the species could spend all night predating on seabirds and arguing the crabs could subsist mostly on seabird flesh. Eggs of Boyd’s Shearwater and Bulwer’s Petrel were also scavenged by giant gecko Tarentola gigas on Raso and Branco Islets. In fact, Lopes et al. (Reference Lopes, Pinho, Santos, Seguro, Mata, Egeter and Vasconcelos2019) confirmed the presence of Cape Verde Shearwater, Bulwer’s Petrel and Red-billed Tropicbird DNA in the giant gecko faeces. The apparent predation of eggs and hatchlings of Boyd's Shearwater, Bulwer’s Petrel, Red-billed Tropicbird and Cape Verde Shearwater by Brown-necked Raven Corvus ruficollis and Neglected Kestrel Falco tinnunculus neglectus on Raso Islet have yet to be confirmed (Isabel Rodrigues pers. comm.). Osprey Pandion haliaetus on Raso Islet appears to prey on Boyd’s Shearwater, Bulwer’s Petrel and Cape Verde Shearwater, because remnants of these species were found in Osprey nests.
Volcanic eruptions can also cause habitat alteration for nesting seabird species on Fogo Island. Historical records of volcanic eruptions on the Fogo Island from 1500 to 2014 show that there were about 31 events (INE 2018). However, only for the 1995 eruption is there reference to the destruction of Fea´s Petrel breeding grounds (Hazevoet Reference Hazevoet1995). However, we do not have proof of nests being destroyed during the most recent eruption on the island (2014), though this might have happened in the past.
A new aspect of human interference is the introduction of electricity (and artificial light) into the remotest locations of Cabo Verde Islands. In 2017, about 90% of the resident population already had access to electricity (INE 2017). In the past, residents of the archipelago used to build bonfires to attract seabirds on their return to the colonies (Ratcliffe et al. Reference Ratcliffe, Zino, Oliveira, Vasconcelos, Hazevoet, Neves, Monteiro and Zino2000), as was done in the Azores archipelago (Monteiro et al. Reference Monteiro, Ramos and Furness1996). Light pollution affects seabird populations worldwide and may contribute to the effective decline of these populations attracted by street lighting (Le Corre Reference Le Corre2008, Rodríguez et al. Reference Rodríguez, García, Rodríguez, Cardona, Parpal and Pons2015a, Rodríguez et al. Reference Rodríguez, Rodríguez and Negro2015b). In Cabo Verde, seabirds disoriented by illumination can after landing be captured either by introduced mammals (e.g. cats) or by humans (Ratcliffe et al. Reference Ratcliffe, Zino, Oliveira, Vasconcelos, Hazevoet, Neves, Monteiro and Zino2000, African Bird Club 2020). Street lighting can also disrupt the trajectory of Cape Verde Petrel in its return to breeding colonies and may constrain the availability of breeding habitat (Militão et al. Reference Militão, Dinis, Zang, Calabuig, Stefan and González-Solís2017). Nowadays, seabirds in the archipelago continue to face the same threats reported in the past by Hazevoet (Reference Hazevoet1995), though human predation appears to have diminished. Predation by alien mammals is still noticeable, especially by rats and cats on Santa Luzia Island (Medina et al. Reference Medina, Oliveira, Geraldes, Melo and Barros2012, Oliveira et al. Reference Oliveira, Oliveira, Melo, Melo and Geraldes2013), cats on all islands where Cape Verde Petrel breeds and dogs on Sal Island (unpubl. data). Recently, green monkeys Chlorocebus sabaeus were introduced onto Santiago and Fogo not far from Cape Verde Petrel breeding colonies, which adds another threat to the list. Overall, further studies are needed to measure the impact of introduced invasive species on seabirds. In general, the current distribution and abundance of seabirds in the archipelago is the result of a combination of factors such as threats (human predation, habitat modification and introduction of invasive species) and habitat characteristics (elevation, distance to forest areas, coastline, human settlements and slope).
Further research
We are confident the historical data included in this work represents an exhaustive compilation of the available knowledge on the past seabird distribution in Cabo Verde. Nevertheless, mapping the current distribution and abundance needs (1) further surveys and counts of nocturnal calls in flight on Sal, Boavista, Santiago, Fogo, Maio, and Brava islands, to confirm whether the abundance patterns found for the Northern Islands are similar to those on the Southern Islands; (2) deployment of automatic recorder units (ARUs) on islands/locations where higher relative abundances were reported, to calculate abundances of the different seabird species from vocalization rates; (3) deployment of ARUs on areas less prospected but with habitat characteristics suitable for the occurrence of seabird species (e.g. steeper cliffs); (4) further detailed prospections of breeding areas and nest counts on all islets and islands; (5) population estimates by capture-mark-recapture methods, in particular for small species such as storm-petrels, whose nests are difficult to find or count.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0959270920000428.
Acknowledgements
This work received financial and logistic support from the project Alcyon – Conservation of seabirds from Cabo Verde, coordinated by BirdLife International and funded by the MAVA Foundation pour la nature (MAVA17022; http://en.mava-foundation.org), through its strategic plan for West Africa (2017-2022). Gilson Semedo acknowledges the support given by the “Fundação para a Ciência e Tecnologia” (FCT) and “Instituto Gulbenkian de Ciência” (IGC) (Portugal, SFRH/BD/135266/2017). We are deeply thankful for the data on seabird occurrence provided by Biosfera (São Vicente Island), Project Biodiversity (Sal Island), Natura 2000 (Boavista Island), Bios CV (Boavista Island), Lantuna (Santiago Island) and Projecto Vitó (Fogo Island). This study benefitted also from funding by the strategic program of MARE, financed by FCT (UID/MAR/04292/2020), through national funds.











