Introduction
Depression is highly prevalent in Parkinson's disease (PD) with estimates ranging between 50 (Reijnders et al., Reference Reijnders, Ehrt, Weber, Aarsland and Leentjens2008) and 75% (Menza et al., Reference Menza, Dobkin, Marin, Mark, Gara and Buyske2009). Depression in PD is associated with faster motor and cognitive decline (Starkstein et al. Reference Starkstein, Mayberg, Preziosi, Andrezejewski, Leiguarda and Robinson1992) and higher mortality (Hughes et al., Reference Hughes, Ross, Mindham and Spokes2004). In addition to higher rates of depression, the prevalence of anxiety disorders in PD has been estimated to range from 25 to 45% (Leentjens et al., Reference Leentjens, Dujardin, Marsh, Martinez-Martin, Richard and Starkstein2008) and the comorbidity of anxiety and depression in PD is very high (Simuni and Fernandez, Reference Simuni, Fernandez, Pfeiffer and Bodis-Wollner2013).
Despite the high prevalence of depression in PD, only 10‒20% of sufferers receive treatment, which is typically anti-depressant treatment and may have limited efficacy for PD patients (Dyduch and Załuska, Reference Dyduch and Załuska2015; Frisina et al., Reference Frisina, Borod, Foldi and Tenenbaum2008). In a meta-analysis that included randomized placebo controlled treatments for depression and anxiety in PD, we found a non-significant moderate effect size for anti-depressants (d = .71) and a significant large effect size (d = 1.57) for cognitive behaviour therapy (CBT) (Troeung et al., Reference Troeung, Egan and Gasson2013). Only two randomized controlled trials (RCTs) of CBT for depression in PD have been conducted to date (Dobkin et al., Reference Dobkin, Menza, Allen, Gara, Mark and Tiu2011; Troeung et al., Reference Troeung, Egan and Gasson2014), which have showed significant decreases in anxiety and depression. However, recruitment for one of these trials was difficult, which is a common issue in PD populations and suggests potential barriers to seeking psychological treatment in the community (Troeung et al., Reference Troeung, Egan and Gasson2014). One difficulty experienced in our CBT group trial (Troeung et al., Reference Troeung, Egan and Gasson2014) was the reading and writing involved, which can be difficult for participants due to the motor symptoms of PD such as tremor, muscle stiffness, and slow movement.
Another behavioural treatment for PD which involves less written strategies than previous CBT protocols for PD (Troeung et al., Reference Troeung, Egan and Gasson2014) is mindfulness-based cognitive therapy (MBCT). This intervention consists of eight weekly group sessions plus daily homework practice aimed at developing mindfulness, and present-focused, non-judgemental awareness of experience (Segal et al., Reference Segal, Williams and Teasdale2012). MBCT includes guided body scan meditations where participants focus on sensations in different parts of their bodies, walking and seated breathing meditations, gentle stretching, and guidance in cognitive decentring as a means of reducing rumination and worry.
Mindfulness-based interventions, including MBCT and its parent intervention mindfulness-based stress reduction (MBSR), have demonstrated efficacy in reducing symptoms of anxiety and depression in general and clinical populations (Gotink et al., Reference Gotink, Chu, Busschbach, Benson, Fricchione and Hunink2015; Hofmann et al., Reference Hofmann, Sawyer, Witt and Oh2010). MBCT was designed to treat recurrent depression and its efficacy in preventing depressive relapse (Piet and Hougaard, Reference Piet and Hougaard2011; Teasdale et al., Reference Teasdale, Segal and Ridgeway2000) has led to it being included in the NICE guidelines for treating recurrent depression (Pilling et al., Reference Pilling, Anderson, Goldberg, Meader and Taylor2009). MBCT aims to reduce unhelpful perseverative cognition (e.g. rumination, worry) by training attention, and research aiming to dismantle components of mindfulness shows that rumination and mindfulness are mediators of clinical outcomes after MBCT (Alsubaie et al., Reference Alsubaie, Abbott, Dunn, Dickens, Keil, Henley and Kuyken2017; Gu et al., Reference Gu, Strauss, Bond and Cavanagh2015). Thus, it is proposed that MBCT has the potential for people with PD to reduce depression associated with rumination (e.g. dwelling on memories of good motor functioning) and anxiety associated with worry (e.g. worrying about motor limitations and concerns over increasing impairment). This rationale is strengthened by extensive evidence that mindfulness-based interventions have efficacy in treating psychological distress associated with chronic health conditions (Bohlmeijer et al., Reference Bohlmeijer, Prenger, Taal and Cuijpers2010) including multiple sclerosis, another movement disorder (Grossman et al., Reference Grossman, Kappos, Gensicke, D'Souza, Mohr, Penner and Steiner2010).
Despite this rationale for using mindfulness interventions, and MBCT in particular, to treat depressive symptoms in PD, there have been few studies to date examining their efficacy for psychological and motor symptoms associated with PD (Advocat et al., Reference Advocat, Enticott, Vandenberg, Hassed, Hester and Grant2016; Cash et al., Reference Cash, Ekouevi, Kilbourn and Lageman2016; Dissanayaka et al., Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016; Pickut et al., Reference Pickut, Vanneste, Hirsch, Van Hecke, Kerckhofs and Mariën2015). Pickut et al. (Reference Pickut, Vanneste, Hirsch, Van Hecke, Kerckhofs and Mariën2015) randomly assigned 27 people with PD either to an 8-week MBSR group intervention (n = 14), or usual care (n = 13). There was a significant decrease of 20% found from pre- to post-treatment on motor symptoms in the intervention group whilst the control group did not demonstrate significant changes. The study did not report on depressive symptoms or psychological disorders, so it is difficult to assess whether there were any participants in the trial with clinical levels of depression or diagnosed psychopathology. Furthermore, the intervention studied (MBSR) has not been designed explicitly to treat depressive symptoms like MBCT has, which actively targets depressive rumination (Segal et al., Reference Segal, Williams and Teasdale2012). In another trial (Dissanayaka et al., Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016), 14 participants with PD received a 6-session modified MBCT over 8 weeks. At post-treatment, there was a significant reduction in anxiety, depression and some common symptoms of PD including stiffness, physical discomfort, tremors, postural instability, and gait dysfunction. Cash et al. (Reference Cash, Ekouevi, Kilbourn and Lageman2016) conducted an uncontrolled trial of MBSR in 29 participants with PD and found significant reductions in depression and improvements in cognitive functioning; however, similar to the Dissanayaka et al. (Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016) study given the lack of control group it is difficult to draw conclusions about efficacy. Advocat and colleagues (Reference Advocat, Enticott, Vandenberg, Hassed, Hester and Grant2016) conducted a randomized controlled trial examining a 6-week MBSR with 57 adults with PD. There were no significant improvements between the MBSR and waitlist groups post-treatment; however, at 6-month follow-up in the mindfulness group there were significant improvements in activities of daily living, mindfulness, health behaviours and stress, potentially indicating a delayed effect of treatment. Despite some of the promise of these initial studies, there is a need for further research to determine the efficacy of mindfulness in PD due to inconclusiveness in the effects of mindfulness-based therapies in PD. Furthermore, while one study has indicated the potential benefits of MBCT in PD (Dissanayaka et al., Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016) it was uncontrolled and although another qualitative study reported participants’ perceived benefits of MBCT (Fitzpatrick et al., Reference Fitzpatrick, Simpson and Smith2010), to date there have been no controlled trials examining MBCT in PD. A recent systematic review echoes this call for further research into mindfulness-based therapies for PD (McLean et al., Reference McLean, Lawrence, Simpson and Mercer2017).
The primary aim of this study was therefore to provide proof-of-concept for a modified MBCT as a treatment for symptoms of anxiety and depression in PD, indicated by significantly greater reductions in anxious and depressive symptoms in the modified MBCT group at post-treatment compared with the waitlist control group. The secondary aim was to examine changes in quality of life. It was hypothesized that, after treatment, participants receiving modified MBCT would have significantly lower anxiety and depression and significantly higher quality of life than waitlist participants.
Method
Trial design
The study was an 8-week, non-blinded, controlled efficacy study for anxiety, depression and quality of life in PD. The research was a registered with the Australian New Zealand Clinical Trials Registry (ACTRN12614000287639) and followed CONSORT guidelines (Schulz et al., Reference Schulz, Altman and Moher2010). Participants were randomized to modified MBCT or a waitlist. Modified MBCT consisted of six group sessions across 8 weeks, with each session lasting for 2 h. Participants randomized to the waitlist received telephone calls every month to assess for depression and suicidality. An a priori power analysis was conducted using G*Power 3.1.8 (Faul et al., Reference Faul, Erdfelder, Lang and Buchner2007). At an alpha of .05, 18 participants per group (a total of 36 participants) were required in order to ensure an 80% chance of detecting a ‘moderate’ (f = .20) interaction between group and time (see Supplementary Material for details).
Participants
Individuals with idiopathic PD were recruited through advertising in Perth, Western Australia, via Parkinson's Western Australia and a local radio station. Inclusion criteria were: (1) meeting the Queen Square Brain bank criteria for idiopathic PD, which is Parkinson's with no identifiable cause (Hughes et al., Reference Hughes, Daniel, Kilford and Lees1992), (2) age 18‒90 years, (3) providing written informed consent, and (4) being able to access transport to attend the treatment. Exclusion criteria were: (1) receiving psychotherapy, (2) cognitive impairment, indicated by scoring below 24 on the Mini-Mental State Examination (MMSE; Folstein et al., Reference Folstein, Folstein and McHugh1975), or (3) having active suicidality using the NHMRC risk assessment guidelines (National Health and Medical Research Council, 2014). Thirty-six eligible participants were randomized to the intervention and waitlist control groups over a 2-month period using computerized block randomization.
Measures
Data were gathered by structured interviews and scales administered by a clinical psychology trainee (S.R.), trained by experienced clinical psychologists (S.E. and R.A.). Pre-randomization screening included the following instruments: (1) Mini-Mental State Examination (Folstein et al., Reference Folstein, Folstein and McHugh1975), to screen for cognitive impairment, (2) Structured Clinical Interview for the DSM-IV (SCID-IV; First et al., Reference First, Spitzer, Gibbon and Williams2002), to assess the presence of affective and anxiety disorders, to screen for substance use and other complex psychiatric disorders (i.e. psychosis), and to assess suicidality, and (3) Movement Disorders Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS; Martinez-Martin et al., Reference Martinez-Martin, Rodriguez-Blazquez, Alvarez-Sanchez, Arakaki, Bergareche-Yarza and Chade2013) to determine diagnosis of PD, which was administered by a neurologist (S.S.) with extensive experience in PD assessment.
Primary efficacy outcomes
To measure the primary outcome of depression, the Geriatric Depression Scale-15 (GDS-15; Sheikh and Yesavage, Reference Sheikh, Yesavage and Brink1986) was used, which is a 15-item self-report measure with high internal consistency (Ertan et al., Reference Ertan, Ertan, Kızıltan and Uyguçgil2005) that was designed to screen for depressive symptoms in older adults and has also been used with PD patients (Weintraub and Stern, Reference Weintraub and Stern2007). Internal consistency in this study was α = .77. To measure the primary outcome of anxiety, the Geriatric Anxiety Inventory (GAI; Pachana and Byrne, Reference Pachana and Byrne2012) was administered, which is a 20-item self-report scale assessing the severity of anxiety disorders (generalized anxiety disorder, simple phobias and panic disorder). This measure has been validated for use in PD (Pachana and Byrne, Reference Pachana and Byrne2012). The internal consistency in this study was α = .87.
Secondary efficacy outcomes
Secondary outcome measures were administered at baseline and post-treatment by a clinical psychology trainee (S.R.). To provide an additional measure of depression, anxiety and stress, the Depression, Anxiety Stress Scale-21 (DASS-21; Lovibond and Lovibond, Reference Lovibond and Lovibond1995) was administered weekly by a clinical psychology trainee (S.R.) during the 8-week treatment. The DASS-21 had an internal consistency of .76 and demonstrates sound construct validity with the DASS-42 for use in PD (Johnson et al., Reference Johnson, Lawrence, Corti, Booth, Gasson and Thomas2016). The internal consistency for the total score in this study was high (α = .90). The Parkinson's Disease Questionnaire-39 (PDQ-39; Peto et al., Reference Peto, Jenkinson, Fitzpatrick and Greenhall1995) is a 39-item self-report scale that was used to examine health-related quality of life (QOL), with lower scores indicating higher quality of life. The scale has good reliability and validity for use in PD (Tan et al., Reference Tan, Luo, Nazri, Li and Thumboo2004). The internal consistency in this study was α = .94.
Statistical methods
A series of generalized linear mixed models (GLMMs) were tested in order to determine whether the intervention group changed on the outcome measures relative to the control group. The GLMMs were implemented through SPSS (version 22) GENLINMIXED procedure and there was one nominal random effect (participant), one nominal fixed effect (group: intervention versus control), one ordinal fixed effect (time: pre-test versus post-test), and Group × Time interaction. The GLMM ‘robust statistics’ option was examined to assess for violations of normality and homogeneity of variance. Violations of sphericity were addressed by changing the covariance matrix from the default of compound symmetry to autoregressive. To optimize the possibility of convergence, a separate GLMM analysis was conducted for each outcome. A significant Group × Time interaction was predicted for all outcomes and Bonferroni adjusted alpha-levels were used.
Description of intervention
MBCT is an 8-week intervention designed to treat recurrent depression, but also has efficacy in reducing anxiety and stress associated with chronic illness (Segal et al., Reference Segal, Williams and Teasdale2012). The protocol used in this trial was developed and initially evaluated by Dissanayaka et al. (Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016), and consisted of a modified MBCT protocol consisting of six group sessions of 2 h duration each, across 8 weeks. Dissanayka et al. (Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016) adapted a brief 3-week MBCT program (Harnett et al., Reference Harnett, Whittingham, Puhakka, Hodges, Spry and Dob2010) which was a condensed version of regular 8-week MBCT (Segal et al., Reference Segal, Williams and Teasdale2002). The protocol used in this study targeted skills such as cultivating present-focused non-judgemental awareness of thoughts, emotions and bodily sensations through guided meditation practice and informal mindfulness exercises that involve bringing mindfulness awareness to everyday activities. The program also included instruction on how thinking patterns maintain depression and anxiety, and how to avoid rumination and worry by non-judgementally observing moment-by-moment experience. The intervention protocol (Dissanayaka et al., Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016) was ‘tailor-made’ for people with PD by taking into consideration aspects of the illness that may impact participation and outcomes (e.g. motor symptoms, increasing memory problems). For example, walking meditation was omitted to accommodate motor difficulties, the language and pace of instructions was adapted to accommodate cognitive difficulties, while the metaphors and examples used in didactic exercises were tailored to symptoms and cognitions common in PD (for further details of the protocol, see Dissanayaka et al., Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016). A set of CDs (Williams, Reference Williams2011) which guide MBCT practice were given to participants to engage in regular mindfulness exercises.
A workbook containing the weekly handouts was provided to each participant. This contained material covered in the weekly session for personal practice at home. Weekly homework tasks were set for participants, which required them to engage in formal meditation based on breathing exercises, and informal meditation based on focusing attention on routine daily tasks. Completion of homework was reviewed in subsequent sessions to assess adherence and guide group discussion around overcoming obstacles.
The modified MBCT intervention was conducted by two clinical psychology trainees (S.R. and independent co-facilitator) who first participated in an MBCT course and were trained in the delivery of the modified MBCT program by experienced clinical psychologists (S.E. and R.A.). The therapists were new to the delivery of MBCT and were supervised weekly by a clinical psychologist (R.A.) with extensive training and experience in delivery and supervision of MBCT. The waitlist control group received a monthly telephone call or email to ensure they remained part of the study, and to check for suicidality using the Australia National Health and Medical Research Council (National Health and Medical Research Council, 2014) risk assessment guidelines.
Results
Participant flow
Of the 38 participants screened for inclusion, two were referred for individual therapy due to the severity of their depression and resulting risk of suicidality. The remaining 36 participants were randomized to the intervention and waitlist control groups. Three participants dropped out of treatment (all three were unable to participate due to other commitments) and six dropped out of the waitlist control (25% dropout), leaving a sample of 27 participants (15 treatment, 12 control; see Fig. 1).
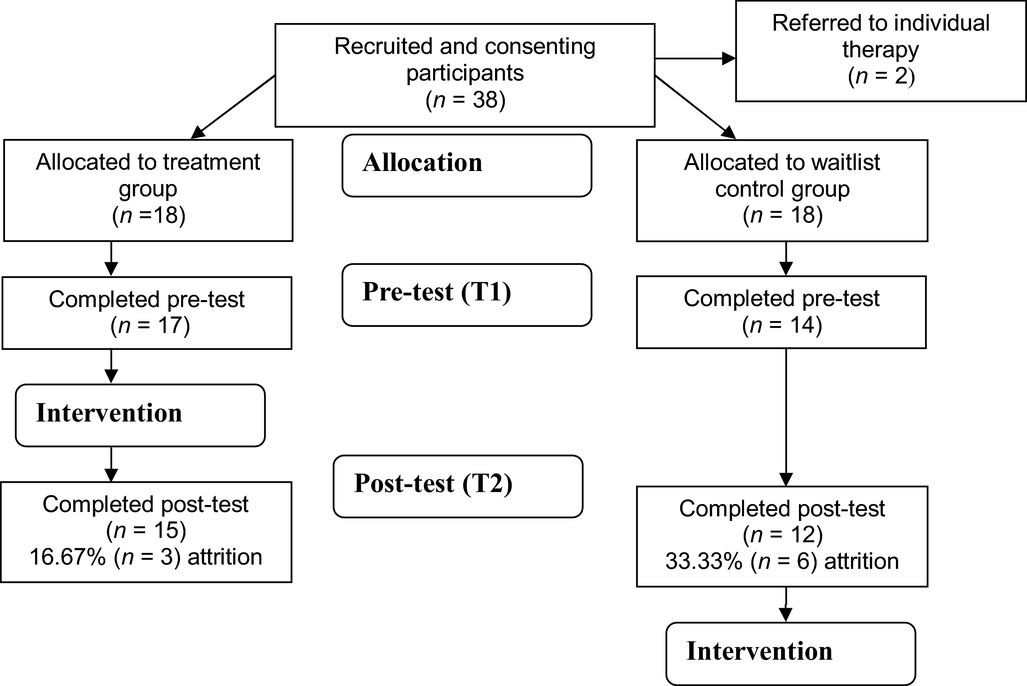
Figure 1. CONSORT (Schulz et al., Reference Schulz, Altman and Moher2010) diagram of participants’ movement during stages of the randomized control trial indicating attrition rates at each stage.
Based on the SCID (Spitzer et al., 1992), at intake 22% of the sample met diagnostic criteria for an affective disorder (18% major depressive disorder, 4% dysthymic disorder) and 19% of the total sample met diagnostic criteria for an anxiety disorder (11% social phobia, 4% post-traumatic stress disorder, 4% generalised anxiety disorder). In the total sample, 4% met diagnostic criteria for both major depressive disorder and social phobia.
Baseline data
Participants were between 40 and 77 years of age (mean = 63.70, SD = 8.76), and 55% were male. In the treatment group there were 67% males, and 60% of the treatment group were married. In the control group there were 58% females, and 91% of the control group were married. All participants in the control and treatment group had a disease severity of greater than 1 on the Hoehn and Yahr, MDS-UPDRS (Martinez-Martin et al., Reference Martinez-Martin, Rodriguez-Blazquez, Alvarez-Sanchez, Arakaki, Bergareche-Yarza and Chade2013), meaning that all participants had at least bilateral or midline disease involvement. There were no significant baseline differences between the intervention and waitlist control group in female/male ratio [χ² (1, N = 27) = 1.69, p = .194], disease severity [χ² (1, N = 26) = .872, p = .647], marital status, or any of the outcome measures [χ² (1, N = 27) = 4.25, p = .235] (see Tables 1 and 2).
Table 1. Statistical group comparison of baseline means

No significant differences between baseline treatment and control group means were found on any of the outcome measures using t-tests. GAI, Geriatric Anxiety Inventory; GDS-15, Geriatric Depression Scale-15; PDQ-39, Parkinson's Disease Questionnaire-39; DASS-21 Depression, Depression Anxiety Stress-Scales-21 (DASS-21) depression subscale; DASS-Anxiety, Depression Anxiety Stress-Scales-21 (DASS-21) anxiety subscale.
Table 2. Descriptive data (adjusted means, SD and n values) comparing treatment and control conditions at all measurement points on each outcome

By convention, item scores were summed within participants; these sums were than averaged across participants to produce a mean outcome score. DASS_Dep, Depression Anxiety Stress-Scales-21 (DASS-21) depression subscale; DASS_Anx, Depression Anxiety Stress-Scales-21 (DASS-21) anxiety subscale; GAI, Geriatric Anxiety Inventory; GDS-15, Geriatric Depression Scale-15; PDQ-39, Parkinson's Disease Questionnaire-39.
Intervention effects
As shown in Table 3, there was a significant Group × Time interaction for DASS-21-Depression (F [1,50] = 23.20, p < .001, partial η2 = .0.32). Least significant difference (LSD) tests showed a significant pre‒post reduction in DASS-21 Depression for the treatment group (t (50) = 7.071, p < .001, Cohen's d = 0.96), but no significant change for the control group (t (50) = 1.86, p = 0.071, Cohen's d = 0.06). There were no significant Group × Time interactions for the GAI (F [1,50] = 3.53, p = .066, partial η2 = 0.07), the GDS-15 (F [1,50] = 2.65, p = .110, partial η2 = 0.05), the PDQ-39 (F [1,48] = 0.50, p = .482, partial η2 = 0.01), or the DASS-21Anxiety (F [1,50] = 0.69, p = .411, partial η2 = 0.01).
Table 3. Results of the omnibus maximum likelihood mixed effects linear regressions for each outcome
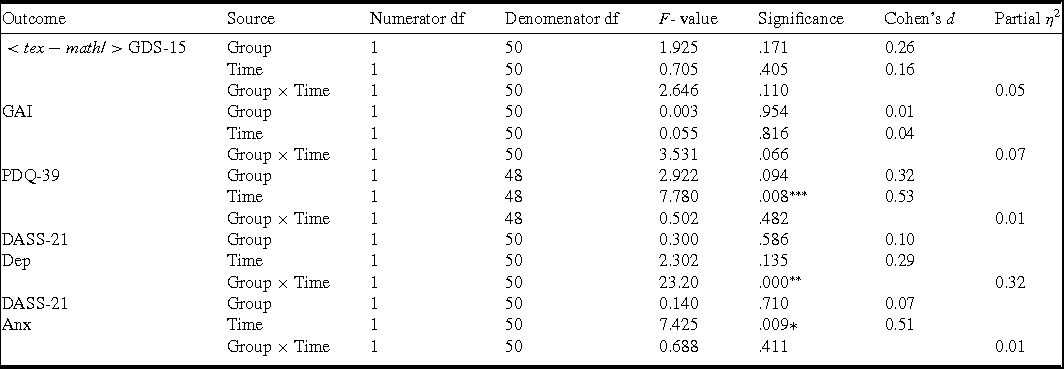
*p < Bonferroni correct alpha-level of .025 (anxiety outcomes), **p < Bonferroni correct alpha-level of .025 (depression outcomes), ***p < .01; GDS-15, Geriatric Depression Scale-15; GAI, Geriatric Anxiety Inventory; PDQ-39, Parkinson's Disease Questionnaire-39; DASS-21 Dep, Depression Anxiety Stress-Scales-21 (DASS-21) depression subscale; DASS-Anx, Depression Anxiety Stress-Scales-21 (DASS-21) anxiety subscale.
Reliable change
Reliable change analysis (Jacobson and Truax, Reference Jacobson and Truax1991) was conducted to determine whether the proportions of people improving, deteriorating, or remaining stable on the DASS-21 depression subscale and GAI differed between the two groups. The proportions are reported in Table 4. For DASS-21 depression, the proportion of participants who improved was significantly higher in the intervention group, and the proportion of participants who deteriorated was significantly higher in the control group (chi-square [1] = 5.49, p = .019). Similarly, for GAI, the proportion of participants who improved was significantly higher in the intervention group, and the proportion of participants who deteriorated was significantly higher in the control group (chi-square [1] = 8.56, p = .003).
Table 4. Reliable change at post-intervention for participants in the intervention and waitlist-control groups
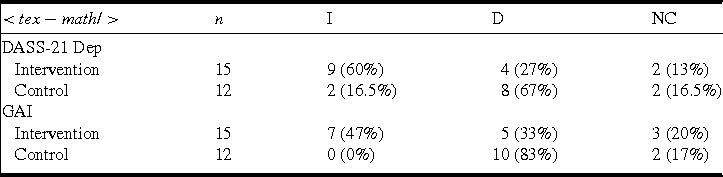
I, improved; D, deteriorated; NC, no change; DASS-21 Dep, Depression Anxiety Stress-Scales-21 (DASS-21) depression subscale; GAI, Geriatric Anxiety Inventory.
Discussion
This proof-of-concept trial aimed to test the efficacy of modified MBCT for reducing psychological distress in people with PD. Our hypothesis that it would decrease symptoms of depression was partially supported. The modified MBCT program, adapted for PD, produced significant reductions in DASS-21 depression scores at both the group level (as demonstrated by the GLMM analysis) and at the individual level (as demonstrated by the reliable change analysis) compared with the control group. Although this was a mixed sample with relatively low levels of baseline depression, we observed a large effect size for modified MBCT in reducing depressive symptoms on the DASS-21. These results should be interpreted cautiously as the same effect was not observed on the other measure of depression, the GDS-15. However, as the GDS-15 is predominantly a screening tool, it is possible that it was more prone to floor effects in this less distressed sample. Providing further support for our primary hypothesis, clinical significance testing showed that participants’ depressive symptoms were more likely to improve if they received modified MBCT and more likely to deteriorate if they received no treatment. Overall, these findings provide preliminary evidence that mindfulness-based therapies may be a useful intervention for depressive symptoms in PD, in line with previous research (Dissanayaka et al., Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016; Pickut et al., Reference Pickut, Vanneste, Hirsch, Van Hecke, Kerckhofs and Mariën2015).
The predictions that there would be a decrease in anxiety and increase in quality of life with modified MBCT were largely unsupported, and in contrast to previous studies which have found significant reductions in anxiety (Dissanayaka et al., Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016). At the group level, there was no significant decrease in anxiety symptoms in the treatment group compared with the control group. This finding may be related to the low level of anxiety at baseline for both groups and that despite some efficacy in reducing anxiety in other populations, MBCT was primarily developed to target cognitive and behavioural processes underlying depressive symptomology (Segal et al., Reference Segal, Williams and Teasdale2012). Indeed, evidence suggests that MBSR, as opposed to MBCT, may better target anxiety and quality of life in people with chronic health conditions (Bohlmeijer et al., Reference Bohlmeijer, Prenger, Taal and Cuijpers2010; Hofmann et al., Reference Hofmann, Sawyer, Witt and Oh2010; Vøllestad et al., Reference Vøllestad, Sivertsen and Nielsen2011). Despite this null finding at the group level, clinical significance testing showed that participants’ anxiety was more likely to improve if they did modified MBCT and more likely to deteriorate if they received no treatment. These inconsistent findings probably highlight not only the floor effects mentioned above but also low power for group statistics in this pilot study due to the small sample. A further reason for the lack of effects on anxiety may also be due to the treatment being modified MBCT, where treatment was shorter, being 6 weeks rather than 8 weeks as in the regular MBCT protocol (Segal et al., Reference Segal, Williams and Teasdale2002, Reference Segal, Williams and Teasdale2012), with no day retreat, resulting in a lower dose of mindfulness than in regular MBCT.
Despite the potential to dilute group effects, we decided to include participants with PD regardless of the presence of clinical diagnoses of depression or anxiety to explore whether modified MBCT could have an effect for participants who did not have a clinical diagnosis but had symptoms of depression and anxiety. Therefore, our sample consisted of participants with a wide range of depression and anxiety scores, with 37% of the total sample having clinical anxiety, and 33% clinical depression at baseline according to the DASS-21. Although we are unable to determine whether modified MBCT had a prevention effect on anxiety and depression as this would require a long follow-up period and a larger number of participants, it is of interest that for the total sample those who did not receive the intervention showed higher scores on depression and anxiety at post-treatment compared with the modified MBCT group. It would be useful for future research to examine if MBCT can prevent the onset of clinical diagnoses of depression, and potentially anxiety, in individuals with PD. This would be consistent with the original rationale behind MBCT, which is to prevent new episodes of depression by targeting rumination, a core cognitive process that maintains depression (Segal et al., Reference Segal, Williams and Teasdale2012).
Several limitations of the study should be pointed out. First, due to drop-out the study was under-powered. Second, the inclusion criteria were broad and did not limit co-morbidities that could potentially influence outcomes (e.g. co-morbid acquired brain injury). Less than half the sample had an anxiety or mood disorder and average symptom severity was low, which limits the generalizability of these results to clinical populations. Furthermore, we only assessed outcome at post-treatment and follow-up assessment at 6 or 12 months post-treatment is required to assess the efficacy of the intervention. Future research should examine the effects of the intervention on measures of mindfulness, as in the present study it was unclear whether the intervention successfully increased mindfulness, the most common mediator of MBCT outcomes (Alsubaie et al., Reference Alsubaie, Abbott, Dunn, Dickens, Keil, Henley and Kuyken2017). Finally, it is important to note that the study had a small sample size and future research should be conducted with a larger sample. However, it is encouraging that the modified MBCT intervention was still associated with a decrease in depressive symptoms despite this.
Limitations notwithstanding, the results are promising as a pilot, proof-of-concept study, and provide impetus for further research, as indicated by the significant reduction in depressive symptoms in the intervention group. Future research using qualitative methods would be useful to further explore participant experiences and inform treatment refinement. Future quantitative research should examine the efficacy of modified MBCT in a larger sample of individuals with PD who are a ‘pure’ clinical sample where all participants have diagnoses of depression and/or anxiety. It would also be useful to examine if a regular MBCT (Segal et al., Reference Segal, Williams and Teasdale2012) confers increased efficacy compared with the shorter, modified version used in this study. MBCT should also be compared with an active treatment such as CBT. Finally, mediation studies should be conducted to test putative MBCT mechanisms such as increasing mindfulness and decreasing rumination and worry (Alsubaie et al., Reference Alsubaie, Abbott, Dunn, Dickens, Keil, Henley and Kuyken2017).
In summary, our findings suggest that modified MBCT holds promise as a potential intervention for depressive symptoms in PD, whilst it is uncertain whether it impacts anxiety and quality of life for people with PD. This treatment appears to be an acceptable intervention for people with PD and may help them to better manage the psychological impact of this challenging disease.
Acknowledgments
The authors thank Dr Nadeeka Dissanayaka, Professor Nancy Pachana and colleagues for providing access to their mindfulness for Parkinson's disease protocol (Dissanayaka et al., Reference Dissanayaka, Idu Jion, Pachana, O'Sullivan, Marsh, Byrne and Harnett2016).
Conflicts of interest: The authors have no conflicts of interest with respect to this publication.
Ethical statements: The authors abided by the Ethical Principles of Psychologists and Code of Conduct as set out by the APA (http://www.apa.org/ethics/code/). Ethics approval for the study was granted by the Curtin University Human Research Ethics Committee, approval number HR 32/2014.
Financial support: We received funding for this study from Parkinson's Western Australia, the School of Psychology, Curtin University, and the Raine Foundation for Medical Research.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S135246581800070X








Comments
No Comments have been published for this article.