He said “What's time? Now is for dogs and apes! Man has Forever!”
— Robert Browning, (Reference Browning1896) A Grammarian's Funeral, p. 425.1. Introduction
Time travel may never be physically possible (Holden Reference Holden2005). For now at least, humans can travel in time only in their minds. Mental time travel is a term we coined to refer to the faculty that allows humans to mentally project themselves backwards in time to re-live, or forwards to pre-live, events (Suddendorf & Corballis Reference Suddendorf and Corballis1997). Past and future travels share phenomenological characteristics and activate similar parts of the brain. Mentally reliving past events is also known as episodic memory in the literature and has been the topic of intense research efforts (e.g., Tulving Reference Tulving1984; Reference Tulving, Terrace and Metcalfe2005). By contrast, mental construction of potential future episodes has only very recently begun to draw attention. Nevertheless, there is growing recognition that mental time travel into the past and future are related, and that the ultimate evolutionary advantage must lie with the capacity to access the future (Dudai & Carruthers Reference Dudai and Carruthers2005a; Suddendorf & Busby Reference Suddendorf and Busby2003b; Reference Suddendorf and Busby2005; Suddendorf & Corballis Reference Suddendorf and Corballis1997; Tulving Reference Tulving, Terrace and Metcalfe2005). Though we may often get it wrong, humans have in general been extraordinarily successful in foreseeing, planning, and shaping the future, and indeed allowing us to influence the earth itself in extraordinary but not always benevolent ways (Dawkins Reference Dawkins2000).
Since present behavior can increase or decrease an individual's future survival chances, one might expect many species to have evolved anticipatory capacities. The world is dynamic, and organisms that can pick up on significant regularities (e.g., fluctuations in food availability) and act in tune with them (e.g., being in the right place at the right time) have an advantage over those that do not. Many organisms actively influence their own futures by creating an environment that suits their needs (so-called niche-construction, Odling-Smee et al. Reference Odling-Smee, Laland and Feldman2003), such as when a beaver dams a stream. However, future-oriented mechanisms vary in flexibility, and relatively inflexible mechanisms will often suffice.
Through natural selection, some species have evolved behavioral predispositions to exploit significant long-term regularities (e.g., seasonal variations). A hibernator, for example, may hoard food for an impending winter even if individually it has never experienced a winter. Such instinctual future-directed behavior serves well, as long as the environmental pattern persists. But even long-term regularities may at times change drastically (e.g., climate change), and organisms fixed to a pattern that no longer prevails are disadvantaged relative to those that have more flexibility. This can be achieved by individual fine-tuning mechanisms, such as critical periods for parameter setting, imprinting, and other forms of learning. Indeed, learning and memory in general may be regarded as future-oriented adaptations that allow an individual, rather than a population, to adjust to local change and track short-term regularities.
Here we propose a taxonomy of how memory systems differ in what they provide for the future. We argue that mental time travel is the most flexible of those memory-based systems, and the most recently evolved. We then review evidence for mental time travel in nonhuman animals, and suggest a framework that identifies subsidiary mechanisms of mental time travel that nonhuman species may or may not possess.
First, though, it is useful to distinguish perceptual systems that detect and track relevant information, from action systems that control behavior itself. Many animal species, as well as human neurological patients and children, show dissociations between what they know in the perceptual domain and what knowledge they can use to control action (Hauser Reference Hauser2003; Sterelny Reference Sterelny2003). Perceptual systems differ in the robustness of their tracking (e.g., use of single versus multiple perceptual channels to track significant aspects of the environment) and storage of information (e.g., knowing what is currently where). Action systems differ in the flexibility or response breadth they provide (Sterelny Reference Sterelny2003); for example, they may include relatively narrow options such as hibernating or storing food, or highly flexible options like cooking and preserving food in diverse ways. Organisms may have sophisticated mechanisms to track temporal information and yet inflexible action systems, and vice versa. Further, the link between the two can be direct, or bottom-up, when perception of a stimulus triggers a response, or they can be top-down, mediated by internal representations (i.e., declarative memory). Top-down mediation offers the opportunity for representations decoupled from the immediate input to drive action flexibly and independently.
2. A taxonomy of future-oriented cognition
Figure 1 shows a widely accepted taxonomy of human memory systems (e.g., Miyashita Reference Miyashita2004; Squire Reference Squire1992), and illustrates how this can serve as a basis for a parallel taxonomy of adaptation to the future.
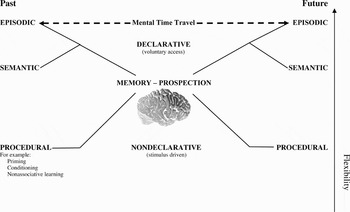
Figure 1. Memory and prospection systems. The common taxonomy of memory systems (left), after Squire (Reference Squire1992), and its proposed prospective counterpart (right).
Non-declarative or implicit memory systems are so called because, in humans, their content cannot be declared or verbalized (Tulving Reference Tulving1985). They allow stimulus-driven prediction of regularities. For example, through association, a conditioned stimulus (e.g., a sound) predicts the future arrival of an unconditioned stimulus (e.g., food) and triggers a future-directed response (e.g., salivation). In operant conditioning a behavioral response predicts a certain outcome (reward). Learning theory has described how organisms use associations to predict the near future, and a growing literature is mapping its neurophysiological basis (e.g., O'Doherty Reference O'Doherty2004; Schultz Reference Schultz2006). Non-associative changes in behavior, such as habituation, also can be understood in terms of expectations (e.g., that the situation stays unchanged). All these non-declarative memory systems allow behavior to be modulated by experience such that the organism gains a future advantage. We may call the resultant future-directed mechanisms “procedural” because flexibility extends only to learning to respond to current indicators of upcoming events. The behavior is stimulus-bound, or better, bound to the perceptual tracking of stimuli.
Declarative or explicit memories provide greater flexibility because they can also be voluntarily triggered top-down from the frontal lobes, rather than bottom-up through perception (Miyashita Reference Miyashita2004). They may be regarded as decoupled representations that are no longer directly tied to the perceptual system. In humans, these memories are conscious and at least partly verbalizable (Tulving Reference Tulving1985; Reference Tulving, Terrace and Metcalfe2005). Declarative memory can be subdivided into semantic memory and episodic memory. Semantic memory contains general knowledge, allowing learning in one context to be voluntarily transferred to another. This capacity provides the basis for inferential and analogical reasoning. Semantic memory may thus enable semantic prospection that is voluntary and not stimulus-bound. Nevertheless, such prospection is restricted in that it builds on a knowledge base that is impervious to particularities of the learning event itself. It is the second component of declarative memory, namely, episodic memory, that gives rise to the notion of mental time travel.
2.1. Toward a definition of mental time travel
Episodic memory, in contrast to semantic memory, provides access to the personally experienced event, rather than just the knowledge extracted from the event. The phenomenological experience of remembering, or what Tulving (Reference Tulving1985) calls autonoetic or self-knowing consciousness, such as recollecting where and when one learned that Wellington is the capital of New Zealand, is different from merely knowing that fact. This distinction is supported by experimental manipulations and imaging studies, and by dissociations in impairments following brain injury (Gardiner et al. Reference Gardiner, Ramponi and Richardson-Klavehn2002; Henson et al. Reference Henson, Rugg, Shallice, Josephs and Dolan1999; Klein et al. Reference Klein, Loftus and Kihlstrom2002b; Tulving Reference Tulving, Terrace and Metcalfe2005). Thus, amnesic patients, such as K.C., may know facts (e.g., the difference between stalagmites and stalactites) and procedures (e.g., how to play chess), without being able to recall a single personally experienced event leading to knowing them (Tulving Reference Tulving, Terrace and Metcalfe2005). Episodic memory is not about regularities, but about reconstructing particularities of specific events that have happened to the individual.
In effect, then, episodic memory implies a mental reconstruction of some earlier event, including at least some of the particularities of that event, such as the principal characters involved, the actions that took place, the setting, and the emotional reactions. Metaphorically speaking, it might be regarded as the result of a mental journey into the past. This idea is readily extended to the future. Based on previous experiences, we can imagine specific events in the future, including the sorts of particularities that have characterized events in the past. Mental time travel into the future might include the planning of some specific event, such as a dinner party, or it might involve the mental anticipation of some event that we know to be scheduled for some future date, such as a job interview. Again, though, there is a distinction between merely knowing that some event will occur, such as that the sun will set, and mentally creating an event, such as a sunset actually experienced, with gradual fading of light, and the blue flash on the horizon as the last image of the sun disappears.
The mental reconstruction of past events and construction of future ones may have been responsible for the concept of time itself, and the understanding of a continuity between past and future. Having a concept of time allows us to understand that past and future are on the same dimension, and what was the future eventually becomes the past (that is, unless the universe comes to an end). Mental time travel allows us to imagine events at different points along this continuum, even at points prior to birth or after death. This means that mental time travel is a generative process, incorporating known elements but arranged in particular ways to create the experience of events that are actually occurring. Even episodic memory may not be a faithful recreation of a past event. False memories have been widely documented (e.g., Loftus & Ketcham Reference Loftus and Ketcham1994) and are readily created in the laboratory (e.g., Roediger & McDermott Reference Roediger and McDermott1995). This means that mental time travel cannot be defined in terms of the veracity of the content. We know what mental time travel is because we can introspectively observe ourselves doing it and because people spend so much time talking about their recollections and anticipations.
A major challenge, though, is to establish a definition, or set of criteria, that might identify mental time travel in nonhuman animals that cannot express their experiences in words. This problem is akin to the decades-long search for behavioral criteria for theory of mind (see sect. 4.3) in nonhuman animals (Heyes Reference Heyes1998; Povinelli & Vonk Reference Povinelli and Vonk2003; Premack & Woodruff Reference Premack and Woodruff1978; Suddendorf & Whiten Reference Suddendorf, Whiten, Sterelny and Fitness2003; Tomasello et al. Reference Tomasello, Carpenter, Call, Behne and Moll2005; Whiten & Byrne Reference Whiten and Byrne1988). Tulving (Reference Tulving, Tulving and Donaldson1972) originally defined episodic memory in terms of the kind of information it appears to store: namely, what happened where and when. This is the so-called www criterion; that is, demonstrated understanding of what, where, and when might be considered sufficient evidence that an imagined event is an instance of mental time travel. For example, if an animal that caches food can be considered to demonstrate understanding of what was cached, where it was cached, and when it was cached, then it has episodic memory for the caching event itself. Similarly, if its behavior indicates specific knowledge of a future event, such as what food it will retrieve, where it will find it, and when it will retrieve it, then it may be said to have travelled mentally into the future to a retrieval event itself. However, although useful, the www criterion is neither necessary nor sufficient for mental time travel. Personal experience, as well as research on false memories, show that one can mentally revisit an event without accurate information as to what, where, and when, and conversely one can know what, where, and when something happened (e.g., one's birth) without remembering the event itself (Suddendorf & Busby Reference Suddendorf and Busby2003b). Thus, Tulving changed his definition to emphasize the phenomenological experience (autonoetic consciousness) of episodic retrieval (Tulving Reference Tulving1985; Reference Tulving, Terrace and Metcalfe2005), and www memory in animals has been more cautiously termed “episodic-like memory” (Clayton & Dickinson Reference Clayton and Dickinson1998).
Phenomenological experience, though, is a private mental capacity, and one may rightfully wonder if one could ever know of another organism's mental world. It is conceivable that neurophysiological criteria may eventually suffice, but there is as yet no agreement as to the conditions defining phenomenological awareness, even in humans. Nevertheless, for mental time travel to have evolved, there must have been something natural selection could work on, that is, some effect on survival or reproduction (unless it was merely an accidental side effect of some other adaptation). The answer we propose is that mental time travel provides increased behavioral flexibility to act in the present to increase future survival chances (Suddendorf & Busby Reference Suddendorf and Busby2003b; Reference Suddendorf and Busby2005). For example, one might prepare for a forthcoming job interview based on past experiences of interviews, and on imagining the questions one may be asked and on one's possible replies to them.
If this argument is correct, then the crux of mental time travel lies in its role in enhancing biological fitness in the future, so that mental time travel into the past is subsidiary to our ability to imagine future scenarios. Episodic memory may also inform semantic prospection by, for example, providing boundary conditions for the scope of generalizations. Indeed, memories for particular contradicting episodes are primed when people retrieve semantic generalizations (Klein et al. Reference Klein, Cosmides, Tooby and Chance2002a). But we argue that the primary role of mental time travel into the past is to provide raw material from which to construct and imagine possible futures. In establishing whether a given behavior is indicative of mental time travel, then, we should consider application of that behavior to the future rather than to the past (Suddendorf & Busby Reference Suddendorf and Busby2005). We therefore advance the following description of conditions that, if met, may entitle us to invoke the construct of mental time travel to explain it.
Mental time travel is evident in voluntary behavior that solves a problem that the organism will encounter at a future point in time, where “future” entails that the problem is not already manifest (as when acting to satisfy a current hunger, for example). To ascertain that a given behavior was driven by mental time travel, it is necessary to rule out chance, innate predispositions, procedural and semantic prospection, or any combination of these. We postulate that the crucial selective advantage mental time travel provides is flexibility in novel situations and the versatility to develop and adopt strategic long-term plans to suit individual selected goals. Thus, paradigms that use transfer tests and cross different domains would be strongest in making cases for mental time travel. Such tests provide evidence for mental time travel into the past only by virtue of the evidence linking past and future mental time travel, to which we turn next.
2.2. Evidence for continuity of past and future mental time travel
Since past is fact and future is fiction, common sense might suggest that different cognitive mechanisms underlie recollection of past events and construction of future ones. There is a fundamental causal asymmetry, and one simply cannot know the future as one knows the past. However, various lines of evidence suggest that mental time travel into the past shares cognitive resources with mental construction of potential future episodes (Suddendorf & Corballis Reference Suddendorf and Corballis1997). Normal adults report a decrease in phenomenological richness of both past and future episodes with increased distance from the present (D'Argembeau & Van der Linden Reference D'Argembeau and Van der Linden2004). The temporal distribution of past events people envisage follows the same power function as the temporal distribution of anticipated future events (Spreng & Levine Reference Spreng and Levine2006). Amnesic patients who are unable to answer simple questions about yesterday's events have been found to be equally unable to say what might happen tomorrow (Klein et al. Reference Klein, Loftus and Kihlstrom2002b; Tulving Reference Tulving1985), and it is not until around age 4 that children are able to accurately answer both such questions (Busby & Suddendorf Reference Busby and Suddendorf2005). Patients with depression who have trouble retrieving specific memories from their past also have trouble imagining specific future episodes (Williams et al. Reference Williams, Ellis, Tyers, Healy, Rose and MacLeod1996). Finally, brain imaging has shown that both remembering the past and imagining the future are associated with frontal and temporal lobe activity, although there are specific areas in the frontal pole and medial temporal lobes that are more involved with the future than with the past (Okuda et al. Reference Okuda, Fujii, Ohtake, Tsukiura, Tanji, Suzuki, Kawashima, Fukuda, Itoh and Yamadori2003).
The constructions of future and past episodes both depend in part on semantic memory, since one must construct events that are consistent with one's general knowledge of the world. Nevertheless, it is the episodic component that provides the particularities that can fit one's future plans precisely to the occasion – the seating arrangement, the clothes one might wear, the menu, the topics of conversation. In allowing us to foresee unique events, mental time travel offers the ultimate step in adaptation to the future. Like human language, it is open-ended and generative, so there is no end to the number of potential future scenarios one might envisage.
Although episodic memory preserves something of the particularities of individual events, it is often unreliable and subject to distortion, as we have seen. In cases of amnesia, moreover, it is episodic memories that are the most vulnerable (Wheeler et al. Reference Wheeler, Stuss and Tulving1997). The fact that episodic memory is fragmentary and fragile suggests that its adaptiveness may derive less from its role as an accurate record of personal history than from providing a “vocabulary” from which to construct planned future events (and perhaps to embellish events of the past). It may be part of a more general toolbox that allowed us to escape from the present and develop foresight (Suddendorf & Corballis Reference Suddendorf and Corballis1997), and perhaps create a sense of personal identity (Schacter Reference Schacter1996). Indeed, our ability to revisit the past may be only a design feature of our ability to conceive of the future (Suddendorf & Busby Reference Suddendorf and Busby2003b).
2.3. Neurophysiological evidence
We have suggested that neurophysiological evidence may eventually serve as a proxy for the phenomenology of mental time travel. There is increasing evidence concerning the neurophysiology of mental time travel in humans, but little from nonhuman animals. Some of this evidence suggests, in fact, that mental time travel may be uniquely human, since the brain areas involved have apparently undergone changes that are not evident in other primates. For example, the key to mental time travel may lie in part in the expansion of the brain, and the prefrontal cortex in particular, in human evolution. Brain size varies between species partly as a function of body size, and an appropriate comparative measure is the encephalization quotient (EQ), devised by Jerison (Reference Jerison1973), which is based on the regression of brain weight on body weight. The human EQ is about three times that of the chimpanzee. Further, it has been claimed that the increase is larger in the prefrontal cortex, known to be critically involved in episodic memory (see review by Wheeler et al. Reference Wheeler, Stuss and Tulving1997) than in other brain areas (Deacon Reference Deacon1997). Although this disproportionate increase has been disputed (e.g., Semendeferi et al. Reference Semendeferi, Damasio and Frank1997; Uylings Reference Uylings1990), Deacon (Reference Deacon1997) argues that other studies have failed to measure prefrontal cortex independently of motor and premotor areas. A more recent study confirms the disproportionate enlargement of the prefrontal cortex in humans, but indicates that it is restricted to white matter, and does not apply to grey matter (Schoenemann et al. Reference Schoenemann, Sheenan and Glotzer2005).
Regardless of the question of size, there is also evidence that the prefrontal cortex has been substantially reorganized in the course of human evolution. For example, Area 13, which appears to be a subdivision of Area 11, is only about half that expected on the basis of brain size, and Semendeferi et al. (Reference Semendeferi, Armstrong, Schleicher, Zilles and van Hoesen1998) suggest that this diminution occurs because other regions of Area 11, along with regions of Area 47, have been enlarged to accommodate a large number of specialized subdivisions. The frontal pole is also considerably enlarged relative to that in apes, especially in the right hemisphere (Semendeferi et al. Reference Semendeferi, Armstrong, Schleicher, Zilles and van Hoesen2001). Summarizing this evidence, Flinn et al. (Reference Flinn, Geary and Ward2005) suggest that these changes to Area 11 and the right prefrontal cortex appear to be involved in “self awareness, social problem solving, the ability to recall personal experiences, and the ability to project oneself into the future” (pp. 30–31).
The involvement of the prefrontal cortex may depend on neural loops that also encompass the basal ganglia and perhaps other regions as well. In one functional magnetic resonance imaging (fMRI) study, for example, brain activity was recorded while human subjects learned actions in a Markov decision task that would bring either immediate or future rewards (Tanaka et al. Reference Tanaka, Doya, Okada, Ueda, Okamoto and Yamawaki2004). In both cases significant activity was seen in the lateral orbitofrontal cortex and striatum, but for actions concerning future rewards, there was additional activation in the dorsolateral prefrontal cortex, inferior parietal cortex, dorsal raphe nucleus, and cerebellum. Regression analysis also suggested graded maps of time scale within the insula and striatum, with ventroanterior regions involved in predicting immediate rewards and dorsoposterior regions in predicting future rewards. In another study involving appetitive conditioning in humans, fMRI recordings showed activity in the orbitofrontal cortex and striatum to be dependent on the time between a present conditioned stimulus and an anticipated reward (O'Doherty et al. Reference O'Doherty, Deichmann, Critchley and Dolan2002; see also O'Doherty Reference O'Doherty2004).
3. Mental time travel in nonhuman animals?
Nonhuman species commonly display behaviors that depend on non-declarative future-oriented systems, and there is strong evidence that some of them can also deploy semantic memory for future-directed action. For example, the chimpanzee Panzee declared where food was hidden outside her enclosure by touching a lexigram denoting the food and directing a naïve human to it by pointing to its location (Menzel Reference Menzel, Terrace and Metcalfe2005). Although this shows that she knew where the food was, it does not prove that she remembered the hiding event itself, just as one can know where the car keys are without remembering the event of putting them there. The “linguistic” outputs of trained apes may demonstrate declarative (i.e., semantic) memory, such as the knowledge of which symbols go with which objects or actions, but they do not include reports of travels down memory lane. They have not provided evidence of mental time travel. There is so far no use of tense, nor any sense that the animals are telling stories about previous or anticipated episodes. In marked contrast, human conversation is replete with references to past and planned future episodes (e.g., Szagun Reference Szagun1978).
3.1. Mental time travel into the past
Despite our earlier reservations, the www criterion for episodic memory has featured prominently in recent comparative research. Though one might expect to find precursors of mental time travel in our closest living relatives, the most vigorously argued case has come, not from apes, but from birds that cache food in various locations and later retrieve it. Scrub jays can select food locations not only according to the type of food, but also according to how long it has been stored. For example, they will recover recently cached worms in preference to nuts, since fresh worms are more palatable; but if the worms have been cached for too long, they will retrieve nuts, because the worms will have decayed and become unpalatable (Clayton et al. Reference Clayton, Bussey and Dickinson2003). Scrub jays seem to know what has been cached, where it was cached, and when it was cached, thus apparently meeting the www criterion. As noted earlier, however, investigators have used the term “episodic-like memory” in acknowledgment that the www criterion need not imply true episodic memory, in part because there need not be any implications about autonoetic consciousness (Clayton et al. Reference Clayton, Bussey and Dickinson2003; Emery & Clayton Reference Emery and Clayton2004).
When humans talk about past events, they typically exchange information about even more “w”s, such as “who did what to whom, and when, and where, and why, and what happened next” (Suddendorf & Corballis Reference Suddendorf and Corballis1997, p. 159). Recent evidence indicates that jays may indeed also store information about who observes them cache (Dally et al. Reference Dally, Emery and Clayton2006b). The birds were more likely to move the food to new locations if a more dominant bird observed the caching than if a less dominant bird did so. The outcome of this re-caching behavior was presumably a reduction in the likelihood of the food being pilfered in future.
This innovative research program on scrub jays has inspired similar work on other species, with varying success (see Table 1 for a summary). Rhesus monkeys, for example, seemed unable to learn that a preferred food was available only after a short interval, but not after a long one (Hampton et al. Reference Hampton, Hampstead and Murray2005), whereas rats were able to learn that a particular arm of a maze contained chocolate pellets after a long delay but not after a short delay (Babb & Crystal Reference Babb and Crystal2005).
Table 1. Recent studies suggesting knowledge of different “w” information in various species that form part of “episodic-like” memory

The cases listed in Table 1 explicitly link their results to one of the “w”s of episodic-like memory. Numerous earlier studies might be included when re-categorized in line with this interpretation (see, e.g., Schwartz & Evans Reference Schwartz and Evans2001). No doubt this table will be expanded and altered further as more comparative research is conducted with the explicit aim of uncovering evidence for episodic-like memory. We believe that this approach may provide one of the first areas in which research systematically maps the types of mental content in different species. Although we look forward to a more comprehensive table, we remain unconvinced that any of these cases shows true mental time travel (Hampton & Schwartz Reference Hampton and Schwartz2004; Roberts Reference Roberts2002; Suddendorf & Busby Reference Suddendorf and Busby2003b).
Suddendorf and Busby (Reference Suddendorf and Busby2003b) put forward various reasons why the data from jays and other species are not yet conclusive. In the present article, we have noted earlier the distinction between the type of information stored and the experience of mentally revisiting an episode that led Tulving to change his definition of episodic memory. The information listed in Table 1 may be known rather than remembered. There is a difference between remembering having put something somewhere, some time ago, and simply knowing right now what is where, and how fresh it is. In fact, episodic-like memory may result from feed-forward mechanisms that need not be about the past at all. Episode A may cause cognitive change B, which may in turn affect later behavior C, without B carrying any information about A itself (Dretske Reference Dretske1982). For example, performance that depends on time since an event may rely on the strengths of memory traces, which fade in time, and may therefore provide a direct cue without implying mental travel through time. Trace strength or some other time-dependent process may act as a clock to determine a “use-by” date for consumption.
One argument against this specific idea that www memory may depend on trace strength is that rats, at least, still show the effects of trace strength in the accuracy of recognition after hippocampal lesions, yet lose the ability to discriminate temporal order (Eichenbaum et al. Reference Eichenbaum, Fortin, Ergorul, Wright and Agster2005), a finding that is also consistent with the hippocampus being a primary site for episodic memory in humans. Even so, the hippocampus may be involved in access to trace strength as a discriminative cue, so failure to make temporal discriminations after hippocampal damage need not rule out the trace-strength hypothesis. Whether or not this specific hypothesis is correct, it should be clear that there are viable alternatives to the notion that evidence for www, or even wwww, memories is evidence for mental time travel. Indeed, even the original proponents of this approach concede that these memories may exist without the jays mentally reconstructing the past (Dally et al. Reference Dally, Emery and Clayton2006b).
A few researchers have tried to tackle the question with different sets of innovative methodologies (Zentall Reference Zentall2006). For example Zentall et al. (Reference Zentall, Clement, Bhatt and Allen2001) taught pigeons to press one or the other of two new keys depending on whether they had previously responded or not responded on another earlier key – which suggests an ability to refer to a previous behavioral episode. Similarly, dolphins appear to remember their own previous behavior, as shown by an ability to respond to commands equivalent to sentences like “do something not recently done,” or “repeat the most recent response” (Mercado et al. Reference Mercado, Murray, Uyeyama, Pack and Herman1998). Rats show evidence for temporal order. When exposed to sequences of four different odors in distinct locations, rats later showed above-chance performance on tests in which they were to choose which of two odors or locations had occurred earlier in the sequence (Eichenbaum et al. Reference Eichenbaum, Fortin, Ergorul, Wright and Agster2005). However, all of these approaches run into problems of interpretation similar to those that afflict the “www” approach. As we suggested earlier here and elsewhere (Suddendorf & Busby Reference Suddendorf and Busby2005), the solution may be to focus on future-directed behavior instead of on episodic memory. We argue that it is action with the future in mind that provides increased selective advantage and is hence visible to evolution. If we are right, it might also be visible to clever researchers (if nonhuman animals have it).
3.2. Mental time travel into the future
Animals meet significant recurrent problems such as seasonal food shortages in different ways (e.g., through migration, torpor, increased fat storage). Caching food is a solution that has evolved among some mammals and birds that provide for their young (Smith & Reichman Reference Smith and Reichman1984). This strategy rests on some memory capacities and suggests orientation to future consumption, but it need not imply that the animal actually envisages that future, or even explicitly plans for it. For example, one study showed that rats continued to cache food in locations in a maze where food was later repeatedly degraded or pilfered, even though they avoided those locations in retrieving the food (McKenzie et al. Reference McKenzie, Bird and Roberts2005). This suggests that they had little sense of what would happen in the future. Similarly, young scrub jays vigorously cache all kinds of objects well before they develop the behavior of retrieving them. When caching and retrieval develop in such predictable fashion, it appears to be based on instinctive or implicitly learned predispositions that may be modulated by semantic memory but need not involve mental time travel (Suddendorf & Busby Reference Suddendorf and Busby2003b).
A possible test of “episodic-like” prospection might run as follows. The birds would first be taught that if they select a cache location under one condition (say, a green light) they will be allowed to retrieve from that location at any time, but if they select under another condition (say, a red light) they must wait at least a day before being allowed to retrieve. Assuming that they could learn this, they are then given the choice of worms or nuts, and the question is whether they would select fresh worms when allowed immediate access, but would anticipate decayed worms when access is delayed, and so choose nuts. If such anticipation were possible, it might indicate an understanding that how long food has been cached projects into the future as well as into the past. However, the same difficulties as with “episodic-like” memory apply. The birds might seem to understand the state of the world tomorrow (rotten worms) only through a combination of predispositions and specific learning algorithms that have evolved in the caching context (Dally et al. Reference Dally, Emery and Clayton2006b; Suddendorf & Busby Reference Suddendorf and Busby2003b).
Whatever the outcome of such an experiment, adult jays have been shown to modulate caching behavior in ways that seem to take the possibility of future pilfering into account. Earlier, we alluded to evidence that they re-cache food if observed by a more dominant bird (Dally et al. Reference Dally, Emery and Clayton2006b). Another study has shown that, when observed caching food, jays re-cache food only if they themselves had previously stolen the food of others. Birds with experience of theft seem to anticipate a future in which their own food might be stolen, and act accordingly (Emery & Clayton Reference Emery and Clayton2001). It takes a thief to know a thief.
Prevention of pilfering is a common adaptive problem for species that store food, and various strategies have evolved to deal with it (for a review, see Dally et al. Reference Dally, Clayton and Emery2006a). Both scatter-hoarders (e.g., ravens) and larder-hoarders (e.g., acorn woodpeckers) may carefully disguise their caches or actively defend their stores (e.g., Bugnyar & Heinrich Reference Bugnyar and Heinrich2006). Strategies of pilferage avoidance are increasingly being documented. For example, Eastern grey squirrels space caches further apart when conspecifics are present and preferentially dig oriented away from them (Leaver et al., in press). Theft is not the only future problem that hoarders may address. Gray squirrels bite out the seeds of white oak (but not red oak) acorns before storage, which prevents germination (Steele et al. Reference Steele, Turner, Smallwood, Wolff and Radillo2001). Treating food before storage (e.g., pine squirrels dry mushrooms in the sun) or keeping it alive but unable to escape (e.g. moles biting worms) are strategies that reduce spoilage and hence increase future survival chances (e.g., Smith & Reichman Reference Smith and Reichman1984).
Are these future-oriented behaviors fundamentally different from non-behavioral species-specific adaptations to the same problem, such as seasonally storing food as blubber or body fat? Perhaps not. Advantageous behavior can lead to evolutionary change (Bateson Reference Bateson2004) and can become instinctive as descendants of animals displaying the behavior face selection pressure for reliable, less resource-intensive shortcuts. New Caledonian crows, for example, manufacture and use tools (Hunt Reference Hunt1996). Although there is evidence for social transmission (Hunt & Gray Reference Hunt and Gray2003), the birds appear to have inherited a predisposition for tool use. They show such behavior without any experience or opportunity for observation (Kenward et al. Reference Kenward, Weir, Rutz and Kacelnik2005). Adaptations for certain types of memory and learning have also been reported in regent honeyeaters. In line with ecological factors (i.e., replenishment rates), these birds easily learn to avoid recently rewarding food locations and to return after long periods, but do poorly when the contingency is the other way around (Burke & Fulham Reference Burke and Fulham2003). Though this learning is appropriately future-oriented to the contingencies of its habitat, such constraints distinguish it from the flexible future-oriented behavior characteristic of human mental time travel.
3.3. The Bischof-Köhler hypothesis
We argued earlier that the selective advantage of mental time travel is the increased flexibility in acting in the present to secure future needs. First, therefore, one has to be capable of conceiving having different future needs, such as imagining being thirsty when currently quenched. One hypothesis, proposed by Bischof-Köhler (Reference Bischof-Köhler, Eckensberger and Lantermann1985) and Bischof (Reference Bischof1985), is that it is in fact an inability of nonhuman animals to flexibly entertain future need or drive states that limits their capacity for mental time travel. That is, nonhuman animals are unable to differentiate future states from present ones (Bischof-Köhler Reference Bischof-Köhler, Eckensberger and Lantermann1985; Bischof Reference Bischof1985; Suddendorf & Corballis Reference Suddendorf and Corballis1997). Superficially, at least, this hypothesis cannot be correct, since many species act to secure future needs, as illustrated by the caching of food. As noted, though, such behaviors may typically be largely instinctive, and experience may only allow changes within specific domain parameters. The Bischof-Köhler hypothesis may still apply to more individual, flexible situations involving non-instinctive behaviors. One study suggests that children anticipate future needs by around age 4 (Suddendorf & Busby Reference Suddendorf and Busby2005), and the challenge is now to document its development in more detail and to test nonhuman species.
Nonverbal tests of the Bischof-Köhler hypothesis have been proposed (Suddendorf Reference Suddendorf1994; Suddendorf & Busby Reference Suddendorf and Busby2005; Tulving Reference Tulving, Terrace and Metcalfe2005), but have yet to be carried out. They involve controlling a current need state, such as ensuring that the subject is no longer thirsty, and then providing an opportunity to secure a future need, such as obtaining a drink for a future situation that will induce thirst. There are various caveats to consider in order to rule out alternative explanations based on instinct or associative learning (Suddendorf & Busby Reference Suddendorf and Busby2005). For example, instinctual explanations can be ruled out if the experimental scenario does not involve species-typical behavior and if similar problems can be solved in a variety of domains. If the same animal were to pass variations of such tests across different needs (e.g., hunger, thirst, temperature) and contexts (locations, time-intervals), the Bischof-Köhler hypothesis would become increasingly untenable.
Mulcahy and Call (Reference Mulcahy and Call2006) recently came closest to implementing such a test. They trained bonobos and orangutans to obtain grapes from an apparatus using a tool. Access to the apparatus was then blocked and the animals were presented with a selection of two suitable and six unsuitable tools which they could take into a waiting room from where the apparatus was still visible. An hour later, they were allowed back into the testing room and given access to the apparatus. In 7 out of 16 trials, on average, the apes carried a suitable tool into the waiting room and returned with it to obtain grapes an hour later. There were strong individual differences in performance, with one orangutan achieving 15 out of 16 correct. This orangutan and the best performing bonobo were then tested again, but with an overnight delay between tool selection and return. They still returned with a suitable tool in more cases than expected by chance. A third experiment showed that the apes could pass the task even when they could not see the apparatus during tool selection. The final control study investigated whether the animals merely associated the tool with the reward. Subjects again received a grape reward if they returned with the right tool, but were not actually given an opportunity to use the tool. Performance in this condition was poorer, suggesting that they did plan ahead in the other studies (Mulcahy & Call Reference Mulcahy and Call2006).
Nevertheless, there are some concerns about this conclusion (Suddendorf Reference Suddendorf2006). The same tools were appropriate over trials, so apes could have just learned to always return with these same tools. This highlights the importance of the final control condition designed to rule out explanations based on associations. However, this control condition was not given to the successful animals of Experiments 1 to 3, but to a new group of four animals. Two of these never brought the tool back and hence could never have experienced the reward that may have facilitated performance in the previous studies. Thus, their data do not inform us about the power of the reward. The other two performed identically to two animals in Experiment 1. Thus, contrary to what the authors claimed, association cannot be ruled out (Suddendorf Reference Suddendorf2006).
But even if subsequent studies confirm that great apes can select and keep a different tool for a specific future use, it does not show anticipation of future needs as proposed by the Bischof-Köhler hypothesis. The “future need” potentially anticipated in these studies refers to the “need” for a tool to satisfy a current desire for the treat, not anticipation of a different internal drive or need state (e.g., such as a future desire that is different from present). The studies did not control or manipulate the drive or need state of the subjects, and it is not unreasonable to assume that they all had a desire for grape rewards throughout testing (Suddendorf Reference Suddendorf2006). Animals that are not capable of conceiving of future drive and need states would have little reason to concern themselves with a remote future, as all they would care about is satisfaction of current needs. More research is required to determine the extent of animal foresight, but at present the limit proposed by the Bischof-Köhler hypothesis has not been falsified.
We are not making these killjoy arguments because of any preconceived notion about the way the world should be. We would be delighted if it could be established that other species have mental time travel. It would be a serious challenge to many humans' anthropocentric worldview and would have profound moral implications (Suddendorf & Busby Reference Suddendorf and Busby2003a). Those species that can be shown to ponder what has happened, and to speculate about what might happen in the future, would require fundamentally different welfare considerations. For example, they would suffer not just from current pain, but also from revisiting past and anticipating future pain (Lea Reference Lea2001). At present, there is no need to abandon the null hypothesis, but neither are we in a position to firmly conclude that other animals do not possess this capacity. We admit that a substantial body of comparative data is required before it can be concluded that any trait is uniquely human (Hauser et al. Reference Hauser, Chomsky and Fitch2002).
Given the selective importance of future-oriented behavior, species are likely to have evolved a wealth of different mechanisms. The growing research interest in the question of animal mental time travel may help identify and describe these mechanisms. Our closest surviving relatives, the great apes, may be expected to have precursors of the human ability (e.g., foresight limited to serving current needs). Mulcahy et al. (Reference Mulcahy, Call and Dunbar2005), for example, have shown that gorillas and orangutans can select an appropriate tool in one location to solve a problem at another location that was not simultaneously visible. There is a range of evidence that great apes can imagine alternative states of the world (see sect. 4.1) and may hence offer a link between animal and human minds (for a review, see Suddendorf & Whiten Reference Suddendorf and Whiten2001). It is possible, then, that antecedents of mental time travel had evolved in the common great ape ancestor. It has been argued that living in fission-fusion societies, as modern chimpanzees do, may have selected for an increased capacity to monitor across time and space the comings and goings of other group members (Barrett et al. Reference Barrett, Henzi and Dunbar2003). Alternatively, ecological pressures on large arboreal apes, like modern orangutans, feeding on spatially and temporally variable food sources (e.g., fruit) might have led to selection for increased tracking of these patterns and planning of travel routes in our common ancestor. But it remains to be established what exactly the nature and extent of modern apes' foresight (and that of other animals) is. We maintain that the data so far continue to suggest that mental time travel is unique to humans. Next we make the case that future research should focus not only on purported instances of mental time travel, but also on subsidiary mechanisms that are typical of human mental time travel and that may be necessary to sustain it.
4. Components of human mental time travel
One reason to question whether mental time travel is possible in nonhuman animals is that flexible anticipation of particular events is no easy feat and may involve a suite of cognitive abilities that may not be available to animals or to young children (Suddendorf & Busby Reference Suddendorf and Busby2003b; Suddendorf & Corballis Reference Suddendorf and Corballis1997). Failures in any one of these may lead to disappointed expectations (a source of much human misery and, we may add, many a punch line) or to no anticipation at all. To evolve a flexible anticipation system, many cognitive components may need to be in place to achieve a level of accuracy that provides a selective advantage sufficient to compensate for the enormous expense of cognitive resources. An appropriate metaphor might be a theater production, which also requires many subsidiary activities apart from the production itself. In the words of Shakespeare, “All the world's a stage,” and this, we suggest, may include the mental world.
In likening mental time travel to a theater production, we are not making any claims about a Cartesian theater of the mind, as discussed by Dennett (e.g., Reference Dennett1995), nor about simulation or any other mechanisms by which this may be instantiated in the brain. Mental simulation of perception and action is of course one way of thinking about conscious thought in general (Hesslow Reference Hesslow2002) and mental time travel in particular (Suddendorf Reference Suddendorf1994), but the point of the analogy here is simply to draw attention to the range of mental capacities that might be considered necessary to engage successfully in mental time travel. We discuss the main theater components and their cognitive analogs in the following sections.
4.1. The stage
To entertain a future event one needs imagination – some kind of representational space in our mind for the imaginary performance. In cognitive psychology the concept of working memory is usually conceived of as such a space (or workbench) where information is temporally combined and manipulated (Baddeley Reference Baddeley1992). Human working memory appears to comprise, in addition to a central executive, two distinct subsystems: a phonological loop and a visuospatial sketchpad. Although non-lingual species may not have anything like the inner voice and inner ear (i.e., the phonological loop), they may very well have an image-based system (i.e., the visuospatial sketchpad).
To represent the future, the stage has to allow combination not only of recently presented material, but also of information stored in long-term memory. It must allow offline processing. Dreaming is an example of offline processing, and mammals appear to have dreams. Offline replay during sleep may be involved in consolidation of declarative memories in the hippocampus (Kali & Dayan Reference Kali and Dayan2004; Wilson & McNaughton Reference Wilson and McNaughton1994). In fact, dreaming or day-dreaming may be regarded as a form of “scenario building” that social mammals may have evolved to allow practice/play without suffering consequences (Alexander Reference Alexander, Mellars and Stringer1989).
To make such offline processing, or mental play, directly relevant to situations, however, one also has to be able to collate it with perceptions of the present real world. So-called secondary representations are thought to allow just that (Perner Reference Perner1991; Suddendorf Reference Suddendorf, Corballis and ea1999). In addition to a primary representation of current reality, a secondary representation of a goal constellation, for example, allows an individual to test potential moves by mental rather than physical trial and error. This capacity is also evident in pretend play (where one entertains an imaginary world while maintaining awareness of the real), in Piagetian invisible displacement tests (where one needs to reason about an invisible past trajectory of a target), and in various other basic cognitive tasks. During the second year of life, human children begin to display evidence for secondary representation in each of these contexts (Perner Reference Perner1991; Suddendorf & Whiten Reference Suddendorf and Whiten2001). A review of the literature found that only our closest relatives, the great apes, have so far provided some evidence for secondary representation in all of these areas (Suddendorf & Whiten Reference Suddendorf and Whiten2001; Reference Suddendorf, Whiten, Sterelny and Fitness2003; Whiten & Suddendorf, in press) and may therefore have the mental stage to imagine past or future events. Though convergent evolution may have produced similar skills in other species (e.g., corvids; see Emery & Clayton Reference Emery and Clayton2004), our own capacity for secondary representation appears to have evolved in the common great ape ancestor some 14 million years ago (Suddendorf & Whiten Reference Suddendorf and Whiten2001).
4.2. The playwright
To generate content, imaginary events need a script or narrative. This requires access to a declarative database – that is, stored information that is not stimulus bound, but can be made available top-down. As noted earlier, some nonhuman species appear to have declarative memory that could serve this function. To generate new content, however, one further needs to be able to combine and recombine existing elements. Like language, imaginary narratives may operate according to the principle of “discrete infinity” (Hauser et al. Reference Hauser, Chomsky and Fitch2002), involving the recursive application of rules to create an unlimited set of potential future scenarios (Suddendorf & Corballis Reference Suddendorf and Corballis1997).
Recursion is a computational procedure that calls itself, or calls an equivalent kind of procedure – as exemplified in sentences with embedded clauses, such as “The malt that the rat ate lay in the house that Jack built.” Recursion lies at the heart of grammar, and enables us to create, from a finite set of elements, a potential infinity of sentences that convey a potential infinity of meanings. Recursion may also be said to underlie other aspects of human thought, including perhaps music, manufacture, navigation, social relationships, and numbering systems (Corballis Reference Corballis1991; Hauser et al. Reference Hauser, Chomsky and Fitch2002). Theory of mind, which is the understanding that others have beliefs, desires, or intentions, for example, can involve several levels of recursion, as in such propositions as I think that you think I think you're stupid, which involves double recursion. Counting, as distinct from subitization or number estimation, involves principles that can be used recursively, enabling us to enumerate indefinitely. We humans, at least, use the concept of time in recursive ways, as in our understanding of such sentences like “Next Thursday he will have arrived in Mexico.” The future perfect is but one of some 30 different tenses in English, and reflects the close relation between language and mental time travel. Children begin to show a recursive, generative capacity in the early preschool years.
There is as yet little, if any, evidence for recursion in any nonhuman species (Corballis Reference Corballis, Sterelny and Fitness2003). Many birds sing songs of considerable complexity, and individual birds may have repertoires of many songs, involving different sequences of notes, but individual songs tend to be fixed and are repeated with remarkable consistency (e.g., Kroodsma & Momose Reference Kroodsma and Momose1991). There has been at least one claim that birds can generate songs using grammatical principles: Hailman and Ficken (Reference Hailman and Ficken1986) reported that the songs of chickadees follow rules governing the selection and sequencing of notes, but those rules appear to conform to a simple finite-state grammar rather than a context-free grammar like that underlying human language (Corballis Reference Corballis1991, p. 140). There is no evidence of recursion.
A recent claim that starlings can learn to recognize recursive patterns of sounds (Gentner et al. Reference Gentner, Fenn, Margoliash and Nusbaum2006) is, in our opinion, without foundation. The experimenters generated recursive sequences of up to eight sounds by embedding pairs of sounds, each consisting of a rattle and a warble, into another pair in recursive fashion. For example, if A i denotes a rattle and B i a warble, two levels of embedding would create the sequence A 3(A 2(A 1B 1) B 2)B 3. Exact sequences were seldom repeated, since the actual sounds were randomly selected from a population of eight rattles and eight warbles. The birds were able to discriminate sequences based on this recursive embedding from sequences not obeying the rule. This need not mean, though, that they understood recursion, since each sequence could be regarded as simply comprising a sequence of rattles followed by a sequence of warbles (e.g., A 3A 2A 1 followed by B 1B 2B 3). That is, the birds may simply have established the number of sequential rattles and the number of sequential warbles, and responded positively if the two were equal. Number perception for auditory sequences in birds may actually surpass that in humans (Thompson Reference Thompson1969), so a strategy of “estimate-and-match” may well have been within the capacity of the starlings. An earlier study showed that tamarins were unable to perform an analogous discrimination task (Fitch & Hauser Reference Fitch and Hauser2004), but even if they had been able to make the discrimination, it need not have implied that they recognized recursion itself (see Corballis [in press] for more detailed discussion).
4.3. The actors
Events typically involve characters, and mental time travel might well have evolved in order to predict (and aid or thwart) the behaviors of others (discussed further on in this section). To represent self and others realistically requires declarative knowledge of individuals and some folk psychology (i.e., theory of mind) to predict how they act (e.g., knowing that they typically act on the basis of their beliefs to fulfill their desires). With such knowledge, one can engage in more sophisticated social competition and cooperation, and one of the most effective ways to do so is to act like an actor: “to see ourselves as others see us, so as to cause them to see us as we would like them to rather than as they would like to” (Alexander Reference Alexander, Mellars and Stringer1989, p. 491). Self-awareness is difficult to define and measure. Alexander (Reference Alexander, Mellars and Stringer1989) argues that one important aspect of self-awareness springs directly from an ability to consider one's self in various alternative versions of future events. This is the notion of “free will,” implying that one can deliberately choose to pursue one of several entertained paths.
It is established that great apes, like 2-year-old children and unlike many other animals tested, can at least recognize themselves in mirrors (e.g., Bard et al. Reference Bard, Todd, Bernier, Love and Leavens2006; Gallup Reference Gallup1970; Patterson & Cohen Reference Patterson, Cohen, Parker, Mitchell and Boccia1994; Suarez & Gallup Reference Suarez and Gallup1981). They have an expectation of what they look like from the outside, and this expectation is rapidly updatable (Nielsen et al. Reference Nielsen, Suddendorf and Slaughter2006). Together with evidence for some understanding of what others can see (Call et al. Reference Call, Hare and Tomasello1998; Hare et al. Reference Hare, Call, Agnetta and Tomasello2000) or did see (Hare et al. Reference Hare, Call and Tomasello2001), this strongly suggests that they can “see themselves as others see them” at least in the literal sense. But it is not so clear whether they care about manipulating how others see them (even in this literal sense, only humans regularly adorn themselves with jewellery and clothes).
Nevertheless, there is a sizable body of evidence to suggest that primates do engage in various forms of tactical deception in social interactions (Whiten & Byrne Reference Whiten and Byrne1988). Primate social knowledge is impressive. Many primates recognize other individuals, as well as their social rank and relations (Seyfarth et al. Reference Seyfarth, Cheney and Bergman2005). Great apes and 2-year-old children also appear to have some limited folk psychology, but the precise nature of their capacities remains to be established (e.g., Suddendorf & Whiten Reference Suddendorf, Whiten, Sterelny and Fitness2003; Tomasello et al. Reference Tomasello, Call and Hare2003). Currently, the available data suggest that they do not have a representational theory of mind which includes the capacity to metarepresent that others may hold representations contradicting their own (Call Reference Call2001; Whiten & Suddendorf Reference Whiten and Suddendorf2001). It is only between ages 3 and 4 that humans pass tests (e.g., false-belief tasks) that demonstrate such understanding (e.g., Perner Reference Perner1991; Wellman et al. Reference Wellman, Cross and Watson2001). We have argued that it may require this level of folk psychology to be able to identify with one's future self, understand that this future self may have mental states that differ from one's current states, and care about them (Suddendorf & Corballis Reference Suddendorf and Corballis1997). As Hazlitt (Reference Hazlitt1805) observed: “The imagination … must carry me out of myself into the feelings of others by one and the same process by which I am thrown forward as it were into my future being, and interested in it” (p. 1).
4.4. The set
The mental play also needs a physical context that operates according to real-world principles. This requires some “folk physics.” Although one prominent researcher recently concluded that not even apes can mentally represent unobservable causal factors such as gravity and force (Povinelli Reference Povinelli2000), many other researchers are not convinced that their folk physics is quite so limited (Allen Reference Allen2002; Hauser Reference Hauser2001; Whiten Reference Whiten2001). Chimpanzees, for example, have passed double invisible displacement (object-permanence) tasks that require reasoning about the movement of objects that are no longer perceptible (Collier-Baker & Suddendorf Reference Collier-Baker and Suddendorf2006).
Especially important to mental time travel is some appreciation of the time dimension itself. Some fundamental timing processes (e.g., circadian rhythms) that link behavior to regular environmental changes are clearly widespread in the animal kingdom and are fairly well understood (e.g., Albrecht & Eichele Reference Albrecht and Eichele2003). Other, more cognitive processes are less well understood. There appear to be various different timing mechanisms in the human brain that track key information about temporal distance, order, and location within time patterns and humans appear to draw on different combinations of those (Friedman Reference Friedman2005).
Proposals for time measurement mechanisms range from discrete accumulating neural pacemakers (e.g., in scalar expectancy theory) to models involving continuous decay strength of memory traces. As noted earlier, fading of memory may itself provide temporal information that could account for the when component of www memory in scrub jays. In humans, cognitively controlled, rather than automatic, tracking of the passage of time typically activates the right hemispheric dorsolateral prefrontal cortex, an area also implicated in working memory (Lewis & Miall Reference Lewis and Miall2006). Lewis and Miall argue that both working memory and judging temporal distance may depend on the same dopamine-modulated neuronal system. Children begin to accurately discriminate distances of past events from around age 4 onwards (Friedman & Kemp Reference Friedman and Kemp1998).
Mental time travel may itself make such timing based on memory-decay functions less reliable. Since mentally constructing and reconstructing episodes reactivates memory traces, it may strengthen these memory traces and make them appear to be “fresher” in memory than warranted by age alone. Other timing mechanisms may therefore have evolved for the construction of a more accurate chronology. Indeed, chronology appears not to be a basic property of human memory, but rather, depends on active repeated construction (Friedman Reference Friedman1993). Some information about the order in which events occurred (e.g., before and after) may, however, be stored in memory. As we saw earlier, even rats show evidence of tracking temporal order (Eichenbaum et al. Reference Eichenbaum, Fortin, Ergorul, Wright and Agster2005).
How internal clocks, order codes, or other processes give rise to adult human concepts of the time dimension remains unclear. Human cultures have developed different semantic representations of time patterns (e.g., hours, days, weeks, months, etc.) that enable people to locate the time of events. Actively reconstructing locations of events in such explicit time patterns is probably the most common human approach to timing (Friedman Reference Friedman1993). Location-based processes are evident in scale effects where judgment of time of day of a past event may be more accurate than judgment of the month in which the event occurred – distance-based mechanisms cannot explain such phenomena. Friedman (Reference Friedman2005) recently reviewed the evidence and concluded that humans use both verbal and image-based processes to reason about the temporal location of events and that children use image codes to differentiate daily events by around age 5, and verbal lists, such as days of the week, by age 7. Children's relatively late acquisition of cultural time concepts suggests that they are not easy to acquire.
Once established, such time patterns offer the opportunity to place future events at particular points in time. Of course, neither order codes nor distance information in memory can explain the development of a temporally differentiated sense of the future. Children begin to show a rough temporal differentiation of future events by around age 5 (for a recent review, see Friedman Reference Friedman2005). Nonhuman animals seem severely limited in learning temporal information, and performance appears to be based on non-declarative mechanisms, rather than any such explicit concepts of time (Roberts Reference Roberts2002). Nonetheless, the work on scrub jays reviewed earlier suggests temporal-tracking mechanisms more sophisticated than previously thought to exist in any nonhuman species.
4.5. The director
Mental time travel is of course not to be mistaken for clairvoyance. The future contains many possibilities, and hence mental time travel involves entertaining different versions of scenarios and evaluating their likelihood and desirability. Like a director trying out alternative ways of presenting a scene, effective mental time travel requires rehearsals and evaluations. This entails some level of dissociation and metacognition (i.e., thinking about thinking), which first emerges in children around age 4 (Perner Reference Perner1991; Suddendorf Reference Suddendorf, Corballis and ea1999; Suddendorf & Corballis Reference Suddendorf and Corballis1997). There is at best limited evidence for very basic metacognitive capacities in nonhuman animals (Hampton Reference Hampton2001; Shettleworth & Sutton Reference Shettleworth, Sutton, Hurley and Nudds2006).
In studies involving extensive training on a simple task, such as determining which of two stimuli is the larger, some monkeys given the option of not taking a trial eventually learn to avoid trials they are likely to fail (Smith et al. Reference Smith, Shields and Washburn2003; Son & Kornell Reference Son, Kornell, Terrace and Metcalfe2005). Such uncertainty monitoring arguably implies metacognition, in that the monkeys may know that they do not know, and may even imply self-reflective consciousness. However, Son and Kornell concede that their results need not mean that monkeys are aware of their judgments. It remains unclear to us how any such performance is “meta” over and above other undisputable and common confidence judgments that animals may hold about their own ability. For example, judging how far it can jump is surely essential for any monkey traversing the canopy. The nature of what is supposed to be (meta-) represented may play a role in comparative cognition. Great apes have shown some signs of competence in regard to basic mental states. Call and colleagues (Call Reference Call2004; Call & Carpenter Reference Call and Carpenter2001), for example, recently provided some evidence that great apes know something about what they have or have not seen, as reflected in whether they do or do not seek further information before acting. But Call (Reference Call, Terrace and Metcalfe2005) notes that it is not known whether any nonhuman species attribute more complex states such as knowledge or beliefs to themselves.
A key role of a director is to orchestrate rehearsals. Humans rehearse significant future events and practice their own performance not only in their minds, but also in action. In fact, this trait would seem essential to any account of the enormous diversity of human expertise. Humans choose to learn and practice things they want to get better at. A function of play in many species may be that it prepares the young for the future (e.g., fighting skills). However, unlike species-typical play, humans choose what to practice and often do so in anticipation of specific future events (e.g., rehearsal for a theater performance). We know of no evidence that nonhuman animals deliberately practice for specific anticipated events (Suddendorf & Busby Reference Suddendorf and Busby2003b).
4.6. The executive producer
Enacting a planned event requires voluntary control, including executive functions such as the ability to inhibit other stimulus-driven responses in favor of one that suits the anticipated events best. Impaired mental time travel capacity following frontal-lobe damage has been associated with impairment of such self-regulation (Levine Reference Levine2004). Even inhibiting a simple response in order to increase one's total future reward is a difficult task for young children, as research on delay of gratification amply illustrates (Mischel & Mischel Reference Mischel and Mischel1983; Moore et al. Reference Moore, Barresi and Thompson1998). Adult humans, on the other hand, regularly forego instant rewards because of a multitude of anticipated long-term gains (e.g., weight loss, longevity, afterlife).
To override current drives in favor of acting to secure some anticipated future outcome, it would help to overestimate the positive or negative effects of future reward or punishment. In fact, humans show a systematic bias towards overestimating the intensity of future affective responses to future events (e.g., the dreaded interview with the bank manager often turns out to be more benign than expected). The nature of this biased “affective forecasting” is currently a topic of investigation in social psychology (e.g., Gilbert Reference Gilbert2006; Wilson & Gilbert Reference Wilson and Gilbert2005). Accurately anticipating one's own future mental states is no easy feat and is often influenced by one's current states – which is why one buys more treats when shopping while hungry (Gilbert et al. Reference Gilbert, Gill and Wilson2002). Even children show this effect (Atance & Meltzoff Reference Atance and Meltzoff2006).
Plans further require prospective memory – remembering to perform a future action at the appropriate time. Purported evidence for prospective memory in nonhuman animals has been disputed (Thorpe et al. Reference Thorpe, Jacova and Wilkie2004). Executive control may be required not only to implement strategic action plans, but also to manage the motivational and goal system itself (Conway et al. Reference Conway, Singer and Tagini2004; Suddendorf & Busby Reference Suddendorf and Busby2005). For mental time travel to become the flexible and effective future-oriented strategy that it is, cognition had to truly take the driver's seat of behavior (Suddendorf Reference Suddendorf, Corballis and ea1999). Modern humans often juggle a multitude of different goals and must decide when to do what to achieve which aims. The development of these sophisticated, resource-intensive, and error-prone mechanisms continues well into adulthood, and we know of no evidence for a comparable capacity in nonhuman animals.
4.7. The broadcaster
Finally, temporally displaced events are not only entertained in private but are often communicated. A play is not just a metaphor for mental time travel, it is often also a public expression of it. More generally, humans use language to exchange and complement their mental travels into the past and their ideas about future events, as well as to cooperatively coordinate plans and strategies (Suddendorf & Corballis Reference Suddendorf and Corballis1997). There is as yet no evidence that nonhuman animals communicate such mental travels. Such communication does not require language, as pantomime and dance can demonstrate. Chimpanzees appear to have some capacity to imitate (Whiten Reference Whiten1998) and to recognize when someone else is copying them (Nielsen et al. Reference Nielsen, Collier-Baker, Davis and Suddendorf2005). Animals with such abilities might in principle also be able to re-enact past episodes, using mime, if they wanted to communicate those to others.
Although language is not necessary for mental time travel, it provides the clearest evidence of it. Indeed, it may not be too far-fetched to suppose that mental time travel was a prerequisite to the evolution of language itself, since, as we saw earlier, language is exquisitely equipped to express events that are distant in both space and time from the present. If we are correct in supposing that mental time travel is uniquely human, then this may explain why language itself is unique to our species, at least in the strong sense defined by Hauser et al. (Reference Hauser, Chomsky and Fitch2002). The adaptive utility of the capacity to transcend the present in an open-ended, flexible manner may have driven these same properties in language (for a discussion, see Corballis & Suddendorf, in press).
4.8. Components of successful mental time travel
In summary, the theater metaphor implies that mental time travel requires a constellation of skills and is not simply an isolated capacity. Adult humans may fail to act now for a particular future event because of a deficiency in any one of these components (e.g., one may fail to predict one's own future mental states, or one may fail to override more immediate impulses). Young children may also fail to do so because one or the other of these components is not yet fully developed. Further study of how these requisite capacities emerge in human development may hold clues to the relationship between them.
Some of the foundations, such as imagination, self-recognition, and semantic memory, begin to emerge early in childhood; whereas other capacities, such as recursive thought and representational theory of mind, develop first in children between ages 3 and 4. It is around this latter time that unequivocal evidence for episodic memory emerges, childhood amnesia ceases, and implicated prefrontal regions mature (e.g., Levine Reference Levine2004). Between ages 3 and 5, children start to reason about future and past states (Atance & Meltzoff Reference Atance and Meltzoff2005; Gopnik & Slaughter Reference Gopnik and Slaughter1991; Suddendorf & Busby Reference Suddendorf and Busby2005), make plans (Atance & O'Neill Reference Atance and O'Neill2005; Hudson et al. Reference Hudson, Shapiro and Sosa1995), delay gratification (Mischel & Mischel Reference Mischel and Mischel1983; Thompson et al. Reference Thompson, Barresi and Moore1997), accurately report events of yesterday and tomorrow (Busby & Suddendorf Reference Busby and Suddendorf2005; Harner Reference Harner1975), and temporally differentiate events in the past (Friedman & Kemp Reference Friedman and Kemp1998) and in the future (Friedman Reference Friedman2000).
Nonhuman animals may also be limited because of deficiencies in any of these components. Although there is evidence in some species (especially from our closest relatives) for basic components, such as imagination and self-recognition, support for most others is very slim. Should a component be present only weakly, if at all, in other species, mental time travel would be severely limited, at best. For example, without a representational theory of mind, foresight might be restricted to serving satisfaction of current needs, as the Bischof-Köhler hypothesis proposes. Our suggestion, then, is that when studying the possibility of mental time travel in other species, the research agenda should include work on these components to human mental time travel. Determining to what extent specific components are necessary and sufficient for what aspects of foresight to emerge will be an important task.
5. Some qualifications
Our theater metaphor and our reference to constructing particular events, as well as the notion of www memory, have connotations that may be misleading. We are not suggesting that humans typically re- or pre-construct full-fledged episodes that are played out like a video recording. As pointed out earlier, episodes are actively constructed and are prone to error. Also, mental time travel often involves only the smallest snippets of events. In fact, the snippets do not have to come from the same place and time; snippets from very different times can be strung together. That is, humans can relate events from different times and identify patterns that help us make sense of the present (e.g., “he did X, and then two days later Y, only to now do Z”) and to predict the future (e.g., “I am going to do A tomorrow, which will deal with his action Y, so I can achieve B next week”).
Rats, on the other hand, even after thousands of trials fail to learn the link between a stimulus and a reward if they are a few seconds apart, and there is no secondary reinforcer (Grice 1946, cited in Roberts Reference Roberts2002). Relating events across time may be one of the key advantages provided by mental time travel into the past, as it offers humans additional information for prediction of events to follow. In other species, solutions to the need to bridge temporally distant events are typically highly specific and lack the flexibility of mental time travel. Whereas most learning links events that occur within a few seconds of each other, rats famously can link taste with nausea even when the illness develops hours after consumption (Etscorn & Stephens Reference Etscorn and Stephens1973; Garcia et al. Reference Garcia, Ervin and Koelling1966). This linkage, however, appears to be highly specific, and is limited to the linking of sickness to taste, and not to auditory or visual stimuli (Garcia & Koelling Reference Garcia and Koelling1966). This temporally extended learning mechanism thus appears to have evolved in response to a recurring problem with strong selective force (those who eat too much of the wrong food die). Humans appear to be less constrained and can (though may not) link virtually anything to anything else across time.
Although we have highlighted the similarities between mental time travel past and future, there are of course also some notable differences. Obviously, the past has in fact happened, whereas the future harbors a lot more uncertainty. For example, the weather may change, or the car may break down. Another potential difference lies in the temporal distribution of the number of events people recall and anticipate. Although there are similarities in the distributions (Spreng & Levine Reference Spreng and Levine2006), the past features the so-called reminiscence bump – by the age of around 70, for example, people tend to recall more events from their twenties than events from their forties and fifties (Rubin & Schulkind Reference Rubin and Schulkind1997). Our view of mental time travel may provide an answer as to why that may be so. If the key function of mental time travel into the past is indeed to inform and allow the anticipation of the future, then it is in the formative years that people have to retain as much event detail as possible in order to abstract facts and make specific predictions. Accumulating a vast pool of episodes of varied experiences provides one with an arsenal of information for future prediction.
As one matures, one also accumulates more semantic facts, and fewer things are surprising. Predictions can increasingly be made by abstract rules (i.e., using semantic prospection) rather than through imagination of individual future events. Retaining details of individual events becomes less important the more over-arching principles have been deduced. Good prediction depends on the right balance of semantic and episodic information, so that one does not get bogged down in too much episodic clutter (e.g., Anderson & Schooler Reference Anderson and Schooler1991). Episodic memory may decline as a consequence of the increase in the extraction of facts and rules, or what some would call crystallized intelligence. Thus, although we have argued that semantic memory and prospection are phylogenetically older than episodic memory and prospection, mental time travel may vastly increase the nature and capacity of semantic future-oriented cognition. Just as semantic and episodic memory interact (Tulving Reference Tulving, Terrace and Metcalfe2005), so may semantic and episodic prospection.
6. Evolutionary considerations
Because behavior that increases future survival has a selective advantage, mechanisms for future-directed behavior are ubiquitous. The now-standard taxonomy of human memory, we suggest, can be extended to incorporate variations in the degree of flexibility each system provides for the future. Mental time travel offers the ultimate level of flexibility, allowing voluntary anticipation of any particular event. This foresight requires several sophisticated cognitive abilities, is resource-intensive and error-prone, and introduces new kinds of mental stress, not least of which is the knowledge of inevitable death.
This is a high price to pay for a system for anticipating the future, which of course can never be known with perfect precision, especially considering the fact that much can be achieved with simpler prospection systems (instincts, procedural and semantic prospection). But mental time travel offers an additional degree of flexibility that, even if the adaptive advantage was in the first instance small, was sufficient to ensure selection and fixation in the population. Haldane (Reference Haldane1927) computed that a variant resulting in a mere 1% in fitness would increase in population frequency from 0.1% to 99.9% in just over 4,000 generations, a time span that fits easily into hominin evolution, or even into the evolution of our own genus, Homo. Even if the initial adaptive advantage may have been small, it is clear that today humans base much of their actions on anticipation of future events, from preparing this weekend's escape to building wealth.
The immense flexibility this foresight provides may have allowed us to successfully adapt to and colonize most habitats on the planet (Suddendorf & Busby Reference Suddendorf and Busby2005). Our future-directed behavior can even go beyond merely individual needs and may carry a concern for the planet itself, along with its inhabitants. That concern may also extend beyond the life span of the individual. As perhaps the only species with such foresight, humans alone may be driven to consciously guide the planet into the future (Dawkins Reference Dawkins2000) and thus be burdened with the responsibility of getting it right. We can identify future threats to our world, be they of our own doing (e.g., the consequences of human-induced global warming) or of external origin (e.g., an asteroid on collision course with Earth). Humans can forecast the outcomes and choose to act now to secure future needs. Cultures have evolved complex moral systems that judge actions as right or wrong partly based on what the actor could or could not have reasonably foreseen to be the future consequences of the act. Law, education, religion, and many other fundamental aspects of human culture are deeply dependant on our shared ability to reconstruct past and imagine future events.
So why might humans, but not other animals, have the capacity for mental time travel? Are there plausible accounts as to how it may have evolved since the split from the last common ancestor of Homo and Pan some 5 or 6 million years ago? The possibility of a gap between the human and animal mind need not contradict Darwinian principles of natural selection. With the global shift to cooler climate after 2.5 million years ago, much of southern and eastern Africa probably became more open and sparsely wooded, exposing the hominins to greater danger from predators and forcing them into a “cognitive niche” (Tooby & DeVore Reference Tooby, DeVore and Kinzey1987), with heavy reliance on social cooperation and communication for survival. With the emergence of the genus Homo, brain size increased rapidly from around 2 million years ago (Wood & Collard Reference Wood and Collard1999), possibly reflecting selection for such interrelated attributes as theory of mind, language, and, we propose, mental time travel.
The earliest hard evidence, quite literally, for foresight comprises stone tools that appear to have been transported for repeated use. Reconstruction of knapping routines (using refit data) suggests that at least by the Middle Pleistocene hominins produced stone tools in one site to use them later at another (Hallos Reference Hallos2005). Bipedal hominins in the deforested savannah may have relied increasingly on throwing stones at predators (e.g., Calvin Reference Calvin1982), as well as using them to bring down prey and cut up carcasses. Great apes do hurl objects, but with little precision. Carrying rocks for use as missiles at some future point may have been vital, and a capacity to plan for this might have been strongly selected for. By 400,000 years ago some hominins had clearly developed aimed throwing, as revealed by wooden spears uncovered in Germany together with butchered horses (Thieme Reference Thieme1997). One possibility, then, is that extensive foresight evolved first in the context of throwing, as hominins faced different predators and prey in the savannah than they did in the trees. Osvath and Gardenfors (Reference Osvath and Gärdenfors2005), for example, marshal evidence for such a scenario leading from the Oldowan tool culture to the evolution of mental time travel.
Similar arguments could be made for other ways in which hominins may have first adapted their environment to suit their future needs. The fact that stone tools provide us with an archaeological record that other behaviors do not may skew our evaluation of their significance. Consider, for example, the use of fire, which is also evident by 400,000 years ago (e.g., Boaz et al. Reference Boaz, Ciochon, Xu and Liu2004) and may well have been used much earlier. Although it is difficult to identify archaeological evidence for mastery of fire, one needs little imagination to envisage the huge selective advantage that it might have bestowed, in defense, attack, cooking, provision of warmth, night-time vision, and so on. Planning capacities could have been selected for as the incidental use of fire gave way to maintenance of fire and, finally, to the making of fire for more controlled and deliberate purposes. Perhaps the ancient Greeks were right: They believed that Prometheus stole fire from heaven to give humans the powers of the gods that set them apart from animals – Prometheus means foresight.
Yet, these victories in gaining control of the natural world may only have been side effects of a quite different evolutionary arms race. Technological advances may have been secondary to abilities that evolved primarily in the social arena. Modern humans certainly use their planning capacities extensively to cooperate with, deceive, and manipulate conspecifics. As noted earlier, much of what humans recall and foresee has to do with “who did what to whom, when, where, and why” (Pinker Reference Pinker, Christiansen and Kirby2003, p. 27). It has been suggested that social rather than ecological pressures drove the evolution of intelligence, not only in humans, but in primates more generally (Humphrey Reference Humphrey, Bateson and Hinde1976). This hypothesis has attracted some strong support in recent years (e.g., Dunbar Reference Dunbar2003; Seyfarth et al. Reference Seyfarth, Cheney and Bergman2005; Whiten & Byrne Reference Whiten and Byrne1988).
One problem for such an account is to explain why the cognitive arms race seems to have persisted longer in humans than in other primates, resulting in apparently unique cognitive skills, including, perhaps, mental time travel. A potential explanation, suggested by Alexander and colleagues (Alexander Reference Alexander, Mellars and Stringer1989; Flinn et al. Reference Flinn, Geary and Ward2005), is that once early hominins obtained a certain level of “ecological dominance,” perhaps partly through technological advances as discussed earlier, they were faced with increased competition from their own species – “humans uniquely became their own principal hostile force of nature” (Alexander Reference Alexander, Mellars and Stringer1989). This may have resulted in a runaway social competition between (and within) groups towards greater intelligence, and enhanced abilities for both cooperation and deception. These may have included the ability to entertain alternative future scenarios (mental time travel), to read others' minds (theory of mind), and to communicate (language).
This “coalitionary arms race” (Flinn et al. Reference Flinn, Geary and Ward2005) also offers an answer to one of the greatest mysteries of human evolution: Why have all the other hominin species become extinct? One only needs to extend the proposal from “intra-species” competition to “intra-genus” competition to see a solution. Our ancestors may have competed with an array of other bipedal species that once graced this planet, and perhaps simply outsmarted them, contributing to their demise (Suddendorf Reference Suddendorf2004). One of the final steps that may have given us a decisive edge over one of the last nonhuman hominins, the Neanderthals, may have been autonomous speech (Corballis Reference Corballis2004). This suggestion that humans played a role in displacing other hominins is in line with recorded human history in that human groups perpetually compete with each other and often violently displace other groups, as well as with various other human characteristics, such as our unique obsessions with inter-group competitive play (e.g., sports). This intra-genus competition may have ultimately been won by human groups because of continued advances in foresight, language, culture, and coordinated aggression, leaving us as the sole survivors of an extraordinary evolutionary arms race. We may be the only species capable of mental time travel because the others competing in our niche have become (or have been made) extinct.
Like the “just-so stories” about throwing or fire, an account emphasizing intra-species or intra-genus competition may exaggerate the importance of a single factor. Claiming that the hostile forces of nature turned into “relative trivialities” (Alexander Reference Alexander, Mellars and Stringer1989) probably underestimates the selective pressure of catastrophic environmental changes such as ice ages, volcanic eruptions, and meteorite impacts on life in general. Such events may well have produced bottlenecks in evolutionary history where social competition was of far less import than ecological factors and cooperation. Rather than endorsing one or the other scenario, we present them here merely to make the point that plausible accounts exist that can explain why humans may have capacities, such as mental time travel, that other species do not have. Darwinian continuity need not demand greater mental powers in nonhuman animals than is currently evident.
ACKNOWLEDGMENTS
Thomas Suddendorf was supported by Australian Research Council Discovery grants (DP0557424 and DP0770113). We are thankful to the eight anonymous reviewers for providing useful comments on an earlier version of this manuscript.




