Introduction
As human societies shifted from hunting and gathering to farming and herding, it is thought that their risk of contracting certain diseases also changed (Cohen & Armelagos Reference Cohen and Armelagos1984; Harper & Armelagos Reference Harper and Armelagos2010; Reinhard et al. Reference Reinhard, Ferreira, Bouchet, Sianto, Dutra, Iniguez, Le Bailly, Fugassa, Pucu and Araújo2013; Mitchell Reference Mitchell and Mitchell2015a). The Near East is the first of the independent centres where agriculture arose. Barley and wheat were domesticated in the Tigris-Euphrates basin; and subsequently, increasingly large settlements developed across the Fertile Crescent approximately 9000 years ago (Armelagos & Harper Reference Armelagos, Harper and Guest2005; Brown et al. Reference Brown, Jones, Powell and Allaby2009). The domestication of crops and the rise of farming and herding necessitated and supported a sedentary lifestyle. Increases in food availability facilitated population expansion, resulting in the first epidemiological transition—the characteristic rise in infectious diseases associated with increased population size and mobility (Armelagos & Harper Reference Armelagos, Harper and Guest2005). New lifeways during the Neolithic probably affected infectious disease transmission in many ways, such as through the accumulation of human waste, the alteration of plant and animal environments, increasing population size and higher population density. It has been argued that intestinal diseases, including parasitic infection, may also have altered at this time. For example, with a changing environment, the differing life cycles of parasite species may have facilitated the spread of some parasites among humans, while hampering the spread of others (Mitchell Reference Mitchell2013).
The three Mediterranean Neolithic-period sites so far subjected to parasitological analysis have revealed a broad range of parasite species. In Neolithic Cyprus, pelvic soil from 11 burials at Shillourokambos (8300–7000 BC) and Khirokitia (7000–6000 BC) contained the eggs of the soil-transmitted helminths (geohelminths), that is roundworm and whipworm, and of species that spend part of their life cycle in cattle, pigs and sheep: namely, Taenia sp. (tapeworm) and Fasciola sp. (liver fluke) (Harter-Lailheugue et al. Reference Harter-Lailheugue, Mort, Vigne, Guilaine, Brun and Bouchet2005). Whipworm, roundworm and pinworm were identified at the Neolithic village of La Draga at Lake Banyoles in Spain (5320–4980 BC), along with species that spend part of their life cycle in cattle, fish, rodents and ants: namely, Taenia saginata, fish tapeworm (Diphyllobothrium sp.), Capillaria sp. and Lancet liver fluke (Dicrocoelium dendriticum) (Maicher et al. Reference Maicher, Hoffmann, Côté, Pérez, Segui and Le Bailly2017). While parasite studies have been undertaken at Neolithic sites in Europe (Anastasiou Reference Anastasiou and Mitchell2015), very little research has investigated the species of intestinal parasites present in the early large village agglomerations of the Near East.
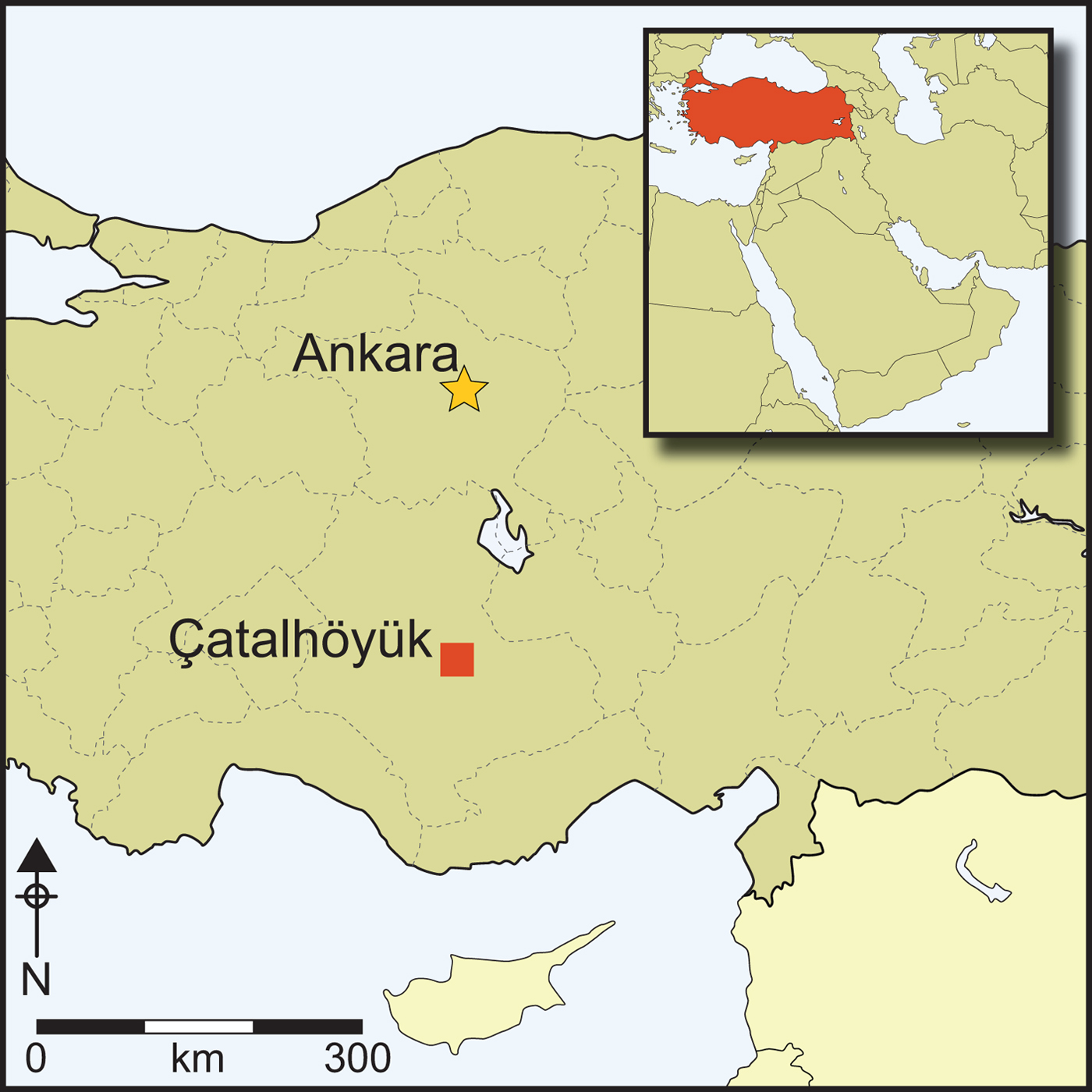
The Neolithic East Mound of Çatalhöyük in south-central Anatolia (Figure 1) was occupied continuously for more than 1000 years from 7100–6000 cal BC (Bayliss et al. Reference Bayliss, Brock, Farid, Hodder, Southton and Taylor2015), during the late Pre-Pottery Neolithic. On the nearby West Mound, occupation of the site continued into the Chalcolithic period until the middle of the sixth millennium BC (Orton et al. Reference Orton, Anvari, Gibson, Last, Bogaard, Rosenstock and Biehl2018). Throughout the occupation of the East Mound, the settlement pattern gradually changed from a dense agglomeration, where people lived in buildings that were constructed directly adjacent to each other with no space for streets in between, to a more open layout during the later occupation phase (Hodder Reference Hodder2014). Throughout the settlement's life span, buildings were clustered in neighbourhoods, often showing long-term continuity of occupation, with multiple episodes of rebuilding in the same location over several generations. Middens accumulated directly adjacent to these building clusters and within abandoned buildings, forming large areas of midden material, including ash and charcoal, animal bone, construction debris and organic waste (Shillito & Matthews Reference Shillito and Matthews2013). Human coprolites and animal dung were occasionally found preserved in these open middens (Shillito et al. Reference Shillito, Matthews, Almond and Bull2011a & Reference Shillito, Bull, Matthews, Almond, Williams and Evershedb). In contrast, building interiors were kept remarkably clean, even at the microscale, with frequent sweeping out and re-plastering of floors and walls on a seasonal basis (Matthews Reference Matthews and Hodder2005; Matthews et al. Reference Matthews, Shillito, Elliot, Bull, Williams, Whittle and Bickle2014).
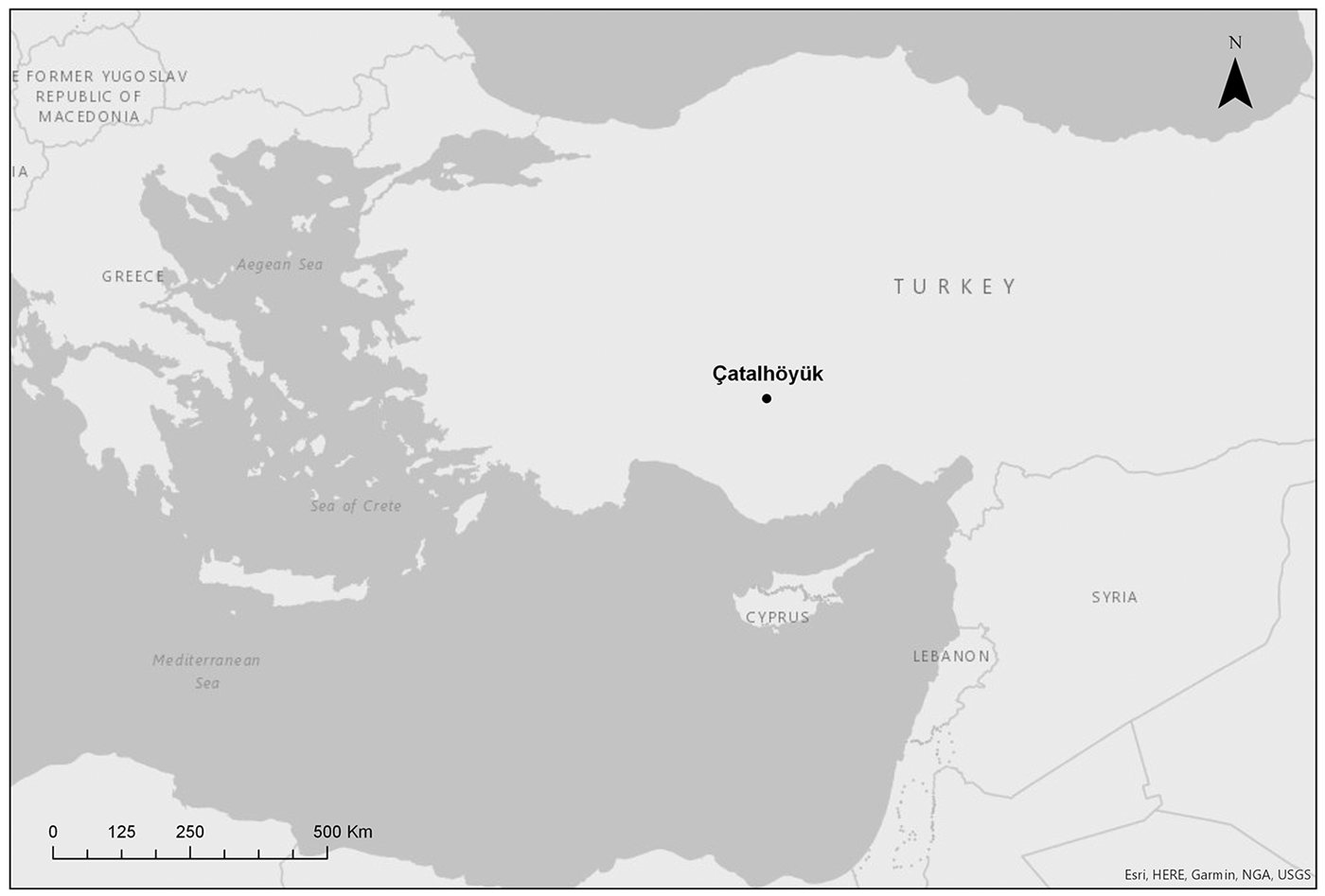
Figure 1. Map showing the location of Çatalhöyük within Turkey (figure from the Çatalhöyük Research Project).
While there has been research on the human skeletal remains from Çatalhöyük to investigate disease and diet in the population (e.g. Hillson et al. Reference Hillson, Larsen, Boz, Pilloud, Sadvari, Agarwal, Glencross, Beauchesne, Pearson, Ruff, Garofalo, Hager, Haddow and Hodder2013; Larsen et al. Reference Larsen, Hillson, Boz, Pilloud, Sadvari, Agarwal, Glencross, Beauchesne, Pearson, Ruff, Garofalo, Hager, Haddow and Knüsel2015; Pearson et al. Reference Pearson, Haddow, Hillson, Knüsel, Larsen and Sadvari2015), intestinal disease is not easily assessed from study of the skeleton. Instead, faecal material preserved in an identifiable archaeological context is required. In societies pre-dating the invention of the toilet in the fourth millennium BC (McMahon Reference McMahon and Mitchell2015), the best targets for analysis are coprolites (preserved pieces of human faeces) and samples of sediment from the pelvic area of burials, where the intestines would have been located during life (Reinhard et al. Reference Reinhard, Confalonieri, Herrmann, Ferreira and Araújo1986; Mitchell Reference Mitchell, Mitchell and Brickley2017a). Analysis of coprolites and pelvic sediment can provide evidence for the species of intestinal parasites that infected individuals in a population and, if sample sizes are large enough, it may also provide an indication of how widespread infection was and whether some people were more heavily infected than others.
Materials
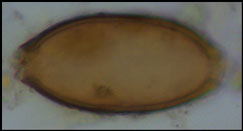
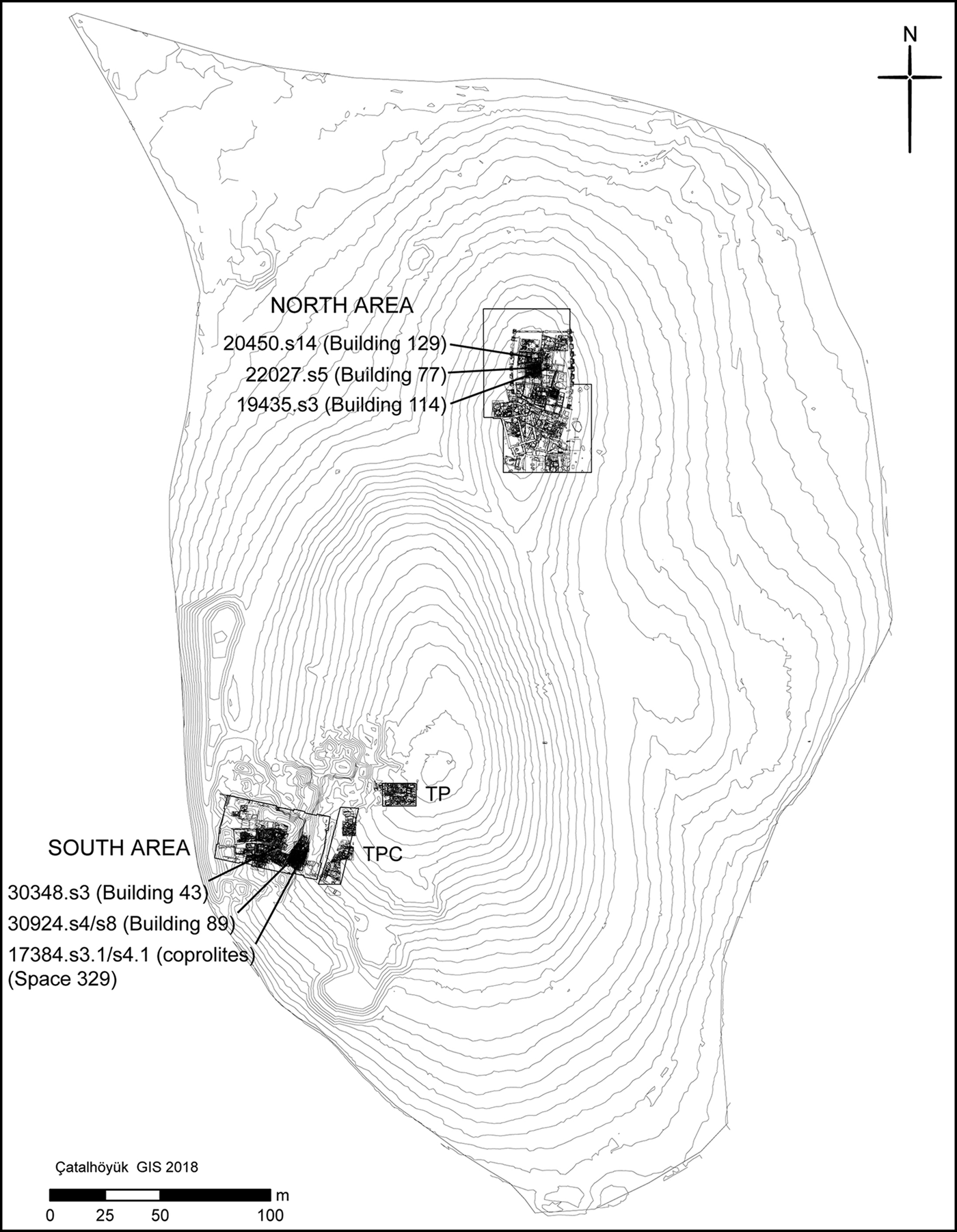
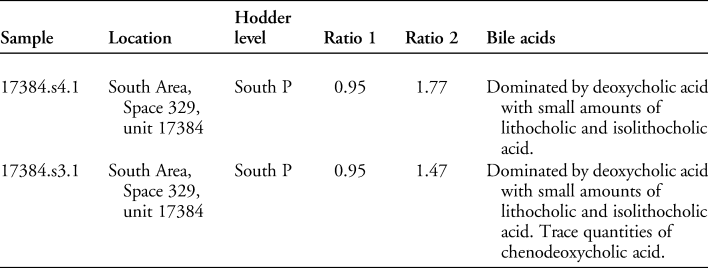
The four coprolites analysed here were recovered during excavation of Space 329, Level South P (Figure 2), dating to c. 6410–6150 BC (from radiocarbon dates interpreted with Bayesian modelling). This corresponds to late or ‘post-peak’ occupation of the site, as defined by Hillson et al. (Reference Hillson, Larsen, Boz, Pilloud, Sadvari, Agarwal, Glencross, Beauchesne, Pearson, Ruff, Garofalo, Hager, Haddow and Hodder2013). Space 329 comprises a series of laminated, midden-like deposits interspersed with charred spots indicating in situ burning activity (Reagan & Taylor Reference Reagan, Taylor and Farid2009). Each coprolite was mineralised (Figure 3), being either hard and solid, or having a crumbly texture with some hard portions.
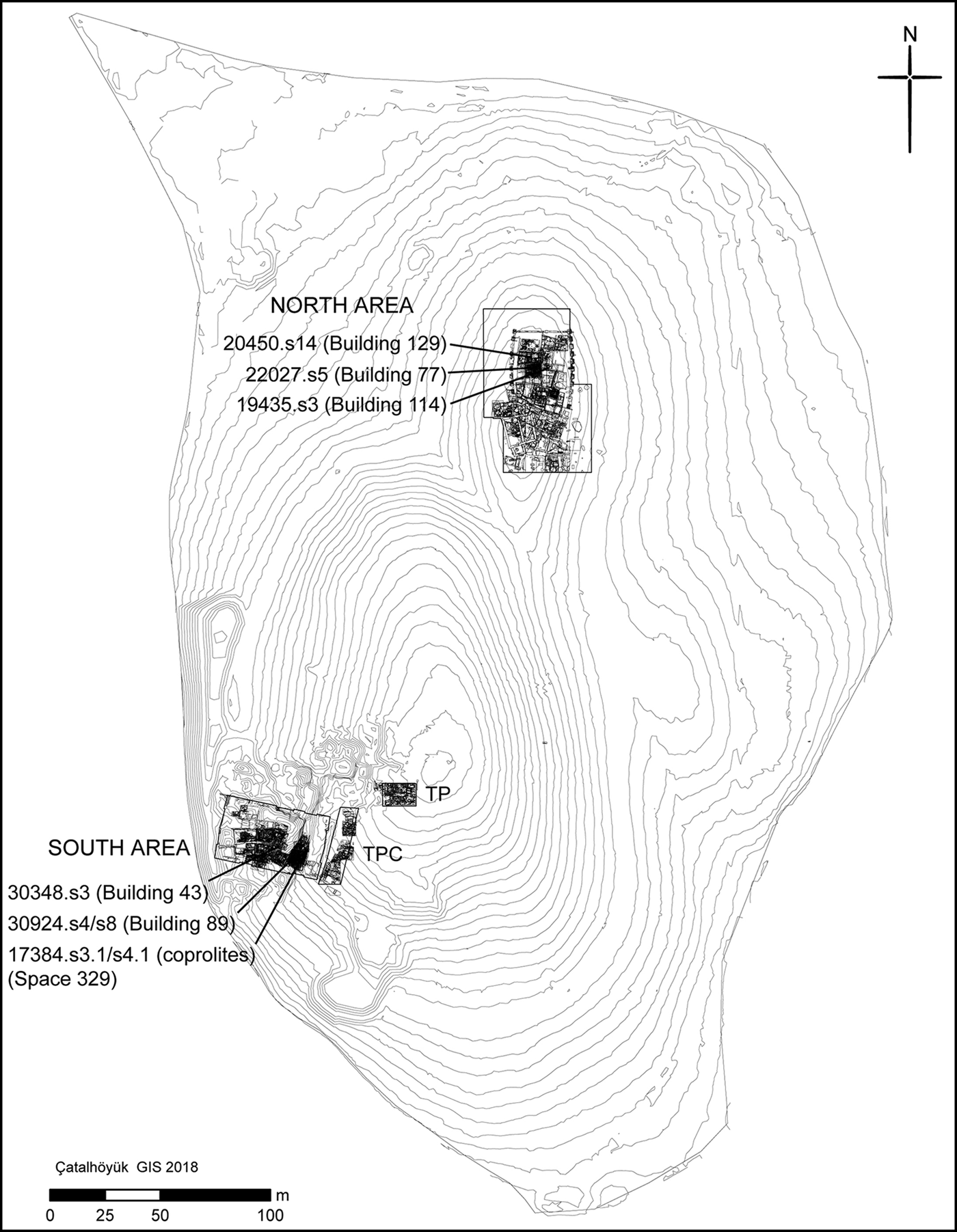
Figure 2. Plan showing the location of the coprolites and burials analysed (figure by Camilla Mazzucato).

Figure 3. Coprolite 15702.s21.1, with dimensions of 41mm in length and 23mm in width (figure by Evilena Anastasiou).
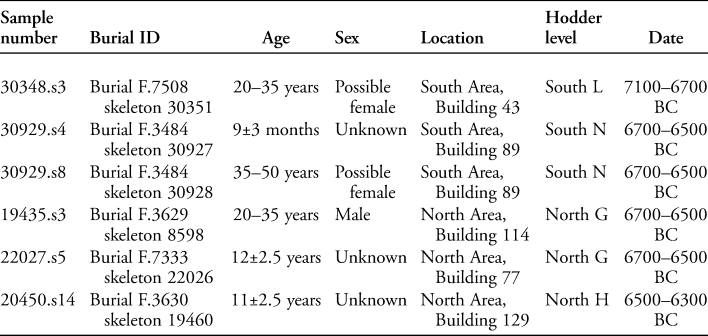
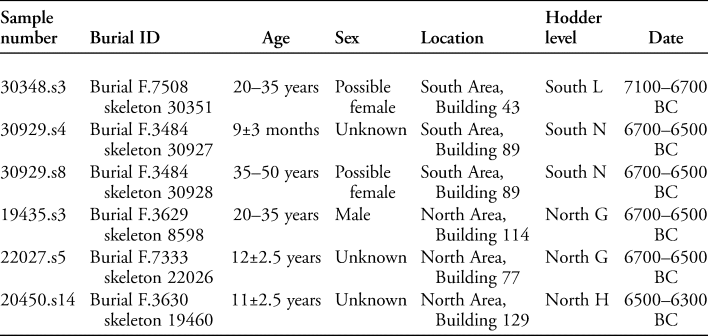
Six human burials were also analysed (for an example, see Figure 4). These individuals were all interred beneath the platforms found within houses (see Figure 2). The burials date from 7100–6300 BC; further details are provided in Table 1. Sediment samples were taken from the area anterior to the sacrum where the intestines would be positioned, and control samples were obtained from within the cranial vault and foot area of each inhumation, where the eggs of intestinal parasites should not be found during life (Reinhard et al. Reference Reinhard, Confalonieri, Herrmann, Ferreira and Araújo1986; Mitchell Reference Mitchell, Mitchell and Brickley2017a).

Figure 4. Skeleton 30928, a possible female, aged 35–50 years, dating from 6700–6500 BC (figure from the Çatalhöyük Research Project).
Table 1. Details of the burials from which pelvic soil samples were taken, including age, sex, location and date.
Methods
One-gram samples of each dried coprolite were weighed, fragmented using a pestle and mortar and disaggregated with 0.5 per cent trisodium phosphate for 72 hours. The mineralised coprolite samples also required a small quantity of dilute hydrochloric acid (10 per cent HCl) to disaggregate the samples. The HCl was added drop by drop to break down calcium carbonates until the gentle bubbling ceased. The pelvic soil samples did not require the use of a pestle and mortar or HCl, and disaggregated completely with 0.5 per cent trisodium phosphate. Once the material was in suspension, each sample was passed through a stack of microsieves with mesh sizes of 300μm, 160μm and 20μm. As intestinal parasite eggs found in this region of the world are typically between 20 and 150μm in size, they are trapped on the 20μm mesh, while larger- and smaller-sized soil particles are removed. Sediment trapped on the 20μm mesh was washed free with ultrapure water and centrifuged to isolate it. The supernatant was then removed, glycerol added and the sample viewed using digital light microscopy at 400× magnification (see Anastasiou & Mitchell Reference Anastasiou and Mitchell2013; Dufour & Le Bailly Reference Dufour and Bailly2013).
Coprolites found to be positive for parasite eggs underwent faecal lipid analysis targeting both sterols and bile acids to determine whether they were of human or animal origin. For faecal lipid analysis, samples were prepared according to methods described in Bull et al. (Reference Bull, Lockheart, Elhmmali, Roberts and Evershed2002). In summary, two internal standards (hyocholic acid and preg-5-en-3β-ol, 0.1mg mL−1 solution) were added to 1g of sieved sample. Samples were processed by microwave-assisted solvent extraction and then saponified. The saponified total lipid extracted was then separated into neutral and acid fractions using an aminopropyl SPE column. The neutral fraction was separated using a silica gel column into less polar and sterol fractions. The sterol fraction was derivitised by the addition of BSTFA/TMCS (99:1 v/v). Excess derivatisation agent was evaporated under a gentle stream of nitrogen and the sample redissolved in ethyl acetate prior to analysis by gas chromatography (GC) and gas chromatography mass spectrometry (GC/MS). The acid fraction was methylated using TMS-DAM in toluene/methanol (4:1 v/v). The methylated acids were further separated using a silica gel column into mono- and dicarboxylic fatty acid methyl ester/diester, and hydroxylated carboxylic acid methyl ester fractions, the latter containing the bile acids. GC/MS peak assignments were made by comparison with known mass spectra (NIST08 and laboratory reference library) and, where possible, authentic standards. Species identifications were made through calculation of sterol ratios in conjunction with bile acid profiles (after Bull et al. Reference Bull, Lockheart, Elhmmali, Roberts and Evershed2002).
Results
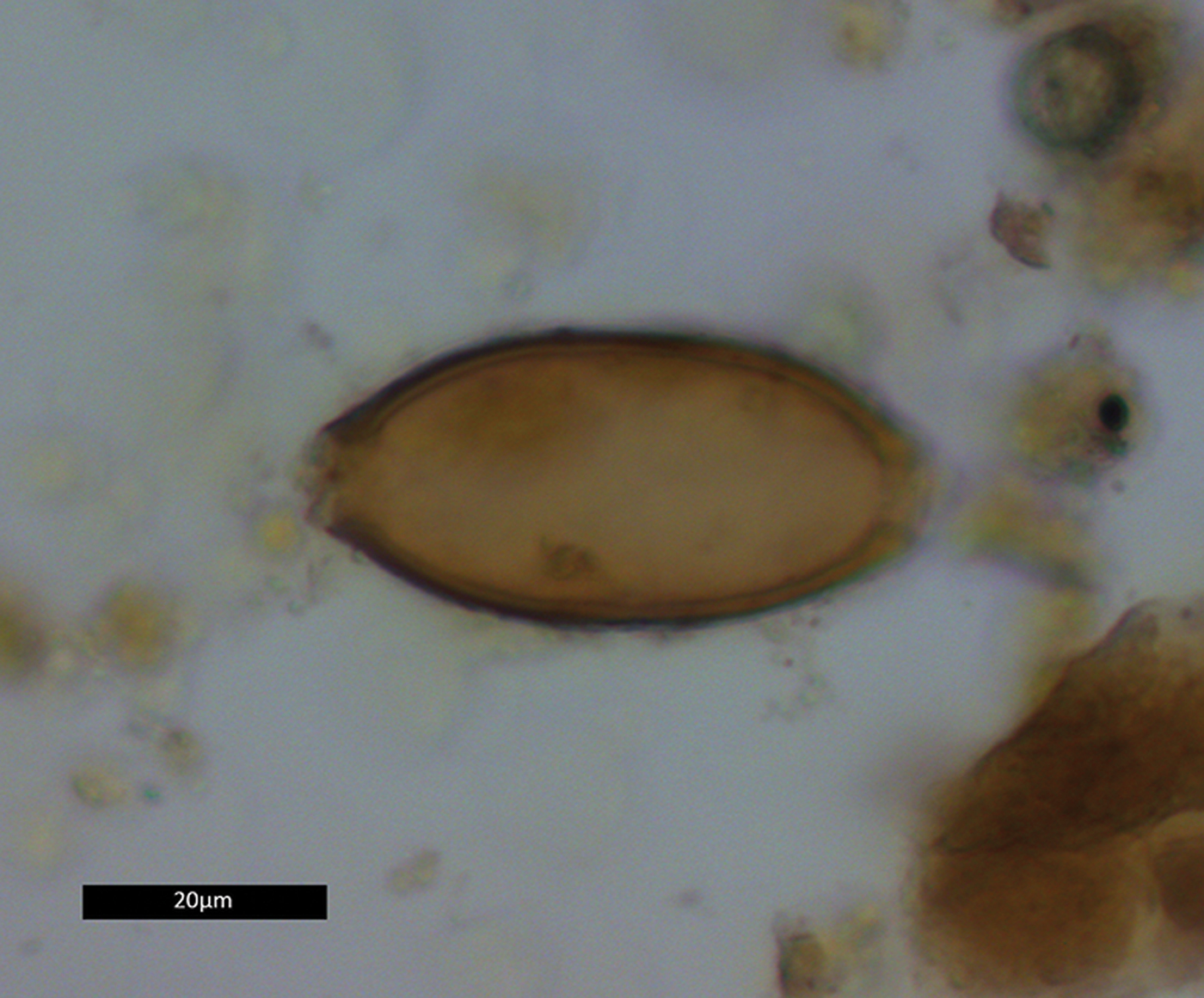
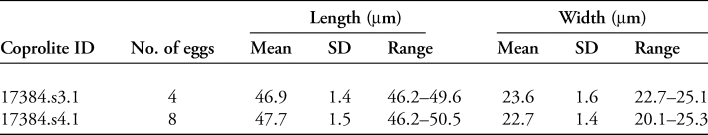
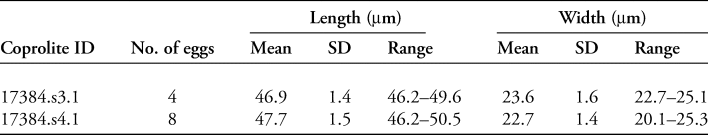
Two coprolites, 17384.s3.1 and 17384.s4.1, tested positive for whipworm eggs (Trichuris sp.) (Figure 5). A total of 12 eggs were recovered: four from s3.1, and eight from s4.1. The eggs were identified by their lemon-shaped form, brown colour, location of apical plugs—although the plugs themselves were lost due to decomposition—and egg dimensions (Table 2). No parasite eggs were identified in the analysed pelvic soil samples.
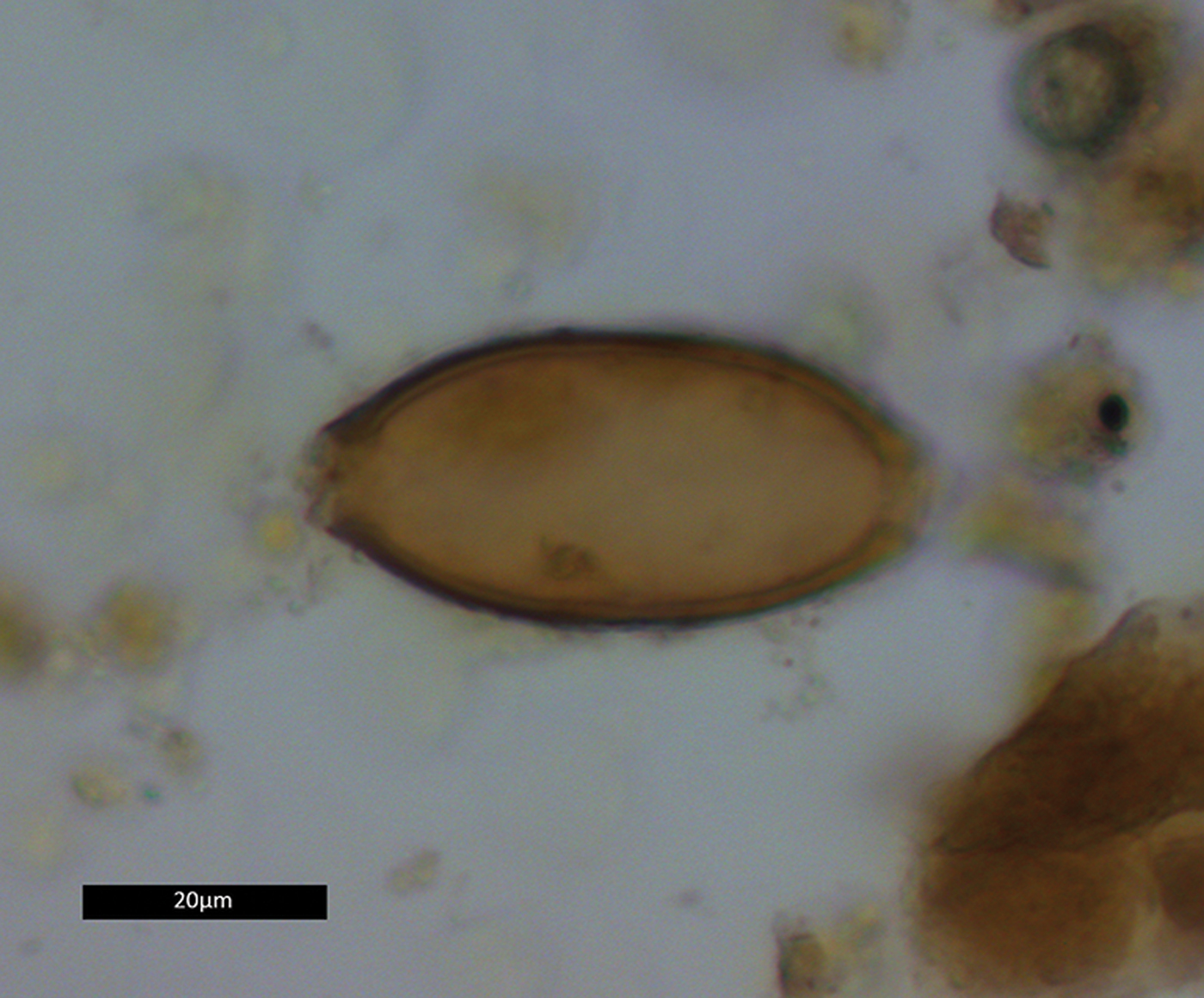
Figure 5. Whipworm egg from coprolite 17384.s4.1. Dimensions 23.1 × 50.2μm (figure by Evilena Anastasiou).
Table 2. Details of the whipworm eggs found in a 1g subsample from each coprolite.
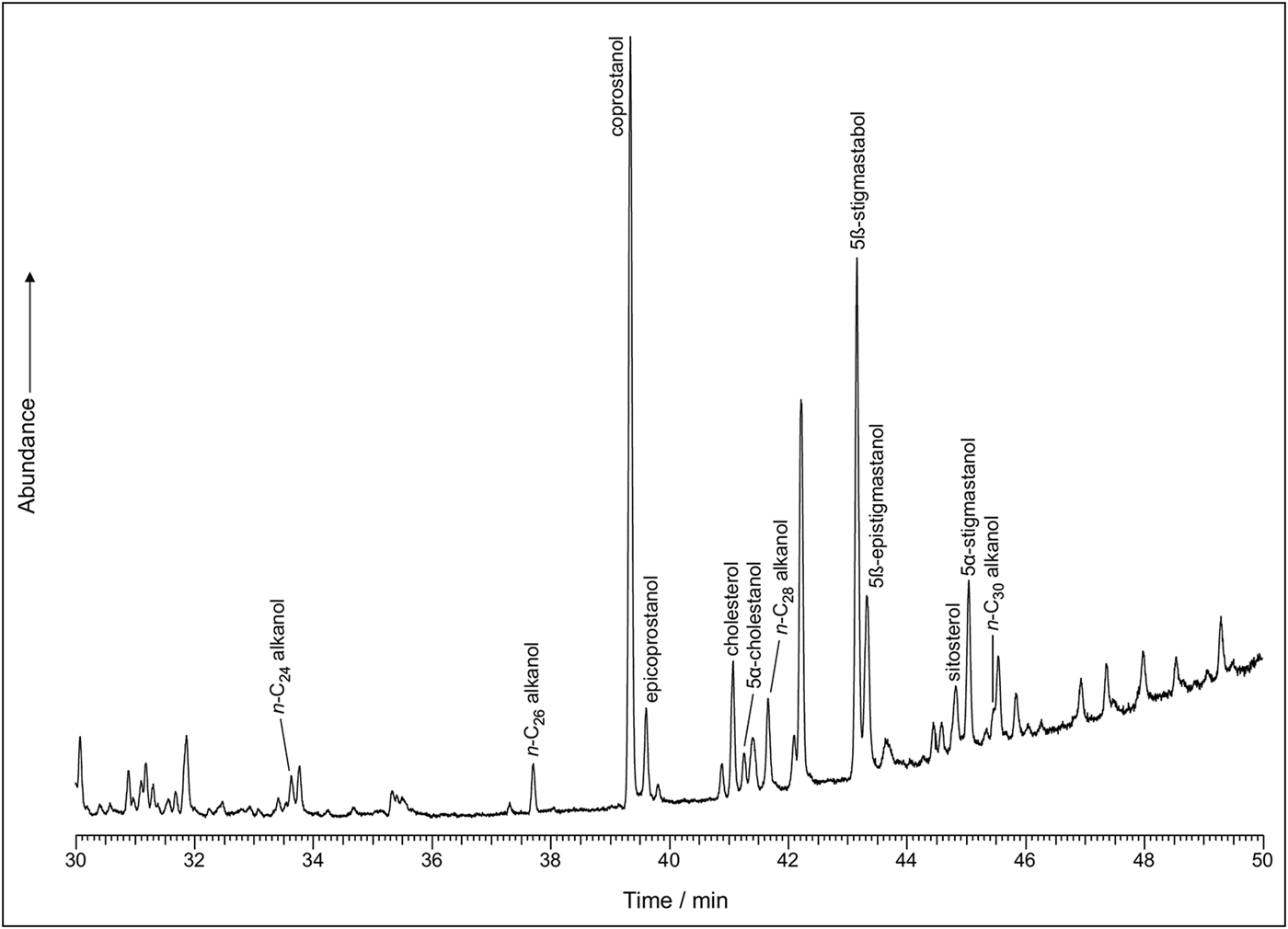
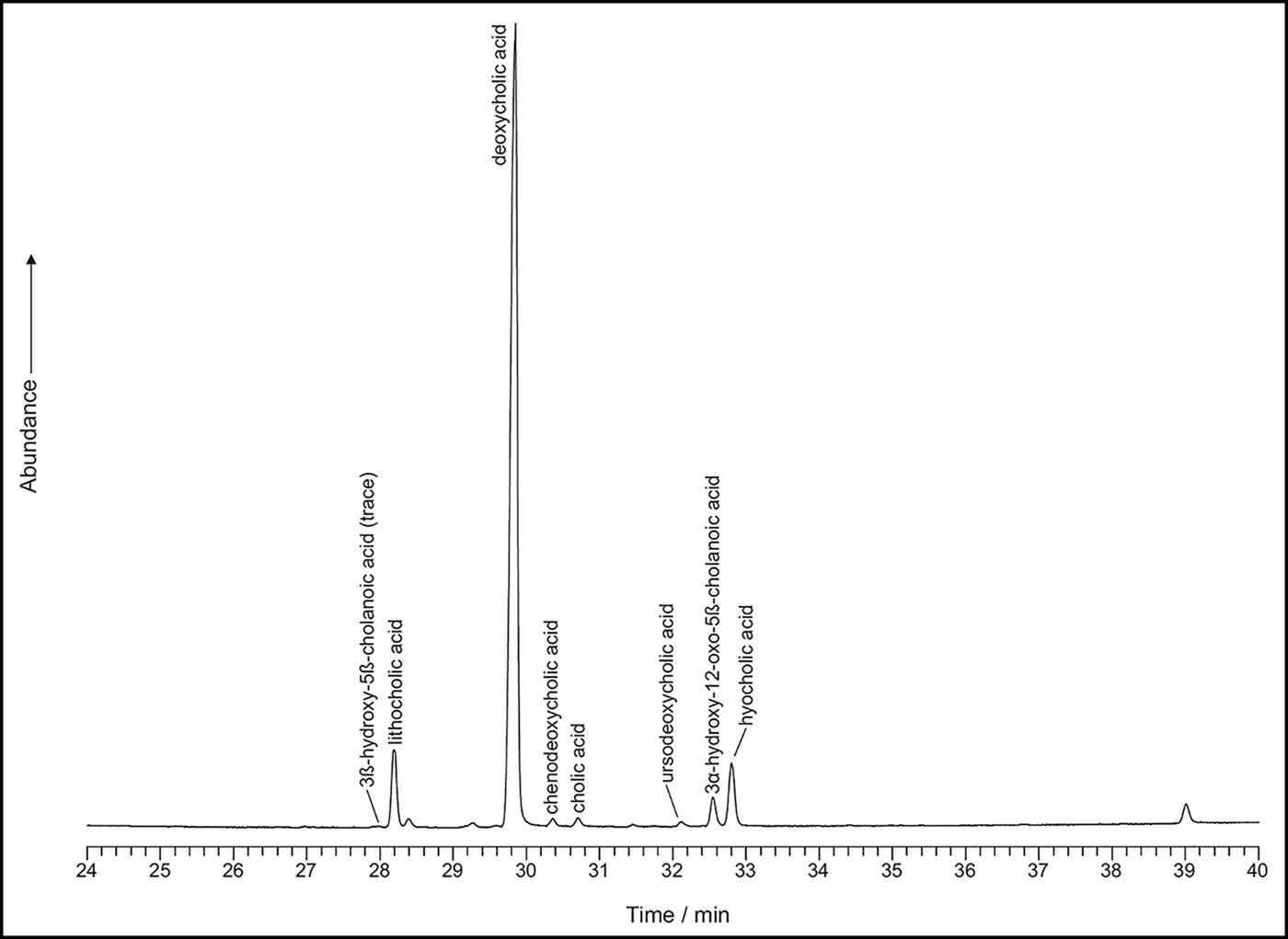
The sterol profiles for both samples show a clear omnivore signal, with a high proportion of coprostanol, smaller proportions of cholesterol and a range of phytosterols (see Figures 6–7). The ratios are presented in Table 3. The bile acid profiles for both samples were dominated by deoxycholic acid, with smaller proportions of lithocholic acids—a signal that indicates a human origin (Bull et al. Reference Bull, Simpson, Van Bergen and Evershed1999, Reference Bull, Lockheart, Elhmmali, Roberts and Evershed2002). Other potential omnivores, such as dog and pig, are excluded, as dogs produce negligible quantities of coprostanol with much higher proportions of cholesterol; the bile acid profile for pigs contains a significant proportion of hyodeoxycholic acid, which is not present in either of the samples analysed.
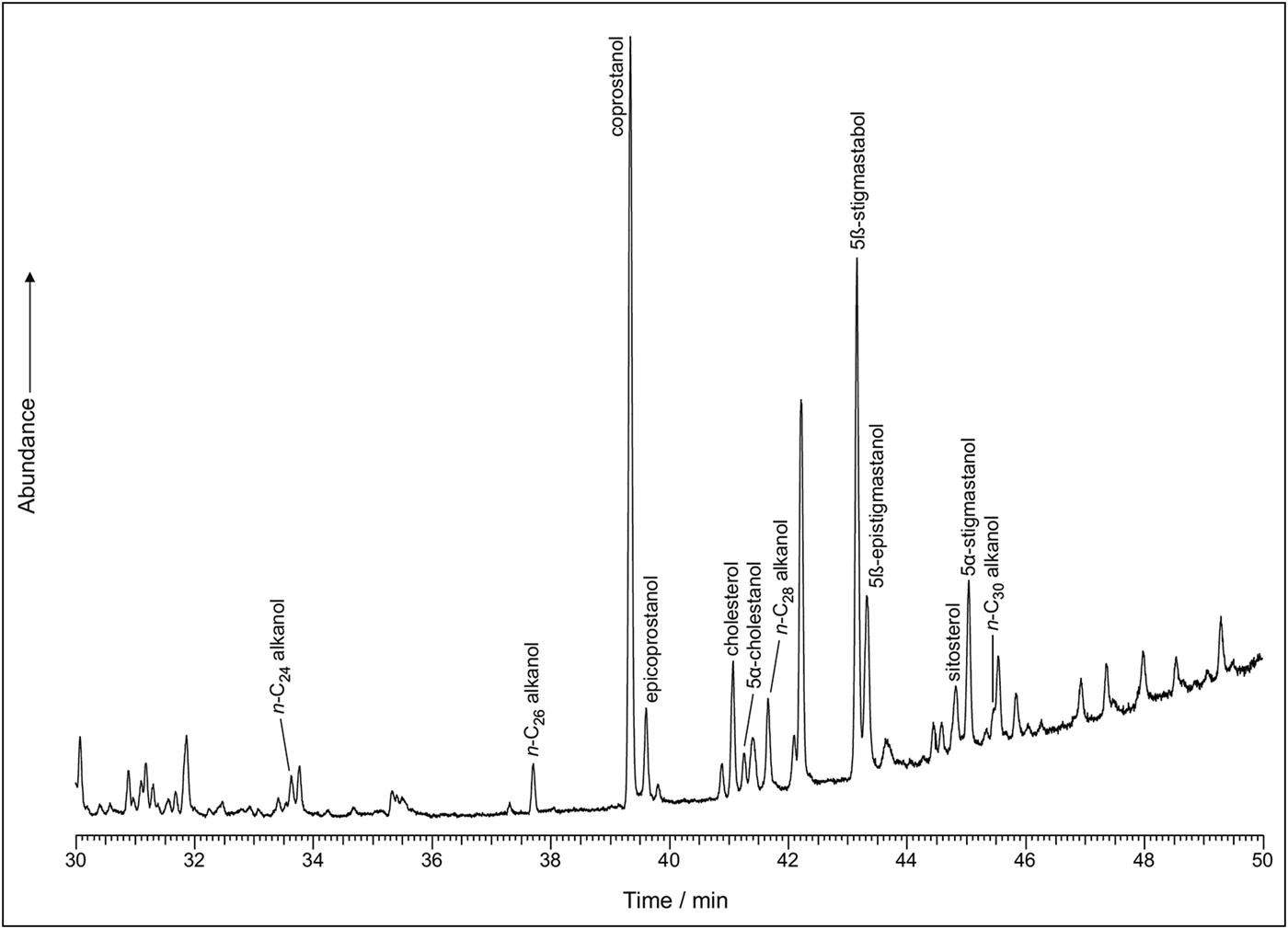
Figure 6. Sterol profile for 17384.s3.1 (figure by Lisa-Marie Shillito & Ian Bull).
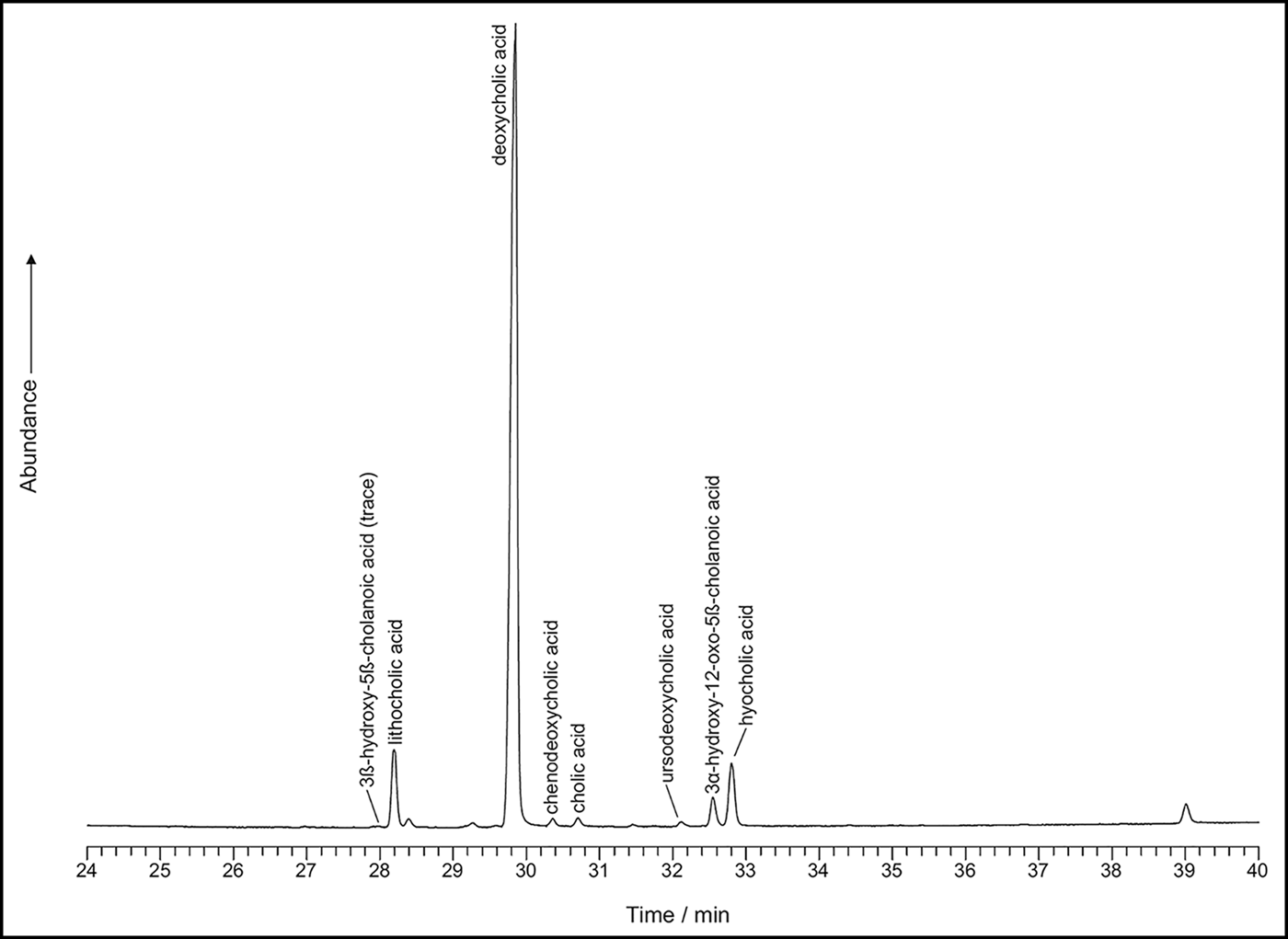
Figure 7. Bile acid profile for 17384.s3.1 (figure by Lisa-Marie Shillito & Ian Bull).
Table 3. Sterol profiles for the two coprolites testing positive for parasite eggs.
Discussion
This is the first evidence for intestinal parasite infection at a Neolithic settlement in the mainland Near East. Two of the four coprolites analysed tested positive for parasite eggs, while the pelvic soil samples from the six burials were all negative. Sterol and bile acid analysis was undertaken to identify whether humans or animals deposited the coprolites. Faecal biomarker analysis, to determine which species produced a particular coprolite, has become a well-established method in archaeology (see Shillito et al. Reference Shillito, Bull, Matthews, Almond, Williams and Evershed2011b, Reference Shillito, Matthews, Bull, Williams, Matthews, Matthews and Mohammadifar2013; Prost et al. Reference Prost, Birk, Lehndorff, Gerlach and Amelung2017). Identification of coprolites gives important information about the host of the parasite and enables researchers to taxonomically identify parasite species that infect different hosts, but whose eggs cannot be distinguished morphologically by microscopic investigation. We did not analyse the pelvic soil samples for faecal biomarkers, as human origin would be clear from skeletal morphology. Sterol profiles of coprolite samples are indicative of carnivore, herbivore or omnivore diet, while bile acids can distinguish between omnivores, such as pigs and humans (Bull et al. Reference Bull, Simpson, Van Bergen and Evershed1999).
In the coprolites analysed here, the high levels of coprostanol combined with lower but still relatively high levels of 5β stigmastanols indicate an omnivore, rather than a carnivore or herbivore signal. The bile acid analysis shows a dominance of deoxycholic acid with smaller amounts of lithocholic acids. This indicates that the faeces were of human origin and not from other omnivores, such as pigs (no hyodeoxycholic acid detected), which might defecate on refuse areas as they scavenge for scraps.
The presence of whipworm eggs in these two coprolites indicates that the faeces forming the coprolites came from humans who were infected with this parasite. It is not possible to determine whether each coprolite came from different individuals or whether both came from the same individual, but from different defecation events. Both human whipworm (Trichuris trichiura) and pig whipworm (Trichuris suis) produce eggs of similar appearance on microscopic investigation. Although pig whipworm eggs do tend to be a little larger than human whipworm, there is some overlap in their size ranges (Beer Reference Beer1976; Mehlhorn Reference Mehlhorn2016). Analysis of sterols and bile acids, however, increases the probability that these eggs are from the human whipworm (Trichuris trichiura).
Whipworm is a parasite that is closely linked to lifestyle and environmental conditions such as sanitation, as it is spread by the faecal contamination of food and water. Human whipworm is thought to have evolved in human ancestors in Africa and spread with early human migrations (Mitchell Reference Mitchell2013); it is a species therefore expected to be present in early populations. Adult worms are 30–50mm in length and they live on the intestinal lining of the large bowel (Garcia Reference Garcia2016: 311). The life cycle involves an infected person defecating, the whipworm eggs then develop in a damp, shady environment for 1–2 weeks, by which time they mature and reach their infective form. If the eggs are then swallowed by another person with their food or water, the eggs hatch in the intestines, develop into adult worms, mate and lay eggs, which mix in with the faeces. Whipworms typically live for up to five years (Weller & Nutman Reference Weller, Nutman and Kasper2014).
The egg concentration recovered from these coprolites was low, at 4–8 eggs g(−1). As egg concentrations are often lower in pelvic soil than coprolites, the absence of eggs in the soil samples may be false negatives. A female whipworm typically produces around 5000 eggs each day (Weller & Nutman Reference Weller, Nutman and Kasper2014). Low egg counts therefore probably indicate that many of the eggs originally present have not survived the last 8000 years. Moreover, it is possible that eggs from other species were present in lower concentrations and are therefore not represented in our samples. Parasite eggs in the soil can be damaged and destroyed by the action of fungi and insects, or washed away by water flowing through the sample over the centuries (Morrow et al. Reference Morrow, Newby, Piombino-Mascali and Reinhard2016). Sample preparation can also damage parasite eggs. The use of concentrated hydrochloric acid (36 per cent) during sample processing, for example, has also been shown to damage the eggs of several species of parasite, such as Capillaria hepatica, although this procedure has little effect on the eggs of most other parasite species (Dufour & Le Bailly Reference Dufour and Bailly2013). While the dilute acid (10 per cent HCl) used here was necessary to disaggregate the mineralised coprolites, it is possible that it may have reduced the number of eggs in the sample.
If the people who deposited these coprolites genuinely had a low number of worms in their intestines, they may have been asymptomatic, and their health may not have been impaired by the infection. For individuals who are infected by many worms, however, research on modern patients shows that whipworm infection can lead to abdominal pain, anaemia, diarrhoea, stunted growth and reduced intelligence in children (Stephenson et al. Reference Stephenson, Holland and Cooper2000; Bethony et al. Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006; Jourdan et al. Reference Jourdan, Lamberton, Fenwick and Addiss2018). Growth has been studied in juvenile skeletons from Çatalhöyük. Comparison of stature and body mass estimates with those from skeletal collections of well-nourished individuals indicates that health and nutrition were adequate to maintain similar rates of growth in children at Çatalhöyük (Hillson et al. Reference Hillson, Larsen, Boz, Pilloud, Sadvari, Agarwal, Glencross, Beauchesne, Pearson, Ruff, Garofalo, Hager, Haddow and Hodder2013). Skeletal indicators of anaemia, such as cribra orbitalia, have been recorded in the human remains, especially in infants (Molleson et al. Reference Molleson, Andrews, Boz and Hodder2005). It is not easy to differentiate whether this anaemia might be related to whipworm infection, to a diet low in iron or folate, or to the presence of other infections, such as malaria.
Past parasite analysis at other Neolithic sites in the Mediterranean region has shown a mixed picture of intestinal infection. There tend to be zoonotic species that spread to humans from animals, and geohelminths, which are spread from one human to another by the contamination of food or water with human faeces. At Çatalhöyük, we have only found evidence for the geohelminth whipworm. Parasite species in Mediterranean populations during the Bronze Age, Iron Age, Roman and medieval periods, however, were dominated by whipworm and roundworm (geohelminths), with the parasite species requiring animals to complete their life cycles becoming increasingly rare (Mitchell Reference Mitchell2015b, Reference Mitchell2017b; Williams et al. Reference Williams, Arnold-Foster, Yeh, Ledger, Baeten, Poblome and Mitchell2017; Anastasiou et al. Reference Anastasiou, Papathanasiou, Schepartz and Mitchell2018; Ledger et al. Reference Ledger, Stock, Schwaiger, Knipping, Brückner, Ladstätter and Mitchell2018). This might be explained by a change in lifestyle from the Neolithic mixed subsistence pattern of farming coupled with the hunting of wild animals and the gathering of wild produce, to later subsistence that was heavily reliant upon farming cereals, eating fruit and herding domesticated animals, such as sheep and goats (Petroutsa & Manolis Reference Petroutsa and Manolis2010; Pokutta Reference Pokutta2017). We should also consider the possibility that preservation conditions in the Mediterranean region may contribute to the apparent reduction of parasite species diversity over time. We would, however, expect that species that have equally robust eggs to whipworm—Taenia tapeworms, for example—could also survive in this environment.
Çatalhöyük is particularly interesting as it represents one of the best-preserved, large Neolithic settlement agglomerations in the Near East. Excavation has demonstrated the population's reliance upon cereal crops and animal herding for subsistence. While plant remains from the site do include legumes and fruit and nuts, the vast majority of domesticated plant remains were of wheat and barley (Ryan Reference Ryan and Hodder2008; Bogaard et al. Reference Bogaard, Charles, Livarda, Ergun, Filipović, Jones and Hodder2013, Reference Bogaard, Filipovic, Fairburn, Green, Stroud, Fuller and Charles2017). Domestic sheep and goats were eaten regularly and were overwhelmingly the most common animal species found at the site, whereas cattle were eaten at feasts (Bogaard et al. Reference Bogaard, Charles, Livarda, Ergun, Filipović, Jones and Hodder2013; Russell et al. Reference Russell, Twiss, Orton, Arzu Demirergi and Hodder2013). While there is some evidence for the consumption of wild deer, wild horse, birds, wild pigs and small freshwater fish, most of the meat consumed was from domesticated sheep and goats (Russell et al. Reference Russell, Twiss, Orton, Arzu Demirergi and Hodder2013; Van Neer et al. Reference Van Neer, Gravendeel, Wouters, Russell and Hodder2013).
Diet at Çatalhöyük appears to resemble more closely that of Mediterranean Bronze Age and Iron Age populations than other Neolithic sites that have previously been studied for parasites in Cyprus and Spain (Harter-Lailheugue et al. Reference Harter-Lailheugue, Mort, Vigne, Guilaine, Brun and Bouchet2005; Maicher et al. Reference Maicher, Hoffmann, Côté, Pérez, Segui and Le Bailly2017). The research presented here suggests that the parasite species present at Çatalhöyük were also unlike those present at the other aforementioned Neolithic sites in the Mediterranean. In a pattern similar to that observed in later periods in the Mediterranean, geohelminths were dominant at Neolithic Çatalhöyük, while zoonotic parasites were not found in the analysed samples. Crowded living conditions, with middens containing human excrement located directly adjacent to houses, probably played a role in the transmission of whipworm, as it is spread by the faecal-oral route; a move away from reliance on wild animals may have decreased the presence of zoonotic species. We do need to consider the limited size of our sample; it is possible that other parasite species might be identified if future work recovers more coprolites from elsewhere at the site. From the evidence acquired so far, however, it appears that the lifestyle and diet of the inhabitants of Çatalhöyük resulted in fewer species of intestinal parasite in the population than were present at other Neolithic sites in the Mediterranean region.
Conclusion
Through analysis of both coprolites and pelvic soil from burials at Çatalhöyük, this study has investigated intestinal parasitic infection in the inhabitants of the large Neolithic settlement over 8000 years ago. The results demonstrate that a proportion of the population was infected with whipworm. While the sample size is small, we have found fewer species of parasite at this site than is the case for studies of other Neolithic sites in Cyprus and Spain. The pattern at Çatalhöyük is more representative of what would be expected at Bronze and Iron Age sites in the Mediterranean. This would suggest that the organised nature of Çatalhöyük—its housing, infrastructure, socio-cultural practices and subsistence strategies—may have reduced the diversity of parasitic infection among the inhabitants of the site, a phenomenon more normally associated with much later time periods.
Acknowledgements
The parasite analysis was supported by a doctoral award from the Social Sciences and Humanities Research Council of Canada (752-2016-2085) and by a Tidmarsh Cambridge Scholarship from the Cambridge Commonwealth, European and International Trust and Trinity Hall College (to M.L.L.). This study received financial support from the French State under the ‘Investments for the Future’ programme, ‘Initiative d'Excellence’, Université Bordeaux (ANR-10-IDEX-03-02) (to C.J.K.). The UK National Environmental Research Council (NERC) provided funding for the mass spectrometry facilities at Bristol (contract no. R8/H12/15).