Introduction
Antarctic seabirds are long-lived marine predators which behave as central-place foragers during the breeding period (Orians & Pearson Reference Orians and Pearson1979, Weimerskirch Reference Weimerskirch2007). However, these animals spend a substantial part of their life not linked to a central place, including during juvenile, immature and interbreeding life-cycle phases. Like in other marine ecosystems, the vast majority of research on southern seabirds’ utilization of marine habitat has focused on the breeding period, and acquiring data about other life-cycle phases is now considered a priority (e.g. Weimerskirch et al. Reference Weimerskirch, Akesson and Pinaud2006, Thiebot et al. Reference Thiebot, Cherel, Trathan and Bost2012). The juvenile phase is especially poorly known in seabirds, and in penguins (Spheniscidae) in particular, despite being a critical period for the birds. Indeed, they need to develop both foraging and anti-predator skills at a time when they are potentially more vulnerable than adults to the different threats that may affect their survival, because of both their inexperience and their larger at-sea distribution (Weimerskirch et al. Reference Weimerskirch, Akesson and Pinaud2006, Trebilco et al. Reference Trebilco, Gales, Baker, Terauds and Sumner2008). Consequently, low survival rates often characterize the juvenile phase, and the rapid acquisition of an efficient foraging behaviour is vital for the juvenile birds, especially in extreme environments such as the Antarctic (Ponganis et al. Reference Ponganis, Starke, Horning and Kooyman1999).
In this study we intended to investigate the habitat used by juvenile emperor penguins (Aptenodytes forsteri Gray) from Pointe Géologie colony, Terre Adélie, Antarctica, during their post-natal dispersal. We were particularly interested in surveying juveniles dispersing from Pointe Géologie since in this colony, the breeding population decreased by 50% during the late 1970s in relation with drastic changes in sea-ice extent, and never recovered since then (Barbraud & Weimerskirch Reference Barbraud and Weimerskirch2001, Jenouvrier et al. Reference Jenouvrier, Barbraud, Weimerskirch and Caswell2009, Barbraud et al. Reference Barbraud, Gavrilo, Mizin and Weimerskirch2011; see also Ainley et al. (Reference Ainley, Ballard, Blight, Ackley, Emslie, Lescroël, Olmastroni, Townsend, Tynan, Wilson and Woehler2010) and Ainley & Ballard (Reference Ainley and Ballard2012) for a potential role of predation). Juvenile emperors may suffer high mortality rates indeed when dispersing, even though travelling in apparently favourable environments (Wienecke et al. Reference Wienecke, Raymond and Robertson2010). It therefore appeared of prime importance to gather data on Pointe Géologie emperor penguins’ at-sea ecology during what could be the most critical period of their life-cycle.
Our main goal was to depict the marine habitat used by these juvenile emperor penguins during their post-natal dispersal, with a special emphasis on their association with sea ice. Adult emperors are known to be confined to waters that are covered at least seasonally by sea ice, while juveniles may exploit much more distant habitat when dispersing from the colonies (Ross Sea: Kooyman et al. Reference Kooyman, Kooyman, Horning and Kooyman1996, Kooyman & Ponganis Reference Kooyman and Ponganis2008; southern Indian Ocean: Wienecke et al. Reference Wienecke, Raymond and Robertson2010). Especially, these studies curiously showed emperor juveniles from different Antarctic regions consistently reach permanently open ocean zones, while it has been shown in other species that juvenile penguins may disperse differently from one colony to another (Thiebot et al. Reference Thiebot, Lescroël, Pinaud, Trathan and Bost2011). Therefore, we intended to measure whether juveniles from Pointe Géologie colony also depend on ice-free areas during their post-natal dispersal, in order to help developing trend models for the population.
Second, the fact that penguins truly exploit their environment in three dimensions to forage (Williams Reference Williams1995) suggests it is essential to investigate simultaneously their horizontal and vertical use of the marine environment in order to get a reliable approach of their foraging habitat (Charrassin & Bost Reference Charrassin and Bost2001). Yet, the use of the water column during post-natal dispersal in juvenile emperor penguins remains inadequatly documented (Ponganis et al. Reference Ponganis, Starke, Horning and Kooyman1999) and has never been analysed in concomitance with spatial (horizontal) data. In this study, we benefited from recent technological developments and used state-of-the-art electronic devices to carry out this first three-dimensional survey.
According to the previous pioneering studies, we expected a much larger at-sea distribution of juveniles compared to breeding adults (i.e. a less tight association with sea ice). We also predicted marked differences in their diving behaviour depending on the marine habitat they use during their journey. More specifically, we predicted the juveniles would use deeper water layers in ice-free zones than in the pack ice, in line with prey availability in these contrasting habitats (Dewitt et al. Reference Dewitt, Heemstra and Gon1990, Lancraft et al. Reference Lancraft, Hopkins, Torres and Donnelly1991, Knox Reference Knox2007).
Materials and methods
Study area and species
Fieldwork was conducted at the Pointe Géologie colony (Dumont D'Urville station, 66°39′S, 140°00′E), Terre Adélie, Antarctica. This coastline extends with a thin continental shelf that abruptly faces abyssal plains (Fig. 1). Based on the major environmental contrasts of this area, we considered four horizontal marine habitats going from south–north (Knox Reference Knox2007). First, the Permanent Pack-Ice Zone (PPIZ), located between the Antarctic coast and the northern limit of pack ice in summer (on 15 March 2010). Second, between the northern limit of pack ice in summer and its northern limit in winter (on 15 September 2010) is the Seasonal Pack-Ice Zone (SPIZ). These two first zones, covered at least seasonally by sea ice, share the westward influence of the Antarctic Coastal Current. Third, is the Ice-free Zone (IFZ), limited to the north by the Polar Front and never covered by sea ice. Finally, up north lies the fourth habitat: the warmer Polar Frontal Zone (PFZ), which is comprised between the Polar and the sub-Antarctic Fronts. The IFZ and PFZ zones are free from sea ice all year round and are under the eastward influence of the Antarctic Circumpolar Current.

Fig. 1 Map of the study region showing the movements performed by the six juvenile emperor penguins satellite-tracked during their post-natal dispersal (colour refers to tag ID as follows: purple for ID 67, red for ID 68, blue for ID 69, turquoise for ID 70, green for ID 71 and black for ID 72). The colony of origin (Pointe Géologie, Terre Adélie) is indicated by the white star. The four natural boundaries separating marine habitats are illustrated: the summer pack-ice edge (white dashed line 1), the winter pack-ice edge (white dashed line 2), the Polar Front (black dashed line 3), and the sub-Antarctic Front (black dashed line 4). Starting from the Antarctic coast, these boundaries serve as northern limit for the four marine habitats considered in our study: the Permanent Pack-Ice Zone (PPIZ), the Seasonal Pack-Ice Zone (SPIZ), the Ice-Free Zone (IFZ) and the Polar Frontal Zone (PFZ), respectively. The isobaths for 200, 1000 and 3000 m are also shown.
Emperor penguins start breeding in the autumn (March–April) when adults arrive on the colonies. Eggs hatch during winter, and chicks fledge in early summer (December) when sea ice suddenly melts and food is locally abundant (Prévost Reference Prévost1961). Chicks leave the colony with a body-mass equivalent to only 60% that of adults, this proportion being the lowest among penguins (Prévost Reference Prévost1961). Juveniles usually remain at sea for three years before returning for their first breeding attempt (Mougin & Van Beveren Reference Mougin and van Beveren1979).
Remote tracking of the animals
On 7 December 2009, we equipped six juvenile emperor penguins with SPLASH tags (Wildlife Computers, Redmond WA, USA) just before their first departure at sea. Individuals most advanced in their fledging were chosen. Tags were fitted to the middle-lower back to reduce drag (Bannasch et al. Reference Bannasch, Wilson and Culik1994), and fixed to the feathers using cyanoacrylate glue (Loctite 401) and cable ties. These oblong devices weighed 62 g in air (0.34–0.44% of a juvenile body mass) and 25.2 g in seawater, and had a cross-sectional area of 3.2 cm2 (< 1% of a bird's cross-sectionnal area). The antenna was 8 cm long, 1.6 mm thick and inclined 45° backwards. Birds were weighed before being released (Table I).
Table I Summary of the six individual surveys of juvenile emperor penguins tracked from Pointe Géologie colony during post-natal dispersal: body mass at equipment, days still on the colony after equipment, tracking duration since equipment, number of weeks without location during transmission day (missed locations), maximum range reached from colony, total linear distance travelled since departure and mean linear distance covered weekly for each individual; mean and standard deviation (SD).

The SPLASH tags are composed of a logger module, collecting diving data, and a transmitter module, that communicates the logged data and the signal for ARGOS location via satellite. In our study we programmed the tags to transmit every eighth day data relative to the seven previous days; several ARGOS locations were acquired each transmission day. Five activity parameters were measured: maximum dive depth, dive duration, time at temperature, time at depth and hourly time the logger was out of water. We focused on those datasets particularly relevant in considering penguins’ habitat use: ambient temperature measured, time-at-depth and time dry. These parameters were collected every 10 s and then archived as percentages calculated on 24 hours among chosen classes, except for the time spent dry which was archived as hourly percentages. Upper thresholds chosen for the 14 classes of ambient temperature values ranged from -3 to > 9°C with 1°C interval. For the time-at-depth percentages, upper bins ranged from 0 to > 400 m, with 10 m intervals for depths < 50 m and 50 m intervals for depths > 50 m up to 400 m. Transmissions of the tags were programmed to pause when haulout exceeded 12 consecutive dry hours.
Analysis of transmitted data
According to the data sampling rate (see above), our investigation was carried out on a weekly basis, using only the location with the best ARGOS class for each week's transmission day. We did not average locations received on the transmission day because we felt that it was better to use the most certain location rather than an average of less certain ones. Distance between the penguins' weekly location and the concomitant pack ice northern edge (≥ 15% ice cover) was measured using daily sea ice concentration data derived from passive microwave imagery (AMSR-E, 12 km resolution, http://nsidc.org/data/, accessed July 2011) at the corresponding longitude. We compared the penguins’ use of marine habitats based on movement as well as activity datasets, using R 2.9.0 (R Development Core Team 2009). When normality was verified (using Shapiro-Wilk normality test), a one-way ANOVA was conducted to detect significant differences between individuals, habitats or depth classes; otherwise we used a Kruskal-Wallis rank sum test. Tukey's ‘Honest Significant Difference’ method was used for post-hoc multiple comparisons of means. The level of significance of all tests was set to 0.05. Values indicated are mean ± SD.
Results
Equipped juveniles left the colony 0–4 days after deployment date (Table I), consistent with most of the juveniles from the colony. The tags transmitted data during an average of 98 days (range: 24–255) after deployment, however, there were 19 gaps in total in the weekly locations before the tags ceased emitting, and no time-at-depth data were transmitted during weeks 14 and 37 after deployment for all tags still functioning at that time.
Horizontal journey of the juvenile penguins
All six individuals dispersed northwards (Fig. 1) in the Australian-Antarctic Basin, immediately after entering the water. Only three weeks after departing from their colony, all of them had crossed the pack-ice edge to warmer waters (Fig. 2). From late December to early January, all tracked juveniles entered the IFZ (Fig. 3), while a first tag (ID 67) ceased transmitting on 31 December. The five juveniles still tracked then reached relatively low latitudes from 58.7°–54.7°S between mid-January and mid-March (Fig. 1). All of them were at that time located in 5–7°C water temperatures (Fig. 2), mainly in the IFZ and PFZ (Fig. 3) north of the Polar Front, more than 1250 km north of the pack-ice edge (Fig. 2). At this time, four of them had reached the South-east Indian Ridge area, while the last one remained in the deeper Australian Antarctic Basin (Fig. 1). Three tags ended transmitting, on 14 January, 11 February and 18 February (IDs 71, 70 and 72, respectively), while all three were still moving northwards. Then, both remaining juveniles engaged in a reverse movement southwards, starting in mid-February and mid-March for IDs 69 and 68, respectively. This southward movement lasted until mid-April when both individuals reached latitudes close to 65°S, near the Antarctic shelf and less than 15 km north of the pack-ice edge. On 15 April, individual ID 68 ceased transmitting while back as close as 199 km to Pointe Géologie. The last individual still tracked from then (ID 69) exhibited completely different bearing by travelling westwards over nearly 3000 km in front of the Antarctic shelf slope, and seemed to associate more closely with the pack ice in 0°C waters (Fig. 2), except from mid-May to mid-June, during which latitude transmitted was twice north of 60°S. In early July, a minimum of 88.6°E was reached in longitude, i.e. approaching the south-eastern tip of the Kerguelen Plateau (Fig. 1). Finally, this penguin suddenly returned back eastwards, into the pack ice (Fig. 2), until the tag stopped emitting on 19 August, at 60.1°S, 103.0°E, after a functioning period of over 250 days.
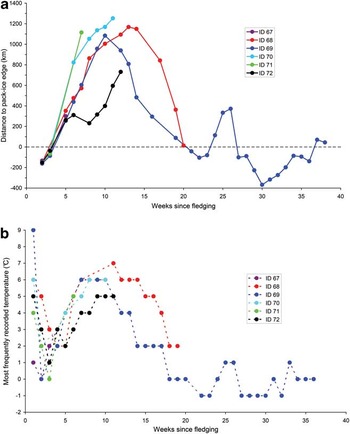
Fig. 2 a. Distance between the weekly location of the juvenile penguins tracked and the northern edge of the pack ice for the same day. Negative values indicate that individuals are situated to the south of the pack-ice edge (into pack-ice zone). b. Ambient temperature recorded by the tags fitted on the juvenile penguins: values are the weekly mode of daily values archived for each individual. In both panels, individual colour coding used is the same as in Fig. 1.

Fig. 3 Number of juvenile penguins located in the different marine habitats for each week of the survey: Permanent Pack-Ice Zone (PPIZ), Seasonal Pack-Ice Zone (SPIZ), Ice-free Zone (IFZ) and Polar Frontal Zone (PFZ).
Use of the Southern Ocean by the juvenile penguins
Average tracking duration was c. 100 days (Table I) and varied considerably between individuals. Maximum range and total linear distance travelled also greatly varied between individuals (1324 ± 610 km and 2579 ± 2385 km, respectively) but only total distance was significantly correlated with tracking duration (Spearman's rho 4 = 0.714, P = 0.14 and rho 4 = 0.943, P = 0.02, respectively). Mean proportion of time spent in the PPIZ was relatively small (Table II) and was restricted to the first locations of the juveniles’ post-natal dispersal. The penguins spent significantly more time in the SPIZ and IFZ than in the PFZ (one-way ANOVA, F 3 = 4.50, P = 0.01; Tukey's multiple comparisons of means, adjusted p-values: P = 0.04 and P < 0.02, respectively).
Table II Number (n) and proportion (%) of locations of the surveyed juvenile penguins in each marine habitat during post-natal dispersal: Permanent Pack-Ice Zone (PPIZ), Seasonal Pack-Ice Zone (SPIZ), Ice-Free Zone (IFZ) and Polar Frontal Zone (PFZ); mean and standard deviation (SD) for the six individuals.

At a finer scale, average distance covered weekly (172 ± 22 km) was not significantly different between individuals (one-way ANOVA, F 5 = 0.61, P = 0.69), however, it varied significantly with the marine habitat crossed (one-way ANOVA, F 3 = 3.78, P < 0.02). This distance covered weekly was significantly higher when the IFZ was crossed (229 ± 83 km; Fig. 4) compared to the PPIZ (117 ± 111 km; Tukey's multiple comparisons of means, adusted p-value: P < 0.01).
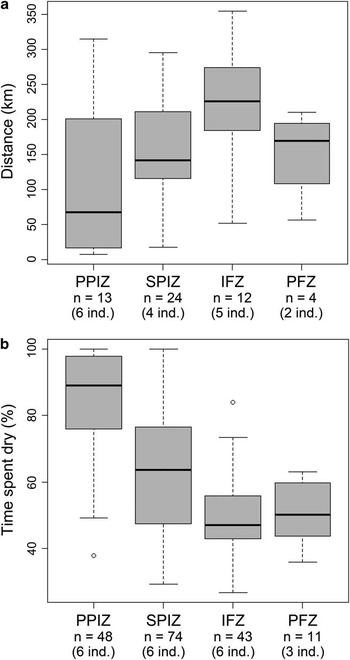
Fig. 4 a. Distance covered weekly, and b. proportion of time with logger dry, for the juvenile penguins according to the marine habitats crossed: Permanent Pack-Ice Zone (PPIZ), Seasonal Pack-Ice Zone (SPIZ), Ice-free Zone (IFZ) and Polar Frontal Zone (PFZ). Number of samples available for each environment as well as number of individuals contributing are indicated under the axis. For each box, bold horizontal bar is the median value, upper and lower sides are the 75th and the 25th percentiles of the distribution, respectively, and the dashed lines extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box. Data outside that range (outliers) are shown as distinct dots.
The time spent not diving also seemed significantly dependent of the marine habitat used (Kruskal-Wallis test, X 23 = 71.02, P < 0.01). These daily periods when the tag was not immerged contrasted significantly between all combinations of habitats (Tukey's multiple comparisons of means, adusted p-values: P < 0.01) except for the PFZ versus the IFZ (Fig. 4). Penguins spent significantly more time not diving when in the PPIZ (84 ± 17%) compared to the SPIZ (63 ± 16%), and significantly less in the ice-free, more oceanic habitats (c. 50% averages both in the IFZ and PFZ).
Vertical activity of the juvenile penguins in relation with horizontal habitats
Over the entire survey, a total of 196 days of time-at-depth activity were transmitted. Data indicated that five out of the six juvenile penguins surveyed used the 0–10 m layer the day following their equipment (the last individual began the fifth day). This enabled us to directly compare these individuals’ behaviour without correcting for potentially different experience between individuals at same dates. Despite our low sample size, our data seemed to indicate general consistency among juveniles in their relative use of the depth classes (Tukey's multiple comparisons of means, adjusted p-values: P > 0.1): hence, individual surveys were thereafter merged.
The time-at-depth dataset indicated a bi-modal use of the water column, whatever the marine habitat (Fig. 5). The first and greatest mode was for 0–10 m deep, and may correspond to travelling dives and shallow feeding: juveniles spent on average over 50% of their time at these shallow depths (up to 58.4 ± 14.4% in the PPIZ), without significant difference in the median time proportions between the habitats (Kruskal-Wallis test, X 23 = 2.62, P = 0.45). Until 50 m deep, all average time fractions decreased below 5%, without significant effect of the habitat (Kruskal-Wallis tests, all P > 0.14). The second and lesser mode in time-at-depth spanned over the 50–100 and 100–150 m classes, and emphasized deeper vertical activity in the more oceanic habitats. First, juvenile penguins spent significantly more time at 50–100 m when in the IFZ (12.4 ± 4.8%) and in the SPIZ (9.85 ± 7.50%) compared to the PPIZ (2.34 ± 2.35%, Kruskal-Wallis test, X 23 = 17.99, P < 0.01, Tukey's multiple comparisons of means, adusted p-values: P = 0.02 and P < 0.01, respectively). Second, juveniles spent more time at 100–150 m when in the PFZ (10.17 ± 2.80%) than elsewhere (Kruskal-Wallis test X 23 = 13.82, P < 0.01). Layers between 150–200 and 200–250 m were much less used, without any such deep dive in the PPIZ and similar time fractions < 3% elsewhere (Kruskal-Wallis tests, X 22 = 4.73, P = 0.09 and X 22 = 1.09, P = 0.58, respectively). Finally, the 250–300 m dive class was seldom used: this occurred only twice in the longest individual survey, respectively in the SPIZ and IFZ (Table III).
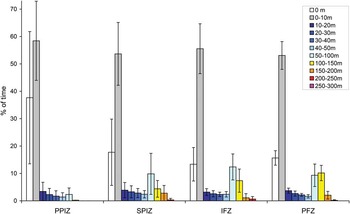
Fig. 5 Time fractions spent at different depth classes by the juvenile penguins surveyed, according to the marine habitat crossed: Permanent Pack-Ice Zone (PPIZ), Seasonal Pack-Ice Zone (SPIZ), Ice-free Zone (IFZ) and Polar Frontal Zone (PFZ).
Table III Detail of the number of individuals involved for each depth class used by the juvenile penguins in our survey. Permanent Pack-Ice Zone (PPIZ), Seasonal Pack-Ice Zone (SPIZ), Ice-Free Zone (IFZ) and Polar Frontal Zone (PFZ).

Discussion
This study represents, to our knowledge, the absolute longest tracking of an emperor penguin (c. 250 days), with also the greatest horizontal movement recorded for this species (c. 7000 km). Total distance travelled was significantly correlated with tracking duration in our study. Thus, these maximal values could be attributed simply to longer lasting batteries, or to lower chances of the instruments being prematurely lost because of their relatively lower impacts to hydrodynamics compared with instruments used in previous studies (see Kooyman & Ponganis (Reference Kooyman and Ponganis2008) and Wienecke et al. (Reference Wienecke, Raymond and Robertson2010)).
Duration of the transmissions and potential impact of the survey on the animals
Given the duration of the longest of our individual surveys, we consider that technical failures are unlikely to be the cause of the early termination of transmissions in other individuals surveyed. It is well known that attachment of satellite tags or data loggers on seabirds, and especially penguins, can greatly impact their foraging performance over prolonged periods (Bannasch et al. Reference Bannasch, Wilson and Culik1994, Bost et al. Reference Bost, Charrassin, Clerquin, Ropert-Coudert and LeMaho2004). However, our longest survey rather suggests that the termination of transmissions in the shortest tracks was due to the natural death of the juvenile penguins at sea (including both starvation and predation) because our method has proven its reliability on other prolonged penguin surveys (e.g. Bost et al. Reference Bost, Charrassin, Clerquin, Ropert-Coudert and LeMaho2004). The two shortest tracks also corresponded to the juveniles with the lowest body mass (Table I), although it is unclear whether lower body mass reflects lesser body condition or more advanced moulting of the chicks. Our range of survey duration together with previous studies in different years and localities (Kooyman & Ponganis Reference Kooyman and Ponganis2008, Wienecke et al. Reference Wienecke, Raymond and Robertson2010) suggests comparable survival rates of juvenile penguins during the first weeks at sea, which may point out a severe limiting factor in population dynamics.
The use of four marine habitats in three dimensions
Even if only two individuals were tracked for durations over 100 days, our data together with previous studies (Kooyman et al. Reference Kooyman, Kooyman, Horning and Kooyman1996, Kooyman & Ponganis Reference Kooyman and Ponganis2008, Wienecke et al. Reference Wienecke, Raymond and Robertson2010) show a greater at-sea distribution range and a looser association with sea ice in juveniles than breeding adults. Moreover, juvenile emperors showed contrasting foraging behaviour according to the habitat used, with deeper dives tending to occur later in the deployments, potentially implying effects of experience on diving and foraging capabilities not directly assessed in our study.
First, the PPIZ was used only in the very early emancipation of juveniles, which never came back to it thereafter. This supports the hypothesis that juvenile penguins remain away from their colonies during post-natal dispersal (Williams Reference Williams1995, Kooyman Reference Kooyman2002), and more specifically that juvenile emperors reach open ocean areas (Kooyman & Ponganis Reference Kooyman and Ponganis2008, Wienecke et al. Reference Wienecke, Raymond and Robertson2010). Indeed, this high latitude habitat is probably avoided during winter due to complete ice cover and reduced daylight at that time, hence limiting the penguins’ foraging ability (Zimmer et al. Reference Zimmer, Wilson, Beaulieu, Ancel and Plötz2008; see also Ainley & Ballard (Reference Ainley and Ballard2012) for predation avoidance hypothesis in such conditions). While in the cold waters of permanent pack ice, juveniles covered the smallest distance weekly (Wienecke et al. Reference Wienecke, Raymond and Robertson2010, this study), a phenomenon associated with much larger fractions than elsewhere of time spent not underwater. Juveniles may indeed take advantage of sea ice to rest and escape from Antarctic predators (Williams Reference Williams1995, Ainley & Ballard Reference Ainley and Ballard2012). They performed relatively shallow dives in this more neritic habitat, with little use of waters deeper than 10 m, only a handful of dives over 100 m and none over 150 m. This diving behaviour was identical to that of juvenile emperors from the Ross Sea at the same stage of their post-natal dispersal (Ponganis et al. Reference Ponganis, Starke, Horning and Kooyman1999). Such shallow dives cannot be interpreted as increased travelling activity (Fig. 3). Rather, it may reflect near-surface feeding on euphausiid and amphipod crustaceans (Ponganis et al. Reference Ponganis, Starke, Horning and Kooyman1999), or foraging right under the fast ice on euphausiids and juvenile Antarctic silverfish Pleuragramma antarcticum Boulenger (Cherel & Kooyman Reference Cherel and Kooyman1998, Ponganis et al. Reference Ponganis, van Dam, Marshall, Knower and Levenson2000). This diet would be fairly consistent with prey juvenile emperors were used to being fed with on the colony (Offredo & Ridoux Reference Offredo and Ridoux1986).
The SPIZ was much more used by the juvenile penguins: first, as a transitory habitat on their northward journey, but afterwards it became the almost exclusive habitat of the five last months of the survey. The strong influence of the Antarctic Coastal Current in this area may have led the prolonged westwards movement observed in the last individual tracked, causing the distance travelled weekly to be greater than in the PPIZ. This habitat was mostly used during the autumn and winter, suggesting sufficient intake rates along the growing pack ice despite the short duration of daylight (down to five hours in July at 63°S). In these cold waters (0–2°C), juveniles tended to exploit more the mesopelagic waters than in the PPIZ: they increased their time spent underwater, while they spent much more time deeper than 50 m, especially at depths greater than 100 m, with dives reaching 200–250 m. In the SPIZ, the myctophid fish Electrona antarctica Günther and Gymnoscopelus braueri (Lönnberg) constitute the dominant prey available to penguins in the upper 500 m of the water column during winter (Lancraft et al. Reference Lancraft, Hopkins, Torres and Donnelly1991). Such prey could thus be targeted by juvenile emperors at this time of the year. Squids may also represent a significant part of this diet in autumn and winter (Ainley et al. Reference Ainley, Fraser, Smith, Hopkins and Torres1991).
During their northward journey, all of the six studied juveniles reached the IFZ, with four of them subsequently reaching the PFZ. The juvenile penguins seemed to forage similarly in these two relatively warmer areas. They showed deep-diving behaviour, with an increased use of deeper water layers and maximum dive depths recorded up to 300 m exclusively in these two habitats, together with the smallest time fractions spent on the sea surface and high distance covered weekly. This suggests a very substantial increase in the foraging effort in relation with prey found at great, specific depths. By analogy with the diving behaviour of king penguins Aptenodytes patagonicus Miller (Charrassin & Bost Reference Charrassin and Bost2001), we can assume that juvenile emperor penguins near the Polar Front fed on small mesopelagic fish (such as myctophids) but also on squids (Kooyman Reference Kooyman2002, Wienecke et al. Reference Wienecke, Raymond and Robertson2010). This would represent a drastic change from the diet fed by their parents on the colony (Offredo & Ridoux Reference Offredo and Ridoux1986) as silverfish are not found north of 60°S (Dewitt et al. Reference Dewitt, Heemstra and Gon1990). This shift in diet would be consistent with the change in both horizontal and vertical habitat exploited.
Why such northerly and temporary exodus?
This study also invites speculation about the adaptive mechanisms driving the juvenile emperor penguins to migrate towards the Polar Front during the first phase of their post-natal dispersal. Previous studies conducted in other regions of Antarctica also highlighted this pronounced northward post-natal dispersal in juvenile emperor penguins (Kooyman et al. Reference Kooyman, Kooyman, Horning and Kooyman1996, Kooyman & Ponganis Reference Kooyman and Ponganis2008, Wienecke et al. Reference Wienecke, Raymond and Robertson2010), which therefore seems to be a fundamental behavioural trait in the species, the reason for which being ‘a mystery’ for these authors (Kooyman & Ponganis Reference Kooyman and Ponganis2008). Interestingly, this successive pattern of north-south-west headings in juvenile dispersal has also been observed in another Antarctic penguin species (Clarke et al. Reference Clarke, Kerry, Fowler, Lawless, Eberhard and Murphy2003). Recent analyses in seabirds’ migratory navigation (Guilford et al. Reference Guilford, Freeman, Boyle, Dean, Kirk, Phillips and Perrins2011) suggested an ‘exploration-refinement hypothesis’ to account for complex population dispersion and interannual fidelity in individual route. In our case, the movement pattern exhibited by juvenile emperors would seem different yet and more driven by innate mechanisms since: 1) it was consistent between individuals tracked around Antarctica so far, while 2) individual route fidelity is irrelevant here since this movement probably occurs only once in an emperor penguin's lifetime. Another invalidated context is that of insufficient daylight (Zimmer et al. Reference Zimmer, Wilson, Beaulieu, Ancel and Plötz2008), since juvenile penguins were situated at their northernmost locations during summer (mid-January to mid-March) and therefore were not escaping from Antarctic polar night at this time: conversely, they were not taking the opportunity of summer permanent daylight to forage at more southerly latitudes. Hence, we propose hereafter three hypotheses we believe to have priority for further investigations on this peculiar movement.
First, an at-sea spatial segregation of conspecifics according to age-class is possible, as shown in other southern oceanic bird and mammal species (Field et al. Reference Field, Bradshaw, Burton, Sumner and Hindell2005, Weimerskirch et al. Reference Weimerskirch, Akesson and Pinaud2006, Trebilco et al. Reference Trebilco, Gales, Baker, Terauds and Sumner2008). In this case, juveniles would show an at-sea distribution radius greater than that of adults: this would suggest high levels of intra-specific competition in the vicinity of the colonies (Ballance et al. Reference Ballance, Ainley, Ballard and Barton2009). Adult individuals, more efficient foragers especially in the PPIZ where access to the water column is limited, could thus drive juveniles to a competive exclusion outside the colony-adjacent areas during the inter-breeding period of adults. Tracking survey of adult emperors after breeding (Kooyman et al. Reference Kooyman, Siniff, Stirling and Bengtson2004) supports this hypothesis: adults remained south of the pack-ice edge, where they experienced extremely high food demand after fasting during moulting, which suggests intense foraging activity before and after moulting. It might therefore be adaptive for the juvenile penguins to leave the pack ice zone where they may be less efficient foragers than the adults, for another biologically productive area: the open-ocean oceanographic fronts (Kooyman Reference Kooyman2002). This hypothesis is also supported by the fact that our studied juveniles came back close to their colonies at a time when adults go onland to mate and breed, and are hence no longer competing for food with the juveniles around the colony. Tracking of adult emperors from Pointe Géologie colony after breeding would allow quantification of this putative spatial segregation in the foraging areas of adults and our surveyed juveniles.
A second factor that may have favoured the emigration of juveniles outside Antarctic coastal waters is the predation risk (reviewed in Ainley & Ballard (Reference Ainley and Ballard2012)). Predation of Antarctic penguins is chiefly by orcas (Orcinus orca (L.)) and leopard seals (Hydrurga leptonyx (de Blainville)), which preferentially operate in the vicinity of the penguin breeding colonies (Ballard & Ainley Reference Ballard and Ainley2005, see Ainley & Ballard (Reference Ainley and Ballard2012) and references therein). Although there is only little evidence for orca predation on emperor penguins (Prévost Reference Prévost1961), individuals with lesser diving capacities and experience such as juveniles during their early dispersal would represent easier prey than adults for these predators. In this context, individuals dispersing towards oceanic waters, where predation risk is diluted, may have been selected. Predation pressure in Antarctic waters is also supported by the higher time fractions found logger dry in the PPIZ, possibly reflecting the fact that juveniles stood on ice floes, above the sea surface in this habitat.
Finally, in the light of the optimal foraging theory (Pyke et al. Reference Pyke, Pulliam and Charnov1977), individuals with low-efficient foraging strategies, such as juveniles, may gain more intake when exploiting areas with low spatio-temporal heterogeneity, as are oceanic versus coastal areas (Hunt & Schneider Reference Hunt and Schneider1987). In this context, and despite larger depths to be exploited, juvenile emperor penguins may benefit from the lower level of prey aggregation in the PFZ compared to the PPIZ or SIZ (Knox Reference Knox2007). Regarding these three non-exclusive hypotheses it must also be considered that juvenile emperor penguins potentially compete with the more abundant, not globally decreasing and greater experienced adult king penguins when exploiting the PFZ waters (Kooyman Reference Kooyman2002), because both species behave similarly in this habitat (Charrassin & Bost Reference Charrassin and Bost2001, Bost et al. Reference Bost, Charrassin, Clerquin, Ropert-Coudert and LeMaho2004).
Regardless of the fundamental causes of the northward migration of juveniles during their early life, a major outcome of our study is that this movement pattern appears highly consistent between localities throughout the Antarctic continent: in the Ross Sea (Kooyman et al. Reference Kooyman, Kooyman, Horning and Kooyman1996, Kooyman & Ponganis Reference Kooyman and Ponganis2008), East Antarctica (Wienecke et al. Reference Wienecke, Raymond and Robertson2010) and Terre Adélie (this study). Our study completed this knowledge by showing a peculiar, more deep-diving behaviour of the juvenile penguins when exploiting this permanently open ocean zone. Ours and earlier findings suggest that additional research, linking ecology to demography, is now needed to understand the impact of oceanographic variability of ice-free habitats, including the PFZ, on juvenile survival and population dynamics of emperor penguins.
Acknowledgements
This work benefited from an Antarctic Science Bursary to AL for purchasing the tags. The Ethics Committee of Institut Polaire Français Paul-émile Victor approved all field procedures. The authors thank M. Kriloff, M. Pelle and D. Filippi for their help in the field, and A. Goarant and C. Péron for their help and advice with analyses. The present work was supported financially and logistically by the ANR 07 Biodiv ‘GLIDES’, the Zone Atelier Antarctique (INSU-CNRS), the Institut Polaire Français Paul-Emile Victor (IPEV, programme nos 394: resp. C.A. Bost, and 109: resp. H. Weimerskirch) and the Terres Australes et Antarctiques Françaises (TAAF) administration. The constructive comments of the reviewers are gratefully acknowledged.










