Introduction
Recent biodiversity inventories associated with the Census of Antarctic Marine Life programme have revealed that many regions of the Southern Ocean are amongst the least known worldwide. Likewise, some groups of benthic macrofauna remain very poorly studied and need more extensive taxonomic effort (De Broyer et al. Reference De Broyer, Danis and Allcock2011) for even approximate estimates of regional richness. In particular, the Antarctic slope has been very rarely sampled (Kaiser et al. Reference Kaiser, Griffiths, Barnes, Brandao, Brandt and O’Brien2011) despite being a potential site for benthos refugia during glaciations. The shelf seas are the best studied regions around Antarctica, but some of these have order of magnitude fewer samples than others.
The Amundsen Sea remains one of the least biologically investigated continental shelves on Earth. Up to now, there are only two studies of benthic macrofauna of this basin (Kaiser et al. Reference Kaiser, Barnes, Sands and Brandt2009, Linse et al. Reference Linse, Griffiths and Barnes2013). Kaiser et al. (Reference Kaiser, Barnes, Sands and Brandt2009) identified a high number of isopod species which were previously unknown, with 96% of individuals belonging to undescribed taxa. The benthos of the Scotia Arc has been studied more extensively (e.g. Griffiths et al. Reference Griffiths, Linse and Barnes2008, Barnes et al. Reference Barnes, Kaiser, Griffiths and Linse2009 and references therein). Some regions of this sea, such as Shag Rocks and South Georgia, can be treated as benthic richness hot spots, with many previously unknown species and steep species-accumulation curves associated with sampling (Barnes Reference Barnes2008). The Scotia Sea is also proving useful for understanding of the origin and zoogeographical patterns of the Southern Ocean benthos (Griffiths Reference Griffiths2010). The chain of the Scotia Arc islands could serve as a dispersion route for marine fauna, and thus may reveal large-scale distribution pathways for many benthic groups (Barnes Reference Barnes2005). This region is also thought to be a key area of marine diversification and a key speciation centre in the Antarctic (see Linse et al. Reference Linse, Cope, Lortz and Sands2007). Gray (Reference Gray2001) highlighted the need for studies of larger areas and latitudinal patterns in the Southern Ocean, especially in the context of species richness or geographical range characteristics. Despite recent intensification of these types of studies most questions concerning the origin, distribution pathways and drivers of biodiversity change events remain unanswered. Some benthic taxa inhabiting shelves offer potentially important insights into historic biodiversity and biogeographical trends. This is particularly the case with investigations of current connectivity between assemblages of invertebrates with low potential for dispersal, such as tanaidaceans (with sedentary adults), and it could advance our understanding of evolutionary processes associated with past land and sea connectivity.
The Tanaidacea are a group of small, peracarid crustaceans, crawling brooders that live in tubes or buried in the sediment for the most of their life. Due to this, and direct development of young, tanaidaceans are thought to have very low mobility and limited dispersal potential. Taxonomic efforts over the past few decades have resulted in the Tanaidacea of the Southern Ocean being fairly well recognized compared with those of seas elsewhere (Błażewicz-Paszkowycz et al. Reference Błażewicz-Paszkowycz, Bamber and Anderson2012). There have been 160 species recorded from the Southern Ocean (De Broyer et al. Reference De Broyer, Danis and Allcock2011). Previous sample collections around the Southern Ocean show that they can be abundant in many benthic habitats at shelf and abyssal depths (Błażewicz-Paszkowycz et al. Reference Błażewicz-Paszkowycz, Bamber and Anderson2012). In the Antarctic, tanaidaceans have even proved a numerically dominant element of some benthic communities (Siciński et al. Reference Siciński, Pabis, Jażdżewski, Konopacka and Błażewicz-Paszkowycz2012), their abundance has been reported to exceed 146 000 ind. m2 (Delille et al. Reference Delille, Guidi and Soyer1985). Estimates by the Census of Antarctic Marine Life programme suggest that the real number of tanaidacean species could be as high as 40 000, forty times higher than the number of currently known species (Appeltans et al. Reference Appeltans, Ahyong and Anderson2012). However, as with most taxa, data for tanaidaceans is geographically very patchy. Some regions of the Southern Ocean, e.g. the Bellingshausen Sea and eastern Ross Sea, have been more thoroughly sampled and analysed compared to others, such as the Amundsen and Scotia seas (Błażewicz-Paszkowycz Reference Błażewicz-Paszkowycz2013). There are around 50 tanaidacean species known from the Scotia Sea, but the majority of these were recorded from the deep sea (e.g. Kudinova-Pasternak Reference Kudinova-Pasternak1975, Sieg Reference Sieg1986, Jóźwiak & Błażewicz-Paszkowycz Reference Jóźwiak and Błażewicz-Paszkowycz2007). Furthermore, there are no biodiversity assessments of Tanaidacea from the Amundsen Sea.
Recently, the Antarctic tanaidacean fauna has attracted scientific attention. The number of new species described from the Southern Ocean is increasing considerably (e.g. Błażewicz-Paszkowycz Reference Błażewicz-Paszkowycz2007, Jóźwiak & Błażewicz-Paszkowycz Reference Jóźwiak and Błażewicz-Paszkowycz2007). Zoogeographical analysis (Błażewicz-Paszkowycz Reference Błażewicz-Paszkowycz2013) has strengthened the hypothesis of deep sea origins of the Southern Ocean tanaidaceans, originally proposed by Sieg (Reference Sieg1988). However, there are still many questions left unanswered with respect to their origin, distribution and diversity around Antarctica. Most Antarctic tanaidaceans have probably evolved from deep water ancestors, as it is thought that only a few could have survived the glaciations in shelf or slope refugia or colonized the Antarctic via the Scotia Arc (Błażewicz-Paszkowycz Reference Błażewicz-Paszkowycz2013). The paucity of the records in some parts of the Southern Ocean thoroughly impede the assessment of tanaidacean zoogeographical patterns.
The aim of the current study was to estimate the tanaidacean species richness in two West Antarctic regions; the Amundsen Sea and Scotia Sea shelves and upper slopes. We also compared the fauna of these two regions, previously unexplored in terms of tanaidacean diversity.
Methods
Study area
The Scotia Arc is a mountain range between South America and the Antarctic Peninsula; some of the peaks form islands. It delimits the basin of the Scotia Sea which spreads for c. 2000 km from the Drake Passage to the east. Among the most prominent islands in the Scotia Arc are (from north to south): Shag Rocks, South Georgia, South Sandwich Islands, South Orkney Islands, and finally, Elephant Island and South Shetland Islands. The Scotia Arc islands span a variety of geological ages and origins. The relatively young South Sandwich Islands (4 m.y.a.) have volcanic origins. In contrast the shelves of South Georgia, South Orkney Islands and Elephant Island are part of the South America and Antarctic Peninsula continental plate. These landmasses were grouped 52 m.y.a. in one land bridge linking the southern tip of South America with the Antarctic Peninsula. The spreading of the land started c. 40 m.y.a. and led to the establishment of the Drake Passage and Antarctic Circumpolar Current (ACC) c. 34 m.y.a., increasing the geographical and thermal isolation of Antarctica; between 20 and 10 m.y.a. the Scotia Sea was formed (Livermore et al. Reference Livermore, Hillenbrand, Meredith and Eagles2007).
The Scotia Sea is characterized by high salinity and its deepest parts reach 4000 m. This region is strongly influenced by the ACC which crosses the Scotia Sea, and all the island shelves are below the Polar Front (the strongest jet of the ACC). The cold deep water from the Weddell Sea flows through the South Scotia Ridge to the north (Barker Reference Barker2001).
The Amundsen Sea is a basin in West Antarctica located along the Marie Byrd Land. It was established during a breakup of Thurston Island and New Zealand from the Antarctic continent. The inner part of the Amundsen Sea shelf is characterized by the presence of deep troughs and furrows shaped during past glaciation and deglaciation periods. In the outer part of the shelf, the sea floor is characterized by numerous iceberg scour marks. It results in a very complex bathymetry with many depressions reaching over 1600 m, as well as less disturbed, shallower (up to 500 m) outer regions of the shelf (Lowe & Anderson Reference Lowe and Anderson2002). The Amundsen Sea is characterized by nearly perennial sea ice cover. The severe ice conditions are a key reason why this basin has been poorly described until recently (Jacobs et al. Reference Jacobs, Jenkins, Hellmer, Giulivi, Nitsche, Huber and Guerrero2012). The Pine Island Glacier, which discharges into the area, has received increased attention and notoriety due to rapid melting and contributions to the global sea level (Smith et al. Reference Smith, Hillenbrand, Kuhn, Larter, Graham, Ehrmann, Moreton and Forwick2011). Pine Island Bay receives a large amount of meltwater flowing from the West Antarctic Ice Sheet, even compared to much larger basins such as the Ross and Weddell seas (Lowe & Anderson Reference Lowe and Anderson2002). The ACC enables exchange between the Pacific and Atlantic water masses in the central part of the Amundsen Sea. Cold waters from the Weddell Sea are transported into the Amundsen Sea by a westward costal current flowing along the continental break of the Antarctic Peninsula. Most shelf areas around West Antarctica are overlain by warm Circumpolar Deep Water, which enters the Amundsen Sea shelf irregularly through troughs in the outer shelf (Jacobs et al. Reference Jacobs, Jenkins, Hellmer, Giulivi, Nitsche, Huber and Guerrero2012).
Sampling
Samples were collected by two expeditions, BIOPEARL 1 and 2 (Biodiversity dynamics: Phylogeography, Evolution And Radiation of Life in Antarctica), of the RRS James Clark Ross in 2006 and 2008 along the Scotia Arc islands, at sites around Livingstone Island, Elephant Island, South Orkney Islands, South Sandwich Islands (Southern Thule), South Georgia and Shag Rocks (Fig. 1), as well as in the eastern Amundsen Sea in the vicinity of Pine Island Bay and close to the shelf break (Fig. 2).
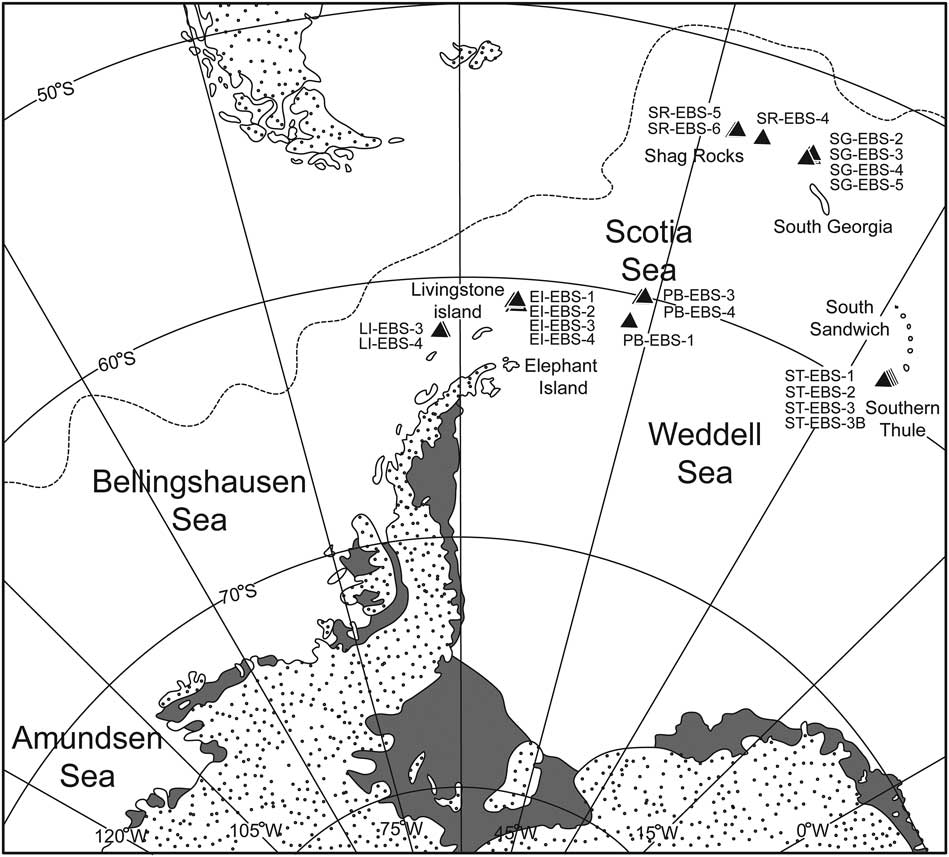
Fig. 1 Distribution of the sampling stations in the Scotia Sea. EI=Elephant Island, LI=Livingstone Island, PB=Palmer Bay (South Orkney Islands), SG=South Georgia, SR=Shag Rocks, ST=Southern Thule (South Sandwich Islands). Grey shading denotes ice.

Fig. 2 Distribution of the sampling stations in the Amundsen Sea. Grey shading denotes ice.
An epibenthic sledge (EBS) was used to sample Tanaidacea (Brenke Reference Brenke2005). The EBS was trawled for 10 minutes at a speed of 1 knot. In the Amundsen Sea, 17 samples were collected at a depth range of 500–1500 m (Table I), and 19 samples were collected in the Scotia Sea at a depth range of 200–1600 m (Table II). The shelf samples were collected at the depth range of 200–500 m, while the slope samples were gathered between 1000–1500 m. Samples were fixed in 96% undenaturated pre-cooled ethanol. After fixation, all samples were frozen for at least 48 hours (-20°C). The material was sieved on a 500 µm mesh. Tanaidacea were identified to the morphospecies level.
Table I Depth and location of samples collected in the Amundsen Sea.

Table II Depth and location of samples collected in the Scotia Sea.

EI=Elephant Island, LI=Livingstone Island, PB=Palmer Bay (South Orkney Islands), SG=South Georgia, SR=Shag Rocks, ST=Southern Thule (South Sandwich Islands).
Data analysis
Data ordination was conducted using non-metric multidimensional scaling (nMDS) of Bray-Curtis similarities on the genus and family level. Since EBS is not a quantitative sampling gear the data were presence/absence transformed according to the instruction provided by Clarke & Warwick (Reference Clarke and Warwick2001) for this type of dataset. One-way ANOSIM permutation tests of the presence/absence transformed Bray-Curtis similarity data obtained from 999 random permutations were also performed using the PRIMER 6.0 package. Species-accumulation curves averaged over 999 permutations were created for the Scotia and Amundsen sea samples using PRIMER 6.0. The curve plotted the cumulative number of different species observed as each new sample was added (Clarke & Warwick Reference Clarke and Warwick2001). Frequency of occurrence (F, percentage of samples where a genus was found in total number of samples) was measured for each genus in both seas.
Results
Species richness
The 549 individuals examined yielded 85 species from 26 genera identified. This included 37 species in the Amundsen Sea and 51 species in the Scotia Sea samples. All the species belonged to suborder Tanaidomorpha. Only three species were common to both basins. Over 90% of the tanaidacean species identified were previously undescribed: 78.3% (29 species) for Amundsen Sea and 96.0% (49 species) for the Scotia Sea. This result increased the number of tanaidacean species known from the Southern Ocean by around 50%. Previously known species were: Akanthophoreus multiserratoides Guerrero-Kommritz, A. weddellensis Sieg, Arthrura monacantha (Vanhöffen), Collettea antarctica (Vanhöffen), Meromonakantha nutae Błażewicz-Paszkowycz, Paranarthrura arctowski Jóźwiak & Błażewicz-Paszkowycz, P. fortispina Sieg, P. meridionalis Sieg. The latter two were found in both seas, while the remaining species were only recorded in the Amundsen Sea.
The most speciose family in the Scotia Sea was Typhlotanaidae (18 species, five genera), followed by Pseudotanaidae (eight species, one genus) and Akanthophoreidae (eight species, one genus). In the Amundsen Sea, the highest number of species were recorded for Pseudotanaidae (ten species, one genus) and Agathotanaidae (six species, three genera). Typhlotanaidae in the Scotia Sea were represented mostly by Typhlotanais sp. (13 species), while in the Amundsen Sea this genus was represented by two species. Akanthophoreus was represented by eight species in the Scotia Sea, and by three species in the Amundsen Sea. Ten genera were found only in the Scotia Sea; most of them were represented by single species. However, Larsenotanais (three species), Tanaopsis (three species), Leptognathia (two species) and Nototanais (two species) were represented by more than one species. Seven genera were only found in the Amundsen Sea (Table III). One genus Agathotanais was recorded in the Southern Ocean for the first time.
Table III Number of species, frequency of occurrence and depth range of tanaidaceans genera recorded in the studied area (current data) and in the Antarctic (current and literature data).

Species-accumulation curves did not reach the asymptotic level and we estimate that further species could be found in both basins with new sampling (Fig. 3). Differences in the number of species were found between the shelf and slope depths in both study areas (Fig. 4). In the Amundsen Sea, 26 species were found at shelf depths, including 11 new species that were restricted to this depth range. On the slope, 24 species were found, including seven new species that were only recorded there. In the Scotia Sea, the number of species on the shelf (42 species) was higher than on the slope (21 species), and the number of species only found at one of those depth ranges was 30 and nine, respectively. The depth range for each genus is provided in Table III for each study area.

Fig. 3 Species-accumulation curves for the Scotia and Amundsen seas.

Fig. 4 Number of species at shelf and slope in the Amundsen and Scotia seas (shelf samples: 200–500 m, slope samples: 1000–1500 m).
There were also differences between the number of species recorded at each site in the Scotia Sea. The highest number of species was recorded at Shag Rocks and most of them were found exclusively at this site. High numbers of species were also found at Elephant Island and Southern Thule. Two genera Typhlotanais and Pseudotanais were present at all Scotia Arc localities. However, most of the sites were characterized by the presence of the species restricted to one site (Table IV). A few species were common to the Scotia Arc sites. Most of the common species were found between Southern Thule and Elephant Island. The tanaids of South Georgia were the most distinct with only one species common to any other site (Table V).
Table IV Number of species at each site along the Scotia Arc.

Table V Number of species common to the sites located along the Scotia Arc.

EI=Elephant Island, LI=Livingstone Island, PB=Palmer Bay (South Orkney Islands), SG=South Georgia, SR=Shag Rocks, ST=Southern Thule (South Sandwich Islands).
The highest frequency in both study basins were noted for Pseudotanais (Amundsen Sea: F=94.1%, Scotia Sea: F=73.6%). High frequency values in the Scotia Sea were also noted for Typhlotanais (F=57.8%) and Akanthophoreus (F=57.8%). In the Amundsen Sea, Paranarthrura also had a high frequency in the studied material (F=64.7%) (Table III).
Similarity of fauna
The nMDS analysis showed two groups of samples at both genus and family level. Samples from the Scotia and Amundsen seas were separated on the plot, although on the family level some of the samples from both seas were mixed in the analysis. The moderate stress values indicated a relatively good 2D representation of multidimensional space (Fig. 5). However, the ANOSIM test showed weak differences for the genus level (r=0.35), while there were almost no differences on the family level (r=0.22). At the same time, no clear pattern associated with geographical or bathymetric distribution of samples in each of the seas studied was observed. The ANOSIM test between the shelf and slope fauna of the both seas did not show significant differences. The global r values for the Scotia and Amundsen seas were close to zero: 0.04 and 0.06, respectively.

Fig. 5 Non-metric multidimensional scaling (nMDS) at genus and family level (Bray-Curtis similarity, presence/absence transformed data).
Discussion
Underestimated diversity
Recently, most of the new species described from the Southern Ocean have come from the deep sea (De Broyer et al. Reference De Broyer, Danis and Allcock2011). The results of ANDEEP project (ANtarctic benthic Deep sea biodiversity: colonization history and recent community patterns) have shown deep sea Antarctic crustaceans to be highly speciose (Brandt et al. Reference Brandt, Gooday and Brandao2007). The slope fauna is poorly understood and the number of studies at this depth range is very limited (e.g. Blake & Narayananswamy 2004, Kaiser et al. Reference Kaiser, Barnes, Linse and Brandt2008). Recent studies of the Amundsen Sea shelf (Kaiser et al. Reference Kaiser, Barnes, Sands and Brandt2009), as well as research from Shag Rocks (Barnes Reference Barnes2008) and South Sandwich Islands (Kaiser et al. Reference Kaiser, Barnes, Linse and Brandt2008), have shown significant underestimation of some groups of benthic macrofauna even at shelf depths. Such studies have highlighted that many regions of the Antarctic are still very poorly studied, despite being newsworthy due to oceanographic, glaciological and atmospheric changes of global significance. The Amundsen Sea shelf remains one of the planet’s least explored seas largely due to ice-loading, the harsh weather conditions (Jacobs et al. Reference Jacobs, Jenkins, Hellmer, Giulivi, Nitsche, Huber and Guerrero2012) and distance to research stations or human habitation. Discovery of previously undescribed species in this area is hardly surprising. The overall high number of species recorded in this basin could be due to high habitat heterogeneity associated partially with the complex bottom topography and high productivity, as was suggested by Kaiser et al. (Reference Kaiser, Barnes, Sands and Brandt2009). In contrast to the Amundsen Sea, the high number of new tanaidacean species found in the Scotia Sea cannot be ascribed to low sample effort. This sea is amongst the most intensively sampled areas in the Antarctic and approaches the level of study of many world seas (except around the US & Europe). The tanaidacean fauna itself has been previously investigated in this region (Kudinova-Pasternak Reference Kudinova-Pasternak1975, Jóźwiak & Błażewicz-Paszkowycz Reference Jóźwiak and Błażewicz-Paszkowycz2007), although records of tanaidaceans from the shelf and slope depths of Scotia Sea are very scarce (Błażewicz-Paszkowycz Reference Błażewicz-Paszkowycz2013). This region is recognized as generally rich in endemic species, presumably due to the great age and isolation of many Scotia Arc islands (Linse et al. Reference Linse, Cope, Lortz and Sands2007, Kaiser et al. Reference Kaiser, Barnes, Linse and Brandt2008). Our study has demonstrated that in both well and poorly studied Antarctic shelf seas there remains considerable unreported benthic biodiversity. We found previously undescribed species in almost all genera recorded in the Scotia Sea. For taxa such as Typhlotanais or Akanthophoreus our samples doubled the number of species known from the Antarctic.
Some authors have recently pondered the question of how many benthic species occur in the Southern Ocean. For some, taxa estimates may be meaningful but, as the current study reveals, for others (such as tanaidaceans) this is a fairly meaningless. Areas such as parts of the Bellingshausen Sea, Amundsen Sea, Cosmonauts Sea, Mawson Sea and even western Weddell Sea are scarcely sampled in general (Griffiths Reference Griffiths2010, De Broyer et al. Reference De Broyer, Danis and Allcock2011) and there are few studies on benthic macrofauna in these regions (Corbera et al. Reference Corbera, Vicente and Sorbe2009, Kaiser et al. Reference Kaiser, Barnes, Sands and Brandt2009). There have been intensive efforts on some specific groups such as polychaetes and bryozoans. However, the richness of others (demonstrably small crustaceans such as tanaidaceans, but also probably cumaceans and isopods) still need further intensive taxonomic study for meaningful regional estimates. The number of ‘white spots’ (where little biodiversity is known) on the map of the Southern Ocean tanaidacean richness is high by global standards (Fig. 6). The current number of benthic macrofaunal organisms known from the Southern Ocean has reached c. 6000 species (De Broyer et al. Reference De Broyer, Danis and Allcock2011). Gutt et al. (Reference Gutt, Sirenko, Smirnov and Arntz2004) estimated the total number of macrozoobenthic species at c. 11 000–17 000 species, although that analysis was based mainly on the larger epibenthos and omitted some important taxonomic groups represented mainly by fine organisms. Our results, as well as some of the above-mentioned studies from the Amundsen, Scotia and Bellingshausen seas, suggest that this assessment might be quite an underestimate when many of the smaller infaunal benthic invertebrates become better sampled.

Fig. 6 Number of tanaidacean species in the Southern Ocean recorded before this study on the shelf and in the deep sea (in brackets) and during this study [in square brackets].
Brökeland et al. (Reference Brokeland, Choudhury and Brandt2007) noted that peracarids are very species rich, both in the deep sea and on the Antarctic shelf. Recent studies have suggested that isopods and amphipods have undergone intensive radiation in the Antarctic (De Broyer et al. Reference De Broyer, Jażdżewski and Dauby2003, Brökeland et al. Reference Brokeland, Choudhury and Brandt2007), at the same time pointing out that groups, such as tanaidaceans and cumaceans, might be less speciose because of a supposed smaller variety in lifestyles and feeding modes, thus a lower evolutionary plasticity. Tanaidomorphs are believed to be mostly benthic detritus feeders or opportunists, living in tubes in soft sediments. However, some studies have demonstrated that the number of ecological strategies among Tanaidacea can be high, including parasitism, various types of predation (Błażewicz-Paszkowycz et al. Reference Błażewicz-Paszkowycz, Bamber and Anderson2012).
To date tanaidaceans have been found in almost all marine benthic habitats (Błażewicz-Paszkowycz et al. Reference Błażewicz-Paszkowycz, Bamber and Anderson2012). Our study confirms that current knowledge underestimates Antarctic, as well as global, tanaidacean diversity (Appeltans et al. Reference Appeltans, Ahyong and Anderson2012). Seventy nine new species recorded in just 38 samples and the associated steep species-accumulation curves suggest high, unexplored diversity of this crustacean order in the Southern Ocean. The low dispersal potential of those crustaceans may be causal to much of this allopatric speciation and leads to their high endemism, and in consequence to both a high species richness and unique characteristics of the Scotia and Amundsen seas tanaidacean fauna. New species were found at all sites along the Scotia Arc (Table IV). The highest number of undescribed species was recorded around Shag Rocks. The tanaidacean fauna at Shag Rocks differed significantly from that around neighbouring South Georgia. This dissimilarity despite close geographical proximity has been noted before in a variety of taxa (e.g. De la Cuadra & Garcia Reference De la Cuadra and Garcia2000, Barnes Reference Barnes2008). It is possible that the currents and narrow, but deep channel between these two shelf areas may form quite an effective barrier to transport, except for stronger swimming nekton.
Most of the species recorded in our study were found only in single samples and at ‘low abundance’ (we appreciate that our samples were not quantitative). A similar observation has been made for the Tanaidacea along the West Australian coast (Poore et al. unpublished). Thus the proportion of species which are ‘rare’ could be very high in this group. The need for gathering data on rare species is a very important element for further biodiversity extrapolations in the Southern Ocean (Gutt et al. Reference Gutt, Hosie and Stoddart2010, De Broyer et al. Reference De Broyer, Danis and Allcock2011). More extensive sampling in scientifically unrecognized areas with devices suitable for collecting smaller macrofauna and meiofauna will probably improve our knowledge on Antarctic fauna more than any other type of sampling at the current time. Such sampling would also greatly improve our species-richness estimations of the overall Southern Ocean. The pattern observed for Tanaidacea at shelf and slope depths in the Scotia and Amundsen seas so far is similar to those typical for the deep sea faunas where the number of singletons is often very high (Blake & Narayanswamy Reference Blake and Narayanswamy2004). An increase in the data for overall biodiversity of the Southern Ocean requires sampling of larger areas, together with more even and wider distribution of the sampling stations, as was already pointed out by Gutt et al. (Reference Gutt, Hosie and Stoddart2010).
The unique character of tanaidacean fauna
Despite both study areas being characterized by relatively warm shelf waters and linked by a strong current (ACC) flowing from one area to the other, our results suggests that tanaidacean faunas of the Scotia and Amundsen seas are quite different at species, but also to a lesser extent at the genus, level. The unique character of the Amundsen Sea can be explained by the geological history, age and hydrology of this basin (Jacobs et al. Reference Jacobs, Jenkins, Hellmer, Giulivi, Nitsche, Huber and Guerrero2012). Kaiser et al (Reference Kaiser, Barnes, Sands and Brandt2009) found the isopod fauna of the Amundsen Sea to be of similarly unique character and they demonstrated substantial differences between the Scotia and Amundsen seas taxonomic composition (Kaiser et al. Reference Kaiser, Barnes, Sands and Brandt2009), although not many differences were noted at familial level. On the other hand, all eight tanaidacean species previously known to science were found in the Amundsen Sea. Most of them are otherwise only known from their type localities in the West Antarctic.
Each of the study islands along the Scotia Arc was characterized by a high number of species restricted exclusively to one site. Even near-neighbouring sites, such as Elephant and Livingstone islands, had very few species common to both island shelves. The distribution of some species of Typhlotanais and Pseudotanais were limited (in our samples) to around a particular island of the Scotia Sea. One interpretation of this is an extensive radiation of those taxa along the Scotia Arc. Some of these archipelagos, e.g. South Sandwich Islands, are isolated and surrounded by abyssal and hadal depths (Kaiser et al. Reference Kaiser, Barnes, Linse and Brandt2008). Distribution of some species may be restricted not only to specific regions of the Antarctic but also to single, isolated sites. However, it could be that tanaidaceans have not been adequately sampled, even in the Scotia Sea, to establish the range of patchy or rare species. To evaluate how far the distribution of particular species is restricted requires implementation of molecular techniques. Dispersal ability at early stages of development was most probably one of the key factors influencing speciation and thus resulting in high endemism of the Southern Ocean benthic fauna (Thatje Reference Thatje2012). Griffiths (Reference Griffiths2010) highlighted that endemism in groups such as cheilosotomatous bryozoans, bivalves, pycnogonids or ascidians may reach between 40–50% and even 74% for gastropods. Values for the tanaidacean fauna are much higher and may exceed 90% or even approach 100%, which makes this taxon very pertinent to the discussion on the biodiversity of Southern Ocean. Comparison of our results with the better studied tanaidacean fauna of the Ross and Weddell seas also revealed considerable differences (Błażewicz-Paszkowycz Reference Błażewicz-Paszkowycz2013).
Our study suggests that it is crucial to include slope depth faunas in future estimations of the Southern Ocean biodiversity. Barnes (Reference Barnes2008) underlined the role of slope refugia in the origin of the Antarctic benthic fauna as well as in future changes associated with climate warming. In the Scotia Sea, the number of new species recorded only on the upper slope was lower than on the shelf, while in the Amundsen Sea, almost half of the new species were found only at slope depths. However, the depths between the 1000 m and 1600 m in the Amundsen Sea are not ‘typical slope’ and those deeper areas are associated with presence of deep scour marks on the shelf of this basin (Jacobs et al. Reference Jacobs, Jenkins, Hellmer, Giulivi, Nitsche, Huber and Guerrero2012). Nevertheless, the possible existence of a distinct slope fauna is still open. Kaiser et al. (Reference Kaiser, Griffiths, Barnes, Brandao, Brandt and O’Brien2011) demonstrated that many isopods were restricted to these depths, but concluded that the hypothesis of a unique slope fauna in the Southern Ocean was not proven and that the patterns differ depending upon taxonomic group and region studied.
Future studies
The Southern Ocean isopod and amphipod richness is believed to be strongly underestimated. However, our study has demonstrated that the current underestimation of tanaidaceans could be even greater. The proportion of previously undescribed tanaidacean species on the shelf and slope of the Scotia and Amundsen seas is as high as in the deep sea sites. Similar observations have been made for the isopod fauna of the Amundsen Sea shelf (Kaiser et al Reference Kaiser, Barnes, Sands and Brandt2009). The actual species richness recognition in groups of macrobenthos characterized by limited dispersal, high level of endemism and high number of rare species is very important for meaningful biodiversity inventories as well as in comprehensive assessment of the Southern Ocean species richness. The cryptic diversity of many Southern Ocean taxa including large and well-studied invertebrates such as Odontaster (Janosik & Halanych Reference Janosik and Halanych2010) but also crustaceans (Held Reference Held2003) has been already demonstrated, and ultimately we expect similar results for Tanaidacea once subjected to similar molecular techniques.
The distribution of particular species of Typhlotanais and Pseudotanais along the Scotia Arc suggests that these archipelagos are not necessarily an easy ‘stepping stone’ route for dispersion. Most of the Antarctic tanaidaceans have a suspected deep sea origin and few seem to come from the Magellanic region, e.g. Paratanais oculatus (Vanhöffen) (Błażewicz-Paszkowycz Reference Błażewicz-Paszkowycz2013). The specific set of species at each island of the Scotia Arc may prove allopatric speciation and support expected restricted distribution of tanaidacean species. The differences between the Scotia and Amundsen seas faunas also strengthen these conclusions. In such circumstances it seems unlikely that populations of Nototanais dimorphus (Beddard), a species that was found in the canals of Tierra del Fuego, as well as Iles Kerguelen and the Ross Sea (Błażewicz-Paszkowycz Reference Błażewicz-Paszkowycz2013) all belong to the one species. Equally the distribution of the other ‘circum-Antarctic’ species should be questioned (Thatje Reference Thatje2012). On the other hand, the unique character of the fauna for each of the Scotia Arc islands, together with presence of species common to sites located at long distances from each other, such as Shag Rocks and Elephant Island, pose new questions. Phylogeographical studies could help to resolve those queries, although obtaining material suitable for molecular studies is not easy with such small crustaceans.
The presence of the ‘white spots’ on the map of the Southern Ocean biodiversity, together with high undersampling of some invertebrate groups still strongly affects reliable analysis of the large scale biodiversity patterns, colonization history and geographical range characteristics. Our study has demonstrated that even intensively sampled areas, such as the Scotia Sea, still have elements of the fauna which are very poorly described. The unique environmental conditions, topography and hydrology of the Amundsen Sea have placed this area among the priority sites for future studies, especially considering its fundamental importance in past and current global climate change. The Amundsen Sea can be considered as a natural laboratory for research on the response of the benthic fauna to rapid climate-related changes observed in this area (Smith et al. Reference Smith, Hillenbrand, Kuhn, Larter, Graham, Ehrmann, Moreton and Forwick2011). Further studies of this area should address the problems of faunal similarity, as well as studies of distribution patterns and zoogeographical links with other regions of the Southern Ocean. Once the species composition is better known the great need for ecological studies can progress more meaningfully, based on a larger number of quantitative samples and variety of environmental variables.
Acknowledgements
The study was supported by a grant of Polish Ministry of Science and Higher Education No. 7984/B/P01/2011/40. We would particularly like to thank Peter Enderlein, Katrin Linse, Stefanie Kaiser and Chester Sands for aid with the EBS deployment and sample sorting as well as the officers, crew and science team on RRS James Clarke Ross on the voyages JR144 and JR179. We also would like to thank the two anonymous reviewers for their helpful comments.
Author contribution
Krzysztof Pabis: general concept, data analysis, writing of the manuscript. Magdalena Błażewicz-Paszkowycz: identification of the material, discussion of the concept and the results and proofreading and comments on the manuscript, discussion of the results with the first author. Piotr Jóźwiak: identification of the material, preparation of the figures. David K.A. Barnes: collection of the material, proofreading and comments on the manuscript.













