Introduction
The ecosystems of the Antarctic continent have unique characteristics because of their distance from direct anthropogenic influence. The continent still has almost pristine environments that depend mainly on the marine ecosystems, particularly by the near-shore waters (Hedgpeth Reference Hedgpeth1977, Rakusa-Suszczewski Reference Rakusa-Suszczewski1980). Because of the variety of factors influencing marine ecosystems, the study of the relationships between organisms and abiotic parameters is necessary to gain an understanding of the whole ecosystem.
In marine ecosystems benthic foraminifera are abundant, ubiquitous and easily sampled (Culver Reference Culver1987) and are traditionally considered good environmental indicators. They are distributed in all marine environments from marshes and bays to abyssal depths, and hundreds of individuals and many different species can be found in a few millilitres of sediment.
Research on recent foraminifera in Antarctic environments flourished in the early part of the 20th century (Mikhalevich Reference Mikhalevich2004) and has been intensively carried out ever since, most recently by Ward & Webb (Reference Ward and Webb1986), Bernhard (Reference Bernhard1987), Ward et al. (Reference Ward, Barrett and Vella1987), Mackensen et al. (Reference Mackensen, Grobe, Kuhn and Fütterer1990), Ishman & Domack (Reference Ishman and Domack1994), Violanti (Reference Violanti1996), Igarashi et al. (Reference Igarashi, Numanami, Tsuchiya and Fukuchi2001), Murray & Pudsey (Reference Murray and Pudsey2004) and Mikhalevich (Reference Mikhalevich2004). Some foraminiferal work was also carried out in the South Shetland Islands (Finger & Lipps Reference Finger and Lipps1981, Ishman & Domack Reference Ishman and Domack1994, Chang & Yoon Reference Chang and Yoon1995, Li et al. Reference Li, Yoon and Park2000, Mayer Reference Mayer2000, Majewski 2005, Majewski et al. Reference Majewski, Lecroq, Sinniger and Pawlowski2007 and Majewski & Tatur Reference Majewski and Tatur2009).
This study attempts to elucidate the relationship between several oceanographic factors with recent foraminifera assemblages in an Antarctic inlet (Ezcurra Inlet) using material sampled during four summers and numerical analyses such as Multi Dimensional Scaling (MDS).
Materials and methods
Study area
The Ezcurra Inlet is located in western Admiralty Bay, the largest bay of King George Island (Fig. 1), South Shetland Islands. Admiralty Bay shoreline is 25.3 km long (Rakusa-Suszczewski Reference Rakusa-Suszczewski1995) surrounded by mountains and icefalls, with steep slopes that reach altitudes of 200–450 m above sea level and submerged depths more than 400 m below sea level at the entrance to Admiralty Bay (Bojanowski Reference Bojanowski1983). The shores of the Ezcurra Inlet coastline are 57.8% mineral material and 42.2% glacial (Marsz Reference Marsz1983). The inlet can be divided into two distinct regions: one at the entrance of the inlet before Dufayel Island, and the other beyond the island in a region containing two coves, Cardozo Cove in the north-west and Goulden Cove in the south-west. Surface currents are generated by local wind fields, which are influenced by irregular and semidiurnal tides, and deep currents are caused by tidal phenomena (Lipski Reference Lipski1987).

Fig. 1 Study area: a. South Shetland Islands, Antarctica, b. Ezcurra Inlet (King George Island) with the sampling stations during four summers. The dark grey represents the areas without glaciers.
Sample collection
During four summers (2002/03, 2003/04, 2004/05 and 2006/07), 24 surface sediment samples of benthic foraminifera were collected with a Van Veen grab sampler in the 15 to 80 m depth range (Table I). For a list of authorities for the species mentioned in this paper see Appendix A.
Table I Locations of the stations sampled at Ezcurra Inlet.

Bottom water content
The bottom water samples were collected using a Teflon Van Dorn sampler. The salinity was determined using an inductive salinometer and calculations proposed by Fofonoff & Millard (Reference Fofonoff and Millard1983) with a precision of ± 0.02. The temperature of each sample was determined directly in the sampler as soon as it was recovered using a mercury-in-glass thermometer. The dissolved oxygen (DO) content was determined using the Winkler method modified by Grasshoff (Reference Grasshoff1983). The hydrogen potential (pH) was determined using a radiometer pH meter following Aminot & Chaussepied (Reference Aminot and Chaussepied1983) (precision ± 0.02). The water was filtered through a GF/F Whatman filter to assess dissolved nutrients (Grasshoff et al. Reference Grasshoff, Kremling and Ehrhardt1999), using a manual method for phosphate and silicate determinations and an automatic method with an AutoAnalyzer II for nitrate and nitrite. The filters were used to determine gravimetrically the suspended particulate matter (Strickland & Parsons Reference Strickland and Parsons1968).
Sediment analyses
Granulometric analyses were carried out using about 30 g of each sample. Hydrogen peroxide (H2O2 10%) was added to this material until the total organic matter (TOM) was completely eliminated. The remaining material was dried at 60°C, weighed, and then washed through a 0.062 mm sieve. The coarser fraction was dried, weighed, and sieved through a series of 12 sieves from 4 to 0.062 mm. The fraction finer than 0.062 mm was put in suspension in 1000 ml of distilled water to which 1 g of sodium diphosphate (Na4P2O7) was added to help deflocculating.
The organic carbon content of sediments was also analysed. Calcium carbonate was removed by adding hydrochloric acid (HCl 10%), and 1 g of the remaining sediment was dried in a centrifuge tube. These samples were rinsed in distilled water and dried. Aliquots were then used to determine total organic carbon (TOC) content using a LECO CNS Analyzer.
Foraminifera laboratory procedures
Samples of the uppermost layer of the sediment (about 2 cm), were stored in a mixture of 4% formaldehyde solution and 1 g of rose bengal (protein stain) in 1 l of distilled water.
For faunal analysis 25 cm3 of sediment was washed through sieves with 0.250, 0.125, and 0.062 mm openings. All specimens were counted and quantitative analysis of the dataset was based on counts of both stained (living) and unstained (dead) specimens. Species identification and counting of dry specimens were done under an optical microscope, and scanning electron micrographs were taken to help with some problematic identification.
Data analyses
The specimens were identified following Gray et al. (Reference Gray, Sturz, Bruns, Marzan, Dougherty, Law, Brackett and Marcou2003), Murray & Pudsey (Reference Murray and Pudsey2004), Mikhalevich (Reference Mikhalevich2004) and Majewski (Reference Majewski2005), and the absolute and relative frequency for each species were calculated. Shannon-Wiener diversity, Simpson dominance and evenness were determined by PRIMER (Clarke & Warwick Reference Clarke and Warwick1994), using the following formulas in which pi is the proportion of the species in the sample and ln pi is the natural log of pi:
1) Shannon Wiener diversity H’ = sum (pi ln pi),
2) Simpson dominance C = sum (pi 2), and
3) Evenness J’ = H’/H’ max.
Matrices were constructed using the Bray-Curtis similarity measure on log (x + 1) to normalize foraminiferal counts. Species-abundance data were calculated for foraminiferal samples (each contributing 1% or more to the assemblage in more than one station) and subjected to Q-mode cluster analysis to define foraminiferal assemblages. The Bray-Curtis distance was used to measure proximity between samples, and Ward’s linkage method was used to arrange samples into a hierarchical dendrogram.
The Indicator Species Analysis (ISA) (Dufrêne & Legendre Reference Dufrêne and Legendre1997) was used to determine exactly which species were unique to groups with significant differences in community assemblage. The ISA was calculated by first computing relative abundance and relative frequency for each foraminifera species by group formed by the Q-mode cluster, then multiplying those scores to give a final indicator value. The statistical significance of the highest indicator value for each species was tested with 10 000 runs of a Monte Carlo randomization procedure. The resulting P-value represents the probability that the calculated indicator value for any species was greater than that found by chance. The ISA was determined using PC-ORD software (McCune & Mefford Reference McCune and Mefford1999).
The foraminiferal data were coordinated by non-metric multidimensional scaling (MDS) (Dufrêne & Legendre Reference Dufrêne and Legendre1997) to emphasize the geometrical aspects of similarity and to enable visualization of complex data in a graphical environment. This approach highlights patterns that might not be apparent in cluster analysis and results in a map of samples in which the placement of samples reflects the similarity of their biological communities and environmental patterns rather than their geographical location. Pearson correlation analyses were performed in order to explore more the relationships between the abiotic parameters with the main species and ecological indices, using P < 0.05 as the significant level.
Results
Environmental parameters
Bottom water
The lowest temperatures and the highest salinity in the bottom water were found in the summer of 2003/04 and the highest temperatures and lowest salinity in the summer of 2006/07 (Table II). The amount of dissolved oxygen was slightly lower and showed a small variability during the summer of 2006/07. The pH presented few differences between the four summers but with more noticeable variability in the summer of 2002/03.
Table II Bottom water variables of the stations sampled at Ezcurra Inlet.

Ammonium had higher values in the summer of 2003/04, whilst nitrite was lowest in the summer of 2004/05 but with greatest variability in 2002/03, and nitrate was lowest in the summer of 2006/07. Phosphate was higher in the summer of 2002/03 and lowest in 2003/04. Reactive silicate was highest and most variable in the summer of 2006/07, whilst suspended matter in the bottom water was also highest in this summer.
Granulometric patterns and sediment geochemistry
The granulometric and geochemical data (Table III and Fig. 2) show that the Ezcurra Inlet has three different kinds of surface sediments. At the entrance of the inlet, large amounts of gravel and very coarse sand were observed. In contrast, at the Goulden and Cardozo coves and near Dufayel Island, the bottom sediments were predominantly fine grained (silt and clay). Muddy sand sediments were found in shallower zones throughout the inlet and at its entrance.
Table III Percentage of grain size and geochemical variables of the bottom sediment of stations sampled at Ezcurra Inlet.


Fig. 2 Grain size distribution at Ezcurra Inlet (King George Island).
The surface sediment appeared to contain lower mean concentrations of total organic matter (TOM) during the first three summers with values lower than 2.5%, with the highest mean concentration of 4.23% measured during the last summer (2006/07). Goulden Cove had the highest concentrations of TOM. All samples had total organic carbon (TOC) content lower than 0.5% with higher levels at Thomas Point and Dufayel Island, and the highest concentration of 0.413% found at OPXXI 11 (2002/03).
Foraminifera fauna
A total of 7304 foraminifera were found (Tables IV–VI and Figs 3 & 4), and only four samples contained less than 25 individual specimens. We found 57 species, 28 of which were agglutinated and 29 of which were calcareous. The main species were the calcareous Bolivina pseudopunctata, Cassidulinoides parkerianus, C. porrectus and Globocassidulina biora and the agglutinated Portatrochammina antarctica, Pseudobolivina antarctica, Spiroplectammina biformis and Adercotryma glomerata.
Table IV Relative abundance of the total (dead + living) foraminifera sampled at Ezcurra Inlet.

Table V Relative abundance of the living foraminifera fauna sampled at Ezcurra Inlet.

Table VI Number of specimens (no. forams), species (richness) and ecological index of the samples collected at Ezcurra Inlet.

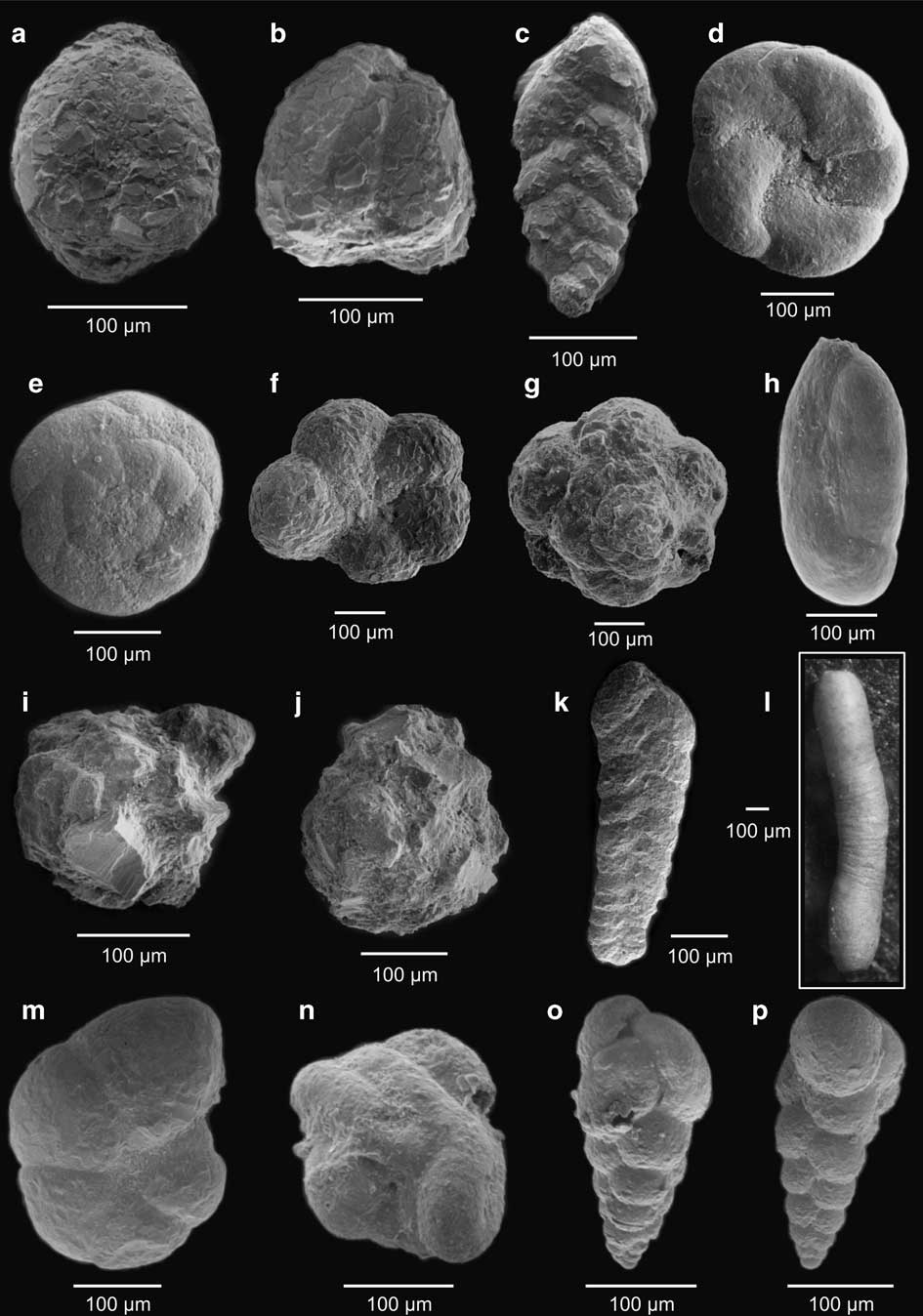
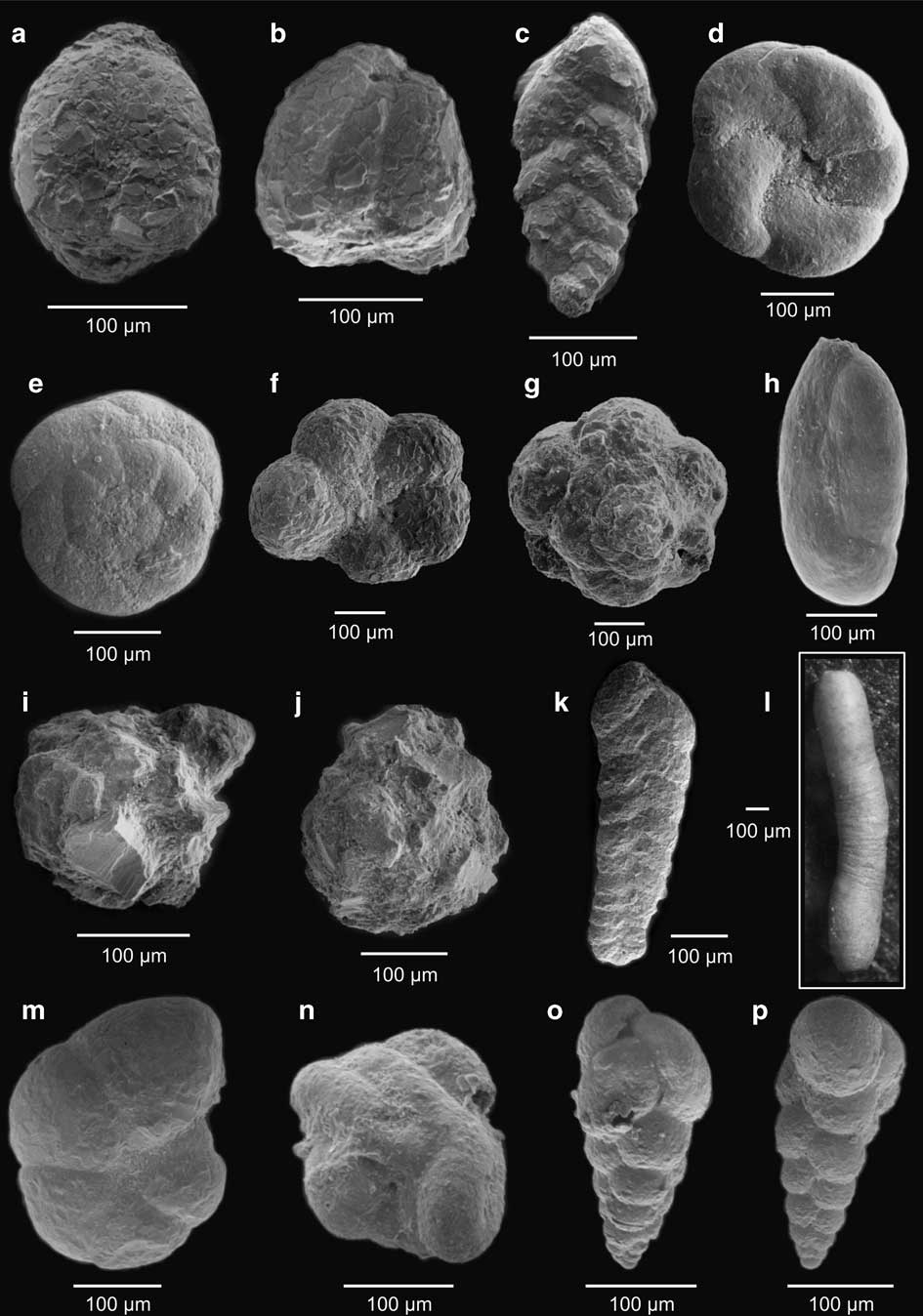
Fig. 3 a, b.Adercotryma glomerata, c.Pseudobolivina antarctica, d, e. apertural and ventral view - Paratrochammina lepida, f, g. apertural and ventral view - Portatrochammina antarctica, h.Miliammina arenacea, i, j.Psamosphaera fusca, k.Spiroplectammina biformis, l.Hippocrepinella hirudínea, m.Haplophragmoides bradyi, n.Glamospira gordialis, o, p.Verneuilina minuta.

Fig. 4 a.Cornospira involvens, b.Spiroplectammina filiformis, c.Proteonina decorata, d.Lagenammina arenulata, e.Textularia earlandi, f.Hemisphaerammina bradyi, g, h.Fursenkoina fusiformis, i.Bolivina pseudopunctata, j.Cassidulinoides parkerianus, k.Cassidulinoides porrectus, l, m. apertural and ventral view - Astrononion echolsi, n.Ammonia becarii, o.Globocassidulina biora, p,q.Procerolagena gracilis, r.Fissurina sp., s.Quinqueloculina lamarckiana.
Table IV shows that the most abundant and richest samples were found during the third summer (2004/05) at OPXXIII 11, with 1430 specimens, and OPXXIII 8, with 34 species, respectively. Most of the 57 species were found during this summer. The last summer (2006/07) was the poorest and least abundant period studied.
The highest diversity values were observed at stations OPXXIII 12 (2.516) and OPXXIII 8 (2.486), while the lowest values were observed at THO 3 (0.456). The latter station also showed the highest dominance (0.774), while the lowest dominance values were found at OPXXIII 12 (0.099) and OPXXIII 13 (0.115).
Numerical analysis
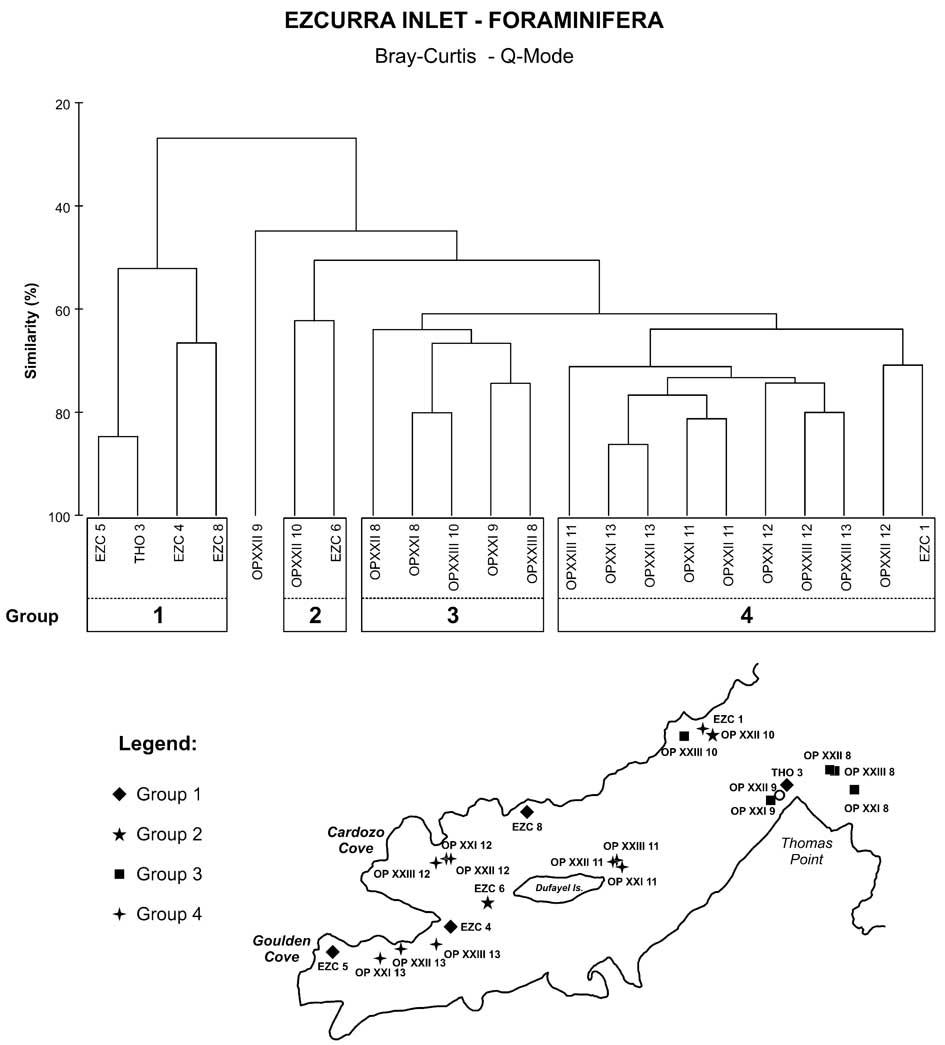
The Q-mode cluster analysis based on the overall dominant species revealed four groups (Fig. 5), and the indicator species analysis (Table VII) defined the significant foraminiferal species of each group. Group 1 was comprised of stations EZC 5, THO 3, EZC 4 and EZC 8, where the most characteristic species was Hemisphaerammina bradyi. Group 2, with only two stations (OPXXII 10 and EZC 6), had a high abundance of Psammosphaera fusca.
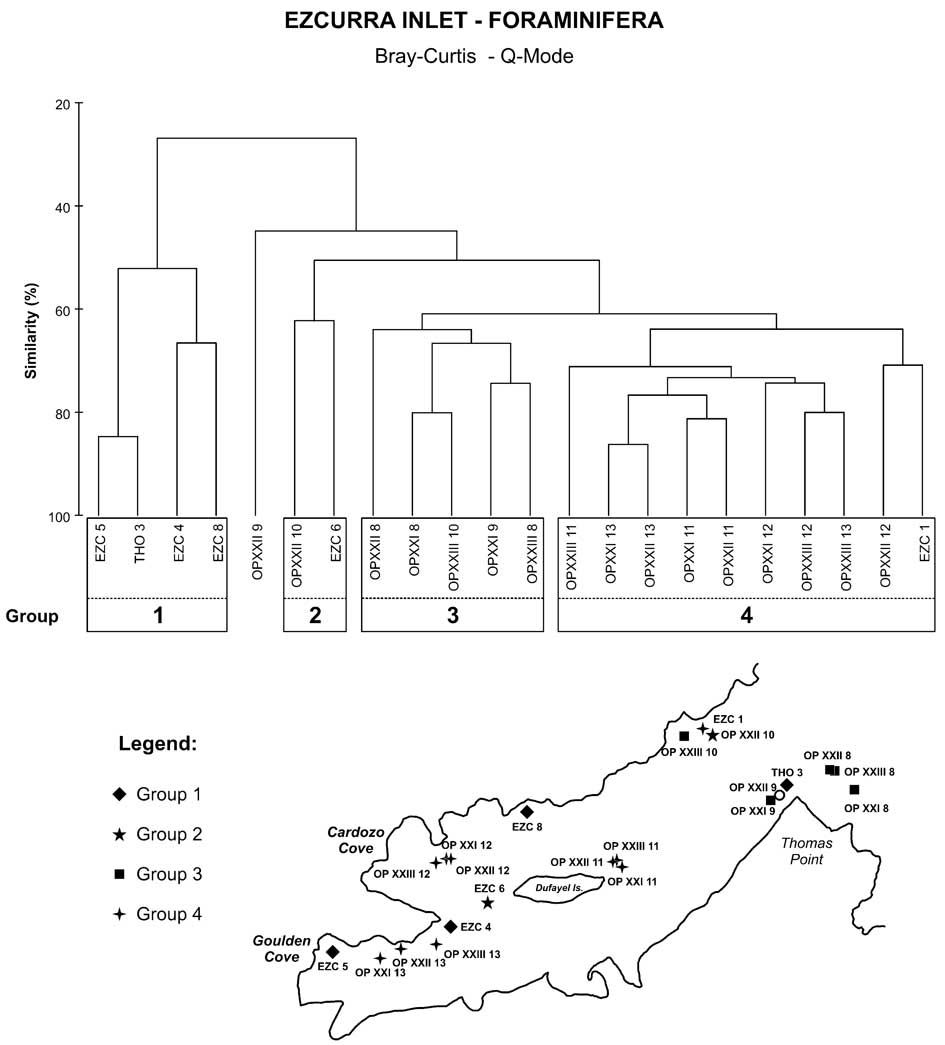
Fig. 5 Dendrogram classifications (Q-mode) showing the groups of station based on faunal composition and its distribution in the Ezcurra map.
Table VII Index of foraminifera species calculated by the Indicator Species Analyses (ISA) based on the dendrogram classification. The indicator species and their groups are in bold.

Group 3 comprised stations located near the entrance of the Ezcurra Inlet, and the dominant species were the calcareous Bolivina pseudopunctata and Fursenkoina fusiformis and the agglutinated Adercotryma glomerata. The station OPXXII 9, where the calcareous Globocassidulina biora dominated (37.1%), was not part of any group.
Most of the stations of Group 4 were from the Goulden and Cardozo coves and the area near Dufayel Island, and the main species in this group were Cassidulinoides parkerianus and Cassidulinoides porrectus.
The MDS analyses performed using the biological data within collected samples showed the same four different groups as revealed in the cluster analysis (Fig. 6). Group 1 showed the lowest values for richness and abundance of foraminifera, and the highest values for temperature and concentrations of silicate and very coarse sand.

Fig. 6 MDS ordination of relative foraminiferal data and oceanographic variables. Dark grey circles represent a quantitative measure of each variable.
Group 2 had the highest concentrations of total organic matter and clay. Group 3 were the deepest and had the highest richness and lowest concentration of clay. Finally, Group 4 had the lowest concentrations of very fine sand, the highest concentrations of medium silt, and the deepest samples. The simple correlation analyses performed on the biological and abiotic data are shown in Tables VIII & IX.
Table VIII Simple correlation between the dominant foraminifera species and the ecological index with oceanographic factors at Ezcurra Inlet. Significant (P < 0.05) correlation coefficients in bold. Significant (P < 0.05) correlation coefficients in boldface. Temp. = Temperature, Sal. = Salinity, DO = Dissolved oxygen, NH4+ = Ammonium, NO2- = Nitrite, NO3- = Nitrate, Si(OH)4 = Silicate, PO43- = Phosphate, SM = Suspended matter, VCSa = Very coarse sand, CSa = Coarse sand, MSa = Medium sand, FSa = Fine sand, VFSa = Very fine sand, CSi = Coarse silt, MSi = Medium silt, FSi = Fine silt, VFSi = Very fine silt, TOM = Total organic matter, TOC = Total organic carbon.

Table IX Simple correlation between the ecological index and the primary foraminifera species sampled at Ezcurra Inlet. Significant (P < 0.05) correlation coefficients in bold.

The clay and nitrite showed a positive correlation with the species Hemisphaerammina bradyi and Hippocrepinella hirudinea, and clay showed a negative correlation with both the richness of foraminifera and Pseudobolivina antarctica. The species Psammosphaera fusca showed a positive correlation with mud sediments (silt and clay) and dissolved silicate.
The very fine sand showed a positive correlation with Portatrochammina antarctica, Adercotrymma glomerata, Verneuilina minuta and P. lepida. These last two species showed a negative correlation with dissolved oxygen. The fine sand showed a negative correlation with evenness.
The temperature showed a negative correlation with richness, diversity, Portatrochammina antarctica and Bolivina pseudopunctata and a positive correlation with Hemisphaerammina bradyi. This species showed a negative correlation with salinity and a positive correlation with pH and suspended matter. The diversity, Bolivina pseudopunctata and Fursenkoina fusiformis showed a positive correlation with depth.
Discussion
Bottom water
The coldest and most saline bottom waters were found during the summer of 2003/04, and the warmer and least saline were found during the summer of 2006/07. The samples collected from shallower water depths in the last summer (2006/07) were more susceptible to the freshwater inputs from glaciers. This might be responsible for the differences found mainly in the lower temperatures, higher salinities, higher silicate and suspended matter found in his summer.
The Antarctic waters are rich in dissolved nutrients at both the surface and the bottom, with high concentrations of nitrate (∼30 μM) and silicate (80 μM) (Rakusa-Suszczewski Reference Rakusa-Suszczewski1995, and Lipski Reference Lipski1987). High oxygen content was observed in almost every bottom water sample collected, with just one outliner from OPXXIII 8 in the summer of 2004/05. This is to be expected as the solubility of oxygen is higher in cold water and it agrees with the results of other studies in Antarctic waters (Szafranski & Lipski Reference Szafranski and Lipski1982, Rakusa-Suszczewski Reference Rakusa-Suszczewski1995). The low nitrite values confirmed the presence of oxygenated water from the nitrification process of the nitrogen cycle. The ammonium signalled organic matter decomposition, common in the summer period and more intensive in OPXXII.
Bottom sediment
The sedimentation patterns in the Antarctic are mainly dictated by its glacial and oceanographic conditions (Ishman & Domack Reference Ishman and Domack1994). The Ezcurra bottom sediments are poorly or very poorly sorted and follows the sediment distribution showed by Majewski (Reference Majewski2005) throughout Admiralty Bay. The bottom sediment of Ezcurra Inlet shows this heterogeneity in its three different regions. Near its entrance there is coarser sediment, between the western glaciers and the Dufayel Island there is a high concentration of silt and clay, and in shallower areas there is muddy sand sediment.
DeMaster et al. (Reference Demaster, Nelson, Nittrouer and Harden1987) showed that the consistently well-oxygenated bottom waters of the high southern latitude shelves result in the rapid degradation of total organic carbon in the surface sediments. In Ezcurra Inlet, the TOC content is less than 0.5%, very similar to other marine superficial sediments across Antarctica and in some Arctic areas (Green Reference Green1960, Gooday et al. Reference Gooday, Bowser and Bernhard1996, Gray et al. Reference Gray, Sturz, Bruns, Marzan, Dougherty, Law, Brackett and Marcou2003). The TOM content follows the same pattern, with low concentrations in most of the samples. The shallower samples, especially from the shallower areas collected during the summer of 2006/2007, showed a higher concentration of TOM.
Foraminifera
Much debate has taken place regarding the origin of the Antarctic biota and its considerable degree of endemism (Dayton Reference Dayton1990, Arntz & Gallardo Reference Arntz and Gallardo1994, Arntz et al. Reference Arntz, Brey and Gallardo1994). The Antarctic foraminiferal assemblages show some endemic species, but other species with worldwide ranges or unknown origins are also present (Gooday et al. Reference Gooday, Bowser and Bernhard1996, Pawlowski et al. Reference Pawlowski, Fahrni, Brykczynska, Habura and Bowser2002, Majewski Reference Majewski2005). The foraminifera species are distributed in a patchy pattern in the shelf zone (Mikhalevich Reference Mikhalevich2004) and normally with an equivalent portion of agglutinated and calcareous forms in shallow water assemblages (Ward et al. Reference Ward, Barrett and Vella1987).
The four summers studied here reflected the different biofacies throughout the Ezcurra Inlet that occur with no great differences between the environmental parameters. The most marked change in annual distribution was observed during the 2006/07 summer, when the samples came from shallower waters, where the foraminifera showed noticeably low diversity with only a few highly dominant species.
The Ezcurra Inlet has a typical Antarctic foraminifera fauna. Most of the studies concerning the assemblages of foraminifera along Antarctic coasts and shelves have found species like Portatrochammina antarctica, Globocassidulina biora, Cassidulinoides parkerianus, C. porrectus, Bolivina pseudopunctata, Spiroplectammina biformis and Adercotryma glomerata, along with low richness and diversity and low abundance of living foraminifera (Chang & Yoon Reference Chang and Yoon1995, Mayer Reference Mayer2000, Li et al. Reference Li, Yoon and Park2000, Majewski Reference Majewski2005).
Ezcurra Inlet assemblages
The typical foraminifera in Admiralty Bay are Portatrochammina antarctica, Cassidulinoides parkerianus, Cibicides refulgens, Spiroplectammina biformis, and Globocassidulina biora, and these are distributed more or less evenly throughout shallow and deep settings (Majewski Reference Majewski2005). The Ezcurra Inlet has assemblages that reflect in particular the shallow area, the entrance and the inner parts, which were defined by MDS analyses, simple correlation and differences in some environmental parameters. The cluster and MDS analyses presented four distinct groups, one of them with just two samples and showing low richness (Group 2). The three remaining groups showed ecological characteristics allowing the definition of three different assemblages: shallow waters (Group 1), inlet entrance (Group 3) and inner coves (Group 4). These assemblages could be defined by richness and abundance of species and their relationship with oceanographic factors are summarized in Table X.
Table X Ezcurra Inlet foraminiferal assemblages and indicator species in relation to environmental sectors and critical variables.

The inlet entrance is in the deepest area with the coldest waters and is characterized by bottom sediment with abundant very fine sand, a large number of specimens and high values of richness and diversity. High diversity has been correlated with environmental stability (Gibson 1966) and also high total abundance (Bernhard Reference Bernhard1988). The main species in this part of the inlet are the calcareous Bolivina pseudopunctata and Fursenkoina fusiformis, and the agglutinated Adercotryma glomerata and Portatrochammina antarctica. These are all typical Antarctic coastal species and have previously been found in Admiralty Bay (Majewski Reference Majewski2005). The calcareous Bolivina pseudopunctata and Fursenkoina fusiformis are commonly observed near the entrances of Admiralty Bay and Bransfield Strait (Ishman & Domack Reference Ishman and Domack1994, Rodrigues Reference Rodrigues2008).
The calcareous species Cassidulinoides parkerianus, Cassidulinoides porrectus, and the agglutinated species Psammosphaera fusca are abundant in mud substrates, particularly between Dufayel Island and the western coves, a restricted area with a depth range of 30–55 m located near glaciers. Around Antarctica, Psammosphaera fusca is known to dominate the continental slope of the Weddell Sea (Milam & Anderson Reference Milam and Anderson1981) and the shelf of Prydz Bay as well as being found in the Antarctic Circumpolar Waters (Schröder-Adams Reference Schröder-Adams1990). Kaminski (Reference Kaminski1985) related a low richness and low diversity assemblage with Psammosphaera spp. and other simple, one-chambered agglutinated foraminifera to an unstable, physically controlled environment favouring opportunistic species (Harloff & Mackensen Reference Harloff and Mackensen1997). Studying the recent benthic foraminiferal assemblages at Admiralty Bay, Majewski (Reference Majewski2005) described a fauna very typical of all its inlets: areas with low-diversity assemblages dominated by Globocassidulina biora and Psammosphaera fusca, but where Cassidulinoides parkerianus and C. porrectus also occur.
The development of the inner inlet zone may have been affected by more intense freezing, iceberg grounding, and freshwater injections with higher silicate content and large quantities of suspended material. This region can be contrasted with the main channel (Majewski Reference Majewski2005).
Shallow waters have low values of richness and abundance, higher suspended matter, high silicate content, high temperature and coarse sediment. The most representative species are Hemisphaerammina bradyi and Hippocrepinella hirudinea. Hemisphaerammina bradyi shows dominance in areas with high concentrations of suspended matter, high pH and low salinity.
The low foraminifera richness and low abundance is characteristic of Antarctic shallow areas (depths up to 20 m) (Bernhard Reference Bernhard1988, Mikhalevich Reference Mikhalevich2004, Majewski Reference Majewski2005). Majewski (Reference Majewski2005) described Hemisphaerammina bradyi as a typical species in Admiralty Bay shallow waters. The correlations between this species and environmental parameters show that the shallow waters are more intensely influenced by local inflow of meltwater. We also suggest bathymetry and near-shore sedimentation have a greater impact on foraminiferal communities than water chemistry or the extension of winter sea ice. The shallow water regions (< 7 m depth) in McMurdo Sound have been characterized as regions of instability (Dayton et al. Reference Dayton, Robilliard and Devries1969), where high proportions of living individuals and low total abundances are common, and where dead tests do not accumulate due to the high energy of the unstable environment (Bernhard Reference Bernhard1988).
Conclusion
The physical and chemical characteristics of the bottom waters of Ezcurra Inlet showed annual differences especially in the last summer studied (2006/07), where the shallower water samples were more susceptible to the freshwater inputs from glaciers and noticeable changes in temperatures, salinity, dissolved silicate and suspended matter. The Ezcurra Inlet has three well-defined recent benthic foraminiferal assemblages. The entrance and inner coves of the inlet had distinct assemblages, whilst the shallow waters assemblage was characterized by samples collected during the summer of 2006/07. Foraminifera species found were very typical of Antarctic coastal and shallow waters, with a low abundance of total and living foraminifers, low diversity and richness, and a proportional number of calcareous and agglutinated species and specimens. Characteristics of the bottom such as grain size and depth seem to be directly or indirectly related to the distribution pattern of the foraminiferal assemblages at Ezcurra Inlet.
Acknowledgements
The field and laboratory work was financially supported by CNPq (Conselho Nacional de Pesquisa, 550349/2002-2 and 558472/2005-2), PROANTAR (Brazilian Antarctic Program) and the Brazilian Navy. Special thanks are due to the crew of the Skua boat for help with the fieldwork. The authors are especially grateful to Adriana R. Perretti, Katia S. Jaworski and Valquiria M. de C. Aguiar for help in the collection of the samples. We also thank Anail Talita P. Alves, Camila M. de Andrade, Teresa L. Diaz and Vitor G. Chiozzini for helping in the foraminiferal and chemistry analysis. We are grateful to Dr Wojciech Majewski and an anonymous referee for their constructive comments, and Dr David Martin for his helpful editorial comments on the manuscript. The research reported in this paper was a part of the lead author’s PhD thesis, which was funded by a CAPES scholarship (Coordenadoria de aperfeiçoamento ao pessoal de Nível Superior).
Appendix A
Species citations for foraminifera mentioned in this paper.
Adercotryma glomerata (Brady)
Ammodiscus incertus (d’Orbigny)
Ammonia beccarii (Linné)
Angulogerina earlandi Parr
Armorella spherica Heron-Allen & Earland
Astrononion echolsi Kennett
Bigenerina minutissima (Earland)
Bolivina pseudopunctata Höglund
Buccella peruviana (d’Orbigny)
Bulimina elongata d’Orbigny
Cassidulinoides parkerianus (Brady)
Cassidulinoides porrectus Heron-Allen & Earland
Cibicides mckannai Galloway & Wissler
Cornuspira involvens (Reuss)
Fursenkoina fusiformis (Williamson)
Gaudryina exilis Cushman & Brönnimann
Globocassidulina biora (Crespin)
Globocassidulina subglobosa (Brady)
Glomospira gordialis (Jones & Parker)
Haplophragmoides bradyi (Brady)
Hemisphaerammina bradyi Loeblich & Tappan
Hippocrepinella hirudinea Heron-Allen & Earland
Hormosinella ovicula gracilis Earland
Lagenammina arenulata (Skinner)
Miliammina arenacea (Chapman)
Miliammina earlandi Loeblich & Tappan
Nodulina kerguelensis Parr
Nonionella bradii (Chapman)
Nonionella iridea Herron-Allen & Earland
Oolina felsinea Fornasini
Oolina lineata (Williamson)
Paratrochammina lepida Brönnimann & Whittaker
Portatrochammina antarctica (Parr)
Procerolagena gracilis Williamson
Proteonina decorata Earland
Psammosphaera fusca Schulze
Pseudobolivina antarctica Heron-Allen & Earland
Pseudononion atlanticum (Cushman)
Pullenia subcarinata (d’Orbigny)
Quinqueloculina patagonica d’Orbigny
Quinqueloculina seminulum (Linné)
Quinqueloculina vulgaris d’Orbigny
Reophax scorpiurus Montfort
Reophax subdentaliniformis Parr
Reophax subfusiformis Earland
Spiroplectammina biformis (Parker & Jones)
Spiroplectammina filiformis Earland
Spiroplectammina subfusiformis Earland
Textularia earlandi Parker
Thurammina corrugata Earland
Trifarina angulosa (Williamson)
Verneuilina minuta Weisner and Tappan