Introduction
The importance of passive dispersal to terrestrial invertebrates at both landscape and regional scales has long been of interest to biologists of Antarctic terrestrial fauna (Gressitt Reference Gressitt1967). Although there is a consensus that the overall patterns of invertebrate distributions in Antarctica ice-free habitats reflect glacial isolation and endemism (Stevens et al. Reference Stevens, Greenslade, Hogg and Sunnocks2006, Convey & Stevens Reference Convey and Stevens2007, Convey et al. Reference Convey, Gibson, Hillenbrand, Hodgson and Pugh2008, Pugh & Convey Reference Pugh and Convey2008), there is growing evidence to support the view that passive dispersal, both in terms of dispersal itself and as a distinct class of interactions with the abiotic environment, is not insignificant. With regard to the former, passive dispersal may contribute to both long and short-term transport of individuals and populations across the matrix of habitat that is not ice-free. Examination of the biogeographic signature of the Antarctic fairy shrimp, for example, makes it hard to come to any other conclusion, other than that phoresy (in particular, avian phoresy) is implicated in its current distribution on the Antarctic Peninsula (Hawes Reference Hawes2009). With regard to the latter, abiotic interactions have traditionally been viewed primarily in terms of physiological interactions with temperature and water availability. However, the physical manifestations of Antarctica's climate - wind, ice, meltwater - all represent distinct phenomena which challenge both the physical and physiological viability of individuals and populations. For example, some of the implications of wind and water capture have been examined in springtails already (Hawes et al. Reference Hawes, Worland, Bale and Convey2007, Reference Hawes, Worland, Bale and Convey2008). Although these experiments were limited to Cryptopygus antarcticus Willem, parallel work in the Arctic confirms that it is a generalized phenomenon in polar habitats (Coulson et al. Reference Coulson, Hodkinson, Webb and Harrision2002, Hawes Reference Hawes2007, Reference Hawes2008).
Gomphiocephalus hodgsoni Carpenter, is one of the most widespread springtails in continental Antarctica with a range that extends over most of southern Victoria Land (Janetschek Reference Janetschek1967, Stevens & Hogg Reference Stevens and Hogg2002). Molecular phylogeography indicates high levels of genetic structure in populations consistent with the effects of isolation and glacial refugia on gene flow (Stevens & Hogg Reference Stevens and Hogg2003, McGaughran et al. Reference McGaughran, Hogg and Stevens2008, Reference McGaughran, Torricelli, Carapelli, Frati, Stevens, Convey and Hogg2010). Studies of continental Antarctic springtails at fine spatial scales (Nolan et al. Reference Nolan, Hogg, Stevens and Haase2006, Hawes et al. Reference Hawes, Torricelli and Stevens2010), in particular, have demonstrated that even small geographic barriers (e.g. streams, ridges, areas of permanent snow) may be effective mechanisms for genetic isolation. Two studies have also suggested, albeit inconclusively, that some aspects of the phylogeographic patterns identified, may be indicative of passive dispersal across such barriers: Stevens & Hogg (Reference Stevens and Hogg2003) found a shared haplotype between Cape Bird and Granite Harbour populations; likewise, Hawes et al. (Reference Hawes, Torricelli and Stevens2010) found a shared haplotype between two sites separated by a gully of ice.
Rafting on water surfaces is a well-known phenomenon in springtails and, in an Antarctic context, may have important ramifications for local, and occasionally long distance, dispersal (Hawes et al. Reference Hawes, Worland, Bale and Convey2008). Previous work (Hawes et al. Reference Hawes, Worland, Bale and Convey2008) has demonstrated the potential for long-term survival of individuals and even production of offspring. More recently McGaughran et al. (in press) have examined short-term survival of Gomphicephalus hodgsoni floating on freshwater and seawater under laboratory conditions. However, although laboratory investigations permit a certain degree of inference about the significance of rafting, field observations have been wanting. The aim of the work described here, was to place these laboratory studies in a field context by examining their applicability to observations of in situ rafting. Thus, although physiological parameters are readily examined under controlled conditions, the actual occurrence of rafting aggregations may be associated with numerous factors that have not been replicated, or even recognized. This paper describes for the first time the results of a series of continuous field observations of rafting aggregations of Gomphicephalus hodgsoni under natural conditions.
Methods
Research was carried out at meltwater pools in the vicinity of Granite Harbour, Victoria Land - the type locality for G. hodgsoni (Davidson & Broady Reference Davidson and Broady1996) from where samples were taken by the Discovery Expedition of 1901–04. Observations for this study were undertaken daily over a ten-day period during January 2009. Rafting aggregations were found in meltwater pools produced by the melting of ice and snow as a result of the warmer temperatures of the summer. The pools were produced by natural inclinations of topography and represented transient capture of free water depending on weather conditions (see Table I) - although not persistent, they would be expected to recur each summer given similar conditions. Five pools were identified within walking distance of the field camp and visited daily over the period. So as not to disturb the aggregations unnaturally, rafts were photographed daily and digital images used to count numbers of springtails and mites. At the end of the observation period, springtails and mites still remaining in pools were removed, checked for mortality, and counted to determine if escape had occurred. The status of each pool (wet, frozen, dry) was noted for each day. General observations were made to corroborate and/or augment previous descriptions of rafting. These included the size and composition of aggregations, their escape from surface capture, and survival at the end of the observation period. In addition to the pools visited daily, a c.1 km transect along the shoreline was walked, looking for evidence of dispersal to the marine environment either in terms of springtails floating in the tide cracks between sea ice and shore, or aerially deposited directly on to the sea ice. For safety reasons, the latter was confined to scanning sea ice which directly abutted the shore.
Table I Comparison of pools and their raft aggregations.
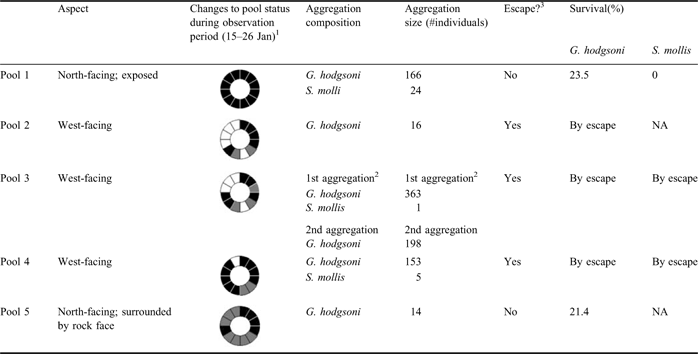
1=rings read clockwise where each slice represents one observation day; black = water, grey = frozen, white = dried out.
2=for pool 3 there were two aggregations, the second captured after the refilling of the pool on 21 Jan.
3=assumes complete capture on pool surface; so ‘escape’ = where changes to pool conditions allow escape.
The results may be considered to be natural history observations that provide ecological context previously lacking in our understanding of rafting in Antarctic springtails. It is noted that no statistical comparisons were made between the pools because such comparisons, while potentially adding a quantitative veneer to observations would, in essence, disguise the stochasticity and individuality of each rafting situation as it was observed. Indeed, any statistical differences identified would be invalidated by the fact that under natural conditions every variable - from source of springtails; to number of springtails; to pool size, topography, aspect and location - is unique.
Results and discussion
Rafting aggregations of G. hodgsoni are comprised of springtails, occasional mites (Stereotydeus mollis), microscopic windblown detritus, and moult exuviae (Table I, Figs 1 & 2). While Strong (Reference Strong1967) has shown the survival of mites after extended seawater immersion in the maritime Antarctic, it is noted that this represents the first recorded observation (although certainly not the first occurrence!) of rafting mites to my knowledge. It should be emphasized that the springtails, in particular, are not entirely passive on the water film. The provision of rafting materials such as moult exuviae and microscopic windblown detritus allows them to move around and considerably extends their survival ability. Also, in an aggregational context, these water traps stimulate intraspecific interactions geared toward improving survival. Moulting, for example, is cued by water availability as well as aggregation (Hopkin Reference Hopkin1997). Other springtails may also be used as rafts, and the sight of springtails surfing on other springtails is a common observation in aggregations (Fig. 2). There are three main windows of escape for trapped animals: opportunistic drifting of aggregations or individuals to the edge of the pool, evaporation of the pool (e.g. Fig. 3) or freezing of the pool (Hawes et al. Reference Hawes, Worland, Bale and Convey2007). However, observations suggest that the last may be rarer, with animals as likely (or more likely) to be trapped by the freezing of surface water (e.g. Fig. 4).

Fig. 1 Typical rafting aggregation. Note how extensive microscopic windblown detritus has been incorporated into the raft (body size = c. 1 mm).

Fig. 2 Close-up of rafting aggregation highlighting moulting (coarse dash line circle), surfing on other springtails (solid line circle) and mite (fine dash line circle) (body size = c. 1 mm).
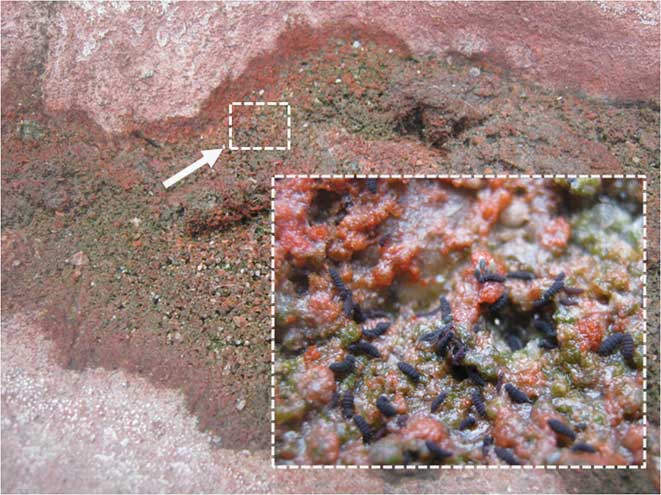
Fig. 3 Dried out pool with ‘escaped’ springtails released by the evaporation of the water (inset shows close-up of springtails; small white arrow and box indicates area magnified) (body size = c. 1 mm).

Fig. 4 Springtail frozen into the ice of a pool (body size = c. 1 mm).
Table I summarizes the results of the time series observations. The most obvious variable is the hydric status of the pools - water-filled, frozen or dried out - which in addition to being related to general meteorological conditions, may vary from pool to pool depending on their aspect and degree of exposure. Thus, for example, pool 1 was open and north facing and was the only pool to retain its status throughout the observation period, while pool 5, although also north facing, was closely surrounded by boulders, so that when it froze, it remained so for days at a time. In general, escape from water capture is shown to be a potentially regular (but localized) occurrence in this context, with the status of the temporary pools frequently changing. However, for those pools where no such change occurs, escape is unlikely and the probability of survival low.
One of the most pressing questions raised by previous experimental work on rafting by Hawes et al. (Reference Hawes, Worland, Bale and Convey2008) was the extent to which they successfully translate into the field ecology of springtails under Antarctic conditions. Thus although the potential for survival, reproduction, and, therefore, dispersal, has been demonstrated; the extent to which such projections are realistic merits further consideration. The conditions of continental Antarctica are considerably more challenging than those of maritime Antarctica, so the case of G. hodgsoni is different to that of Cryptopygus antarcticus (see Hawes et al. Reference Hawes, Worland, Bale and Convey2008). In particular, G. hodgsoni experiences lower temperatures and reduced water availability (flushes of vegetation which are not uncommon on the Antarctic Peninsula are extremely rare and through most of its range, it occupies what are effectively polar desert landscapes). Aggregations, likewise, at least in terms of rafting - compare for example typical raft aggregations shown here and in Hawes et al. (Reference Hawes, Worland, Bale and Convey2008) - are considerably smaller and probably generally reflective of smaller populations. Certainly, for example, some variation in tolerance and motility might be expected between families like Hypogastruridae (G. hodgsoni) and Isotomidae (C. antarcticus) as a result of body-shape and the degree of furca reduction. However, although the observations described here concern one species, the broad findings remain relevant to springtails throughout Antarctica. Indeed, unpublished pilot experiments (TCH) have found short-term survival of rafting in Friesea grisea (Schäffer) in the maritime Antarctic and Gressittacantha terranova Wise at Terra Nova Bay. While Pryor (Reference Pryor1962) noted the presence of Isotoma klovstadi Carpenter floating on temporary pools produced by meltwater runoff. It is also worth noting, that Coulson et al. (Reference Coulson, Hodkinson, Webb and Harrision2002) demonstrated the potential for oceanic rafting in a range of representative species (including isotomids and hypogastrurids) in the high Arctic.
In addition to the springtails observed in meltwater pools, examinations of the tidewater cracks of seawater that existed between the land and sea ice, revealed a number of springtails floating on the surface. Although there was nowhere for them to go - the sea ice blocked further carriage elsewhere and the area is enclosed within a bay - the potential for marine dispersal was evident. Inspection of the sea ice surface along the same observational transect - looking at sea ice which directly touched the land (without tidewater cracks in between) revealed two instances of springtails that had been aerially deposited on to the sea ice from the land (Fig. 5). It has been previously noted that aerial dispersal of Antarctic springtails may also be an important interacting factor with water capture (Hawes et al. Reference Hawes, Worland, Bale and Convey2007). Of course, realistically, even for springtails that are less isolated - such as in the maritime Antarctica - the word to emphasize is ‘potential’. This potentiality is a product of numerous factors, many of them stochastic (such as topography, distance to suitable habitat etc) - although it does increase with time. For the moment, it must be noted that although none of the observations presented here represent movements of significance to populations, they do contribute to our understanding and characterization of the factors that make such events so stochastic.

Fig. 5 Springtail aerially deposited on the sea ice; glove shown for scale, with successive close-ups (a–d) showing the location of the springtail (body size = 1 mm).
The present study also emphasizes the extent to which water capture is a class of environmental interaction in its own right for Antarctic springtails. Even our understanding of its physiological implications remains incomplete. In this context, it is worth noting that the observations of rafting aggregations also suggested an additional previously unrecognized (Hawes et al. Reference Hawes, Worland, Bale and Convey2008) influence on the viability of rafting springtails: the recognition that the physiological challenge of survival may not only be determined by responses to water and temperature, but also by continuous direct exposure to sunlight with the concomitant danger of extended exposure to ultraviolet radiation (UV-R). A separate paper (Hawes et al. unpublished data) will examine the potential for UV-R damage in G. hodgsoni; but for now it is sufficient to note, that capture on the water surface effectively prevents access to mitigating cover, so that springtails on the water surface may be directly exposed for days at a time to 24 h UV-R. Given the variability of Antarctic weather, such exposure may be intermittent and unpredictable, but it is nonetheless recurrent and unavoidable for such captured springtails.
The significance of rafting to springtails, to dispersal in particular, has been previously highlighted (Hawes et al. Reference Hawes, Worland, Bale and Convey2008). Continuous field observations of rafting in G. hodgsoni agree with these findings but also show the ways in which the size and composition of rafts are dependent on (or perhaps reflective of) the local faunal assemblage. They also emphasize two additional ecological parameters - the stochasticity of the raft environment and potential for extended exposure to UV-R. Thus, although water capture is an accidental phenomenon, it is a recurring and widespread component of springtail ecology in Antarctica and, irrespective of its implications to dispersal, should be considered as a common and distinct class of ecological interaction with the Antarctic abiotic environment.
Acknowledgements
Thanks to David Wharton and Craig Marshall for their support; Steven Clarke and Michelle Liddy for field assistance, and Antarctica NZ; to the reviewers for their helpful comments.








