Introduction
The global diversity of free-living protists is not known, although estimates range from < 30 000 (Mora et al. Reference Mora, Tittensor, Adl, Simpson and Worm2011) to over 1 million species (Adl et al. Reference Adl, Leander, Simpson, Archibald, Anderson and Bass2007, Cotterill et al. Reference Cotterill, Al-Rasheid and Foissner2008, Larsen et al. Reference Larsen, Miller, Rhodes and Wiens2017), with many in between (Appeltans et al. Reference Appeltans, Ahyong, Anderson, Angel, Artois and Bailly2012, Pawlowski et al. Reference Pawlowski, Audic, Adl, Bass, Belbahri and Berney2012, de Vargas et al. Reference de Vargas, Audic, Henry, Decelle, Mahe and Logares2015). Improved understanding of protistan diversity of soils in ice-free regions around Antarctica (c. 0.5% of the continent; Burton-Johnson et al. Reference Burton-Johnson, Black, Fretwell and Kaluza-Gilbert2016) can help to refine these estimates. As the most climatically extreme and isolated continent on Earth, Antarctic soils are home to many phylogenetically and physiologically unique species (Rogers Reference Rogers2007, Vyverman et al. Reference Vyverman, Verleyen, Wilmotte, Hodgson, Willems and Peeters2010, Convey et al. Reference Convey, Chown, Clarke, Barnes, Bokhorst and Cummings2014). These soil communities are on the verge of experiencing major shifts in the face of climate change (Amesbury et al. Reference Amesbury, Roland, Royles, Hodgson, Convey and Griffiths2017), and some of the more specialized species may be at risk of extinction (Frenot et al. Reference Frenot, Chown, Whinam, Selkirk, Convey and Skotnicki2005, Hughes et al. Reference Hughes, Pertierra, Molina-Montenegro and Convey2015), including heterotrophic protists (HPs) that play key roles in nutrient cycling and community structure. Conservation of these at-risk, scientifically intriguing species is therefore a high priority (Chown & Convey Reference Chown and Convey2007), yet a checklist of HP species for Antarctica does not exist. Here, we present a comprehensive summary of the diversity of Antarctic HPs in order to establish a baseline for conservation efforts and a framework for future protist biodiversity research in Antarctica's ice-free regions.
As a group, HPs possess a high degree of morphological, physiological, evolutionary and ecological diversity (Doolittle et al. Reference Doolittle, Feng, Tsang, Cho and Little1996, Couteaux & Darbyshire Reference Couteaux and Darbyshire1998, Geisen et al. Reference Geisen, Mitchell, Adl, Bonkowski, Dunthorn and Ekelund2018). They play unique and essential roles in soil ecosystems, including promoting prey diversity and mobilizing nutrients to higher trophic levels (Corliss Reference Corliss2004, Clarholm Reference Clarholm2005, Anderson Reference Anderson2012, Rønn et al. Reference Rønn, Vestergård and Ekelund2012, Wilkinson et al. Reference Wilkinson, Creevy and Valentine2012). In Antarctica, these protists have been studied for over 100 years (Richters Reference Richters1908, Sudzuki Reference Sudzuki1979, Roland et al. Reference Roland, Amesbury, Wilkinson, Charman, Convey and Hodgson2017). The most recent review of this diversity listed 50 zooflagellates, 15 gymnamoebae, 60 testate amoeba and 75 ciliates, or 200 total species, yet the studies were heavily biased towards the Antarctic peninsula, South Orkney Islands and other maritime Antarctic islands (Smith Reference Smith1996).
The Antarctic continent provides a range of environmental conditions that are important to consider when discussing species surveys and management (Terauds et al. Reference Terauds, Chown, Morgan, Peat, Watts and Keys2012, Convey et al. Reference Convey, Chown, Clarke, Barnes, Bokhorst and Cummings2014). Generally, the northern peninsula is warmer and wetter relative to continental sites and hosts large swaths of moss beds and input from seabirds and other marine mammals. This region is more immediately susceptible to anthropogenic climate change and the invasion of non-native species (Hughes & Convey Reference Hughes and Convey2010). Coastal continental sites (i.e. East Antarctica, Dronning Maud Land, Enderby Land) are colder and dryer but still experience moisture, chemical and biological input from the sea. Intra-continental sites (i.e. Transantarctic Mountains, Ellsworth Land and Mountains, south and north Victoria Land, Prince Charles Mountains) host ice-free regions that are among the driest and coldest on Earth and are often used as analogues for other planets (e.g. Mars) (Doran et al. Reference Doran, Lyons and McKnight2010, Heldmann et al. Reference Heldmann, Pollard, McKay, Marinova, Davila and Williams2013). Assessing HP diversity in the coldest and driest regions is especially important to the conservation of highly unique ecosystems, which may hold a higher proportion of endemic organisms with unique physiologies.
Here, we do not differentiate between Antarctic regions (Terauds & Lee Reference Terauds and Lee2016) since: 1) no checklist exists for the continent as a whole, 2) Antarctica is largely isolated from the rest of the world and its HP diversity may reflect this at the continental scale, and 3) HP biogeography and dispersal rates between regions in Antarctica are unknown and efforts to characterize diversity at smaller scales will benefit from a comprehensive reference. A review that explores HP diversity at the regional scale is in preparation (Thompson et al. unpublished).
Methods
This checklist focuses on continental and peninsular Antarctica, but includes the South Shetland Islands and Elephant Island due to their proximity to the northern tip of the peninsula. We reviewed all the studies that our literature search recovered on HPs in these regions since the beginning of formal research in Antarctica. The earliest was published in 1907 (Richters Reference Richters1907) and the most recent was published in 2018 (Park et al. Reference Park, Min and Kim2018). Searches were performed using variations on the keywords ‘Antarctica’, ‘terrestrial’, ‘moss' and ‘soil' coupled with ‘protist’, ‘protozoa’, ‘ciliate’, ‘Ciliophora’, ‘testate’, ‘Thecamoebian’, ‘Rhizopod’, ‘Arcellacean’, ‘Testacean’, ‘amoeba’, ‘flagellate’, ‘Cercozoa’, ‘Excavata’, ‘Euglenozoa’, ‘Mycetozoa' and ‘slime mould' in Web of Science, SCOPUS and Google Scholar, and by following citation chains in all of the articles found (Ing & Smith Reference Ing and Smith1983, Putzke et al. Reference Putzke, Pereira and Terezinha Lopes2004). Although we feel confident that we have captured the vast majority of relevant articles (we found no additional articles cited by the reviewed literature that we were not aware of or could not obtain electronically), this search will have failed to recover any articles that were not readily accessible through digital means.
Where a paper performing original research (e.g. Smith Reference Smith1992) included taxonomic entries from previous studies, only novel records from the original research were added to the list. Whenever possible, we found the previous studies referenced and pulled their records directly in order to control for unclear sampling origin or other inconsistences (e.g. Hada Reference Hada1964; see ‘Results' section).
To account for taxonomic changes that have occurred since many of the reviewed studies were published, we include a brief taxonomic history for each species entry. To ensure the accuracy of this taxonomic history, an additional search was performed using Web of Science, SCOPUS and Google Scholar to verify the most recent accepted taxonomic position and list pertinent nomenclatural changes. Resources used to construct the checklist are included as supplementary material. Indented names are not currently considered valid but represent original descriptions, past classifications or misspellings that appear in the literature. The geographical origin and author of each record presented by the checklist will be included in another publication (Thompson et al. unpublished).
Results
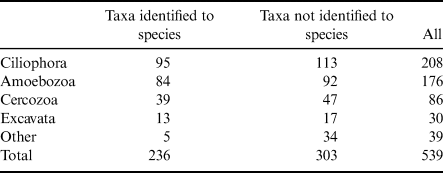
In our review of 54 studies on HP diversity in Antarctica, we recovered a total of 539 taxa (Table I). Of this total, 236 were identified to species: 95 Ciliophora, 84 Amoebozoa (including 7 species of slime mould), 39 Cercozoa, 13 Excavata, 3 Stramenopiles, 1 Apicomplexan (Colpodella edax) and 1 incertae sedis (Polypseudopodius bacteroides). An additional 303 taxa not identified to species were recorded, 194 of which were identified as far as genus. The 109 remaining include the records from those studies that did not identify specimens past the morphological phylum level (i.e. ciliate, flagellate, testate amoeba), as well as unclassified sequences from molecular studies.
Table I. Taxonomic summary of terrestrial heterotrophic protists in continental and peninsular Antarctica. Records were pulled from the results of a literature review; counts include all reliable records from all publications. Taxa not identified to species may be identified to any other taxonomic level, although most commonly they were identified to genus or phylum. ‘Other' includes heterotrophic stramenopiles, non-ciliophoran alveolates and heterotrophic protists of uncertain phylogenetic position.
SAR: Stramenopile (Chrysophyceae)
Oikomonas mutabilis Kent, 1880
Oikomonas termo (Müller, 1773)
Monas termo Müller, 1773 (orig.)
Heterochromulina termo (Ehrenberg) (syn., no year)
Oikomonas termo (Müller, 1773) Kent, 1880 (reclass.)
Oikomonas termo Ehrenberg, 1838 (error in authorship)
Oicomonas termo Ehrenberg, 1838 (error in authorship, misspelling)
SAR: Stramenopile (Dictyochophyceae)
Actinomonas mirabilis Kent, 1880
SAR: Alveolata (Apicomplexa)
Colpodella edax (Klebs, 1892)
Bodo edax Klebs, 1892 (orig.)
Heteromita angusta Dujardin, 1841 (syn.)
Bodo caudatus Stein, 1878 sensu Hänel, 1979 (syn., in part) see Parabodo caudatus
Spiromonas angusta (Dujardin, 1841) Kent, 1881 (syn.)
Bodo celer Klebs, 1892 (syn., no year)
Colpodella angusta (Dujardin, 1841) Simpson and Patterson, 1996 (syn.)
Colpodella edax (Klebs, 1892) Simpson and Patterson, 1996 (reclass.)
SAR: Alveolata (Ciliophora)
Acineria uncinata Tucolesco, 1962
Acineria uncinata Dujardin, 1841 (error in authorship)
Acuholosticha paranotabilis (Foissner, Agatha and Berger 2002)
Uroleptus paranotabilis Foissner, Agatha and Berger 2002 (orig.)
Cuadiholosticha paranotabilis (Foissner, Agatha and Berger, 2002) Berger, 2006 (reclass.)
Acuholosticha paranotabilis (Foissner, Agatha and Berger 2002) Li et al., 2017 (reclass.)
Anteholosticha rectangular Jung, Park and Kim, 2016
Anteholosticha sigmoidea (Foissner, 1982)
Holosticha sigmoidea Foissner, 1982 (orig.)
Anteholostigma sigmoidea (Foissner, 1982) Berger, 2003 (reclass.)
Blepharisma hyalinum Perty, 1849
Blepharisma hyalinum Perty, 1852 (error in year)
Bryophyllum loxophylliforme Kahl, 1931
Bryophyllum tegularum Kahl, 1931
Adumbratosticha tetracirrata (Buitkamp and Wilbert, 1974)
Holosticha tetracirrata Buitkamp and Wilbert, 1974 (orig.)
Caudiholosticha tetracirrata (Buitkamp and Wilbert, 1974) Berger, 2003 (reclass.)
Adumbratosticha tetracirrata (Buitkamp and Wilbert, 1974) Li et al., 2017 (reclass.)
Cinetochilum margaritaceum (Ehrenberg, 1831)
Cyclidium margaritaceum Ehrenberg, 1831 (orig.)
Cinetochilum margaritaceum (Ehrenberg, 1831) Perty, 1852 (reclass.)
Cinetochilum margarcliclum (Ehrenberg) (misspelling)
Codonella cratera (Leidy, 1877)
Difflugia crater Leidy, 1877 (orig.)
Codonella lacustris Entz, 1885 (syn.)
Codonella cratera (Leidy, 1877) Imhof, 1885 (reclass.)
Colpoda californica Kahl, 1931
Colpoda cucullus (Müller, 1773)
Kolpoda cucullus Müller, 1773 (orig.)
Colpoda cucullus (Müller, 1773) Gmelin, 1790 (reclass.)
Colpoda cuculla (Müller, 1773): Hada, 1967 (misspelling)
Colpoda ecaudata (Liebmann, 1936)
Cyclidium ecaudatum Liebmann, 1936 (orig.)
Balantiophorus minutus Schewiakoff sensu Watson, 1945 (syn.)
Colpoda ecaudata (Liebmann, 1936) Foissner, Blatterer, Berger and Kohmann, 1991 (reclass.)
Colpoda inflata (Stokes, 1884)
Tillina inflata Stokes, 1884 (orig.)
Colpoda rouxi Kahl, 1926 (syn.)
Colpoda inflata (Stokes, 1884) Kahl, 1931 (reclass.)
Colpoda inflata (Stokes, 1885) Kahl, 1931 (reclass., error in year)
Colpoda maupasi Enriques, 1908
Colpoda fastigata Kahl, 1931 (syn.)
Colpoda matritensis Ocariz, Rico and Munoz, 1965 (syn.)
Colpoda steinii Maupas, 1883
Colpoda steini Maupas, 1883: Sudzuki, 1979 (misspelling)
Tillina saprophila Stokes, 1884 (syn.)
Colpoda saprophila (Stokes, 1884) (syn.)
Colpoda duodenaria Taylor and Furgason, 1938 (syn.)
Colpoda steni (misspelling)
Colpoda dragescoi Chardez, 1981 (syn.)
Cyclidium glaucoma Müller, 1786
Cyclidium muscicola Kahl, 1931
Cyrtohymena candens (Kahl, 1932)
Steinia candens Kahl, 1932 (orig.)
Steinia simplex Dragesco, 1966 (syn.)
Cyrtohymena candens (Kahl, 1932) Foissner, 1989 (reclass.)
Cyrtohymena citrina (Berger and Foissner, 1987)
Steinia citrina Foissner, 1985 (nomen nudum)
Steinia citrina Berger and Foissner, 1987 (orig.)
Cyrtohymena citrina (Berger and Foissner, 1987) Foissner, 1989 (reclass.)
Cyrtolophosis acuta Kahl, 1926
Cyrtolophosis mucicola Stokes, 1885
Dichilum cuneiforme Schewiakoff, 1889
Dichilium cuneiforme Schewiakoff (misspelling)
Dichilum cunciforme (misspelling)
Dichilum cuneiforme Schewiakoff, 1892 (error in year)
Drepanomonas revoluta Penard, 1922
Drepanomonas borzai Lepsi, 1948 (syn.)
Drepanomonas sphagni Kahl, 1931
Enchelys polynucleata (Foissner, 1984)
Enchelydium polynucleatum Foissner, 1984 (orig.)
Enchelys polynucleata (Foissner, 1984) Foissner, Agatha and Berger, 2002 (reclass.)
Epispathidium papilliferum (Kahl, 1930)
Spathidium papilliferum Kahl, 1930 (orig.)
Epispathidium papilliferum (Kahl, 1930) Foissner, 1984 (reclass.)
Fuscheria lacustris Song and Wilbert, 1989
Fuscheria terricola Berger, Foissner and Adam, 1983
Gastronauta derouxi Blatterer and Foissner, 1992
Gonostomum affine (Stein, 1859)
Oxytricha affinis Stein, 1859 (orig.)
Plagiotricha (Gonostomum) affinis Stein, 1859 (syn.)
Stichochaeta affinis (Stein, 1859) Gourret and Roeser, 1888 (syn.)
Gonostomum algicola Gellért, 1942 (syn.)
Gonostomum bryonicolum Gellért, 1956 (syn.)
Gonostomum ciliophorum Gellért, 1956 (syn.)
Gonostomum spirotrichoides Gellért, 1956 (syn.)
Gonostomum geleii Gellért, 1957 (syn.)
Gastrostyla affine (Stein, 1859) Borror, 1972 (syn.)
Trachelostyla bryonicolum (Gellért, 1956) Borror, 1972 (syn.)
Trachelostyla ciliophorum (Gellért, 1956) Borror, 1972 (syn.)
Trachelostyla geleii (Gellért, 1957) Borror, 1972 (syn.)
Trachelostyla spirotrichoides (Gellért, 1956) Borror, 1972 (syn.)
Trachelostyla canadensis Buitkamp and Wilbert, 1974 (syn.)
Trachelostyla affine (Stein, 1859) Small and Lynn, 1985 (syn.)
Gonostomum singhii Kamra, Kumar and Sapra, 2008 (syn.)
Grossglockneria acuta Foissner, 1980
Halteria grandinella (Müller, 1773)
Trichoda grandinella Müller, 1773 (orig.)
Halteria grandinella (Müller, 1773) Dujardin, 1841 (reclass.)
Hemiurosomoida longa (Gelei and Szabodos, 1950)
Oxytricha longa Gelei and Szabodos, 1950 (orig.)
Urosomoida longa (Gelei and Szabodos, 1950) Foissner et al., 1991 (reclass.)
Hemiurosomoida longa (Gelei and Szabodos, 1950) Singh and Kamra, 2015 (reclass.)
Heterourosomoida lanceolata (Shibuya, 1930)
Oxytricha lanceolata Shibuya, 1930 (orig.)
Heterourosomoida lanceolata (Shibuya, 1930) Singh and Kamra, 2015 (reclass.)
Holosticha pullaster (Müller, 1773)
Trichoda pullaster Müller, 1773 (orig.)
Oxytricha pullaster (Müller, 1773) (syn.)
Kerona pullaster (Müller, 1773) (syn.)
Amphisia micans (Engelmann, 1862) (syn.)
Oxytricha micans Engelmann, 1862 (syn.)
Holosticha micans (Engelmann, 1862) (syn.)
Oxytricha alba Fromentel, 1876 (syn.)
Amphisia multiseta Sterki, 1878 (syn.)
Holosticha simplicis Wang and Nie, 1932 (syn.)
Keronopsis retrovacuolata (Tucolesco, 1952) (syn.)
Holosticha kessleri var. aquae-dulcis Buchar, 1957 (syn.)
Keronopsis litoralis Gellért and Tamas, 1958 (syn.)
Holosticha danubialis Kaltenbach, 1960 (syn.)
Holosticha retrovacuolata Tucolesco, 1962 (syn.)
Holosticha coronata Vuxanovici, 1963 (syn.)
Holosticha minima Vuxanovici, 1963 (syn.)
Holosticha rhomboedrica Vuxanovici, 1963 (syn.)
Holosticha rhomboedrica f. eliptica Vuxanovici, 1963 (syn.)
Holosticha rhomboedrica f. lata Vuxanovici, 1963 (syn.)
Holosticha rostrata Vuxanovici, 1963 (syn.)
Holosticha rostrata f. pitica Vuxanovici, 1963 (syn.)
Holosticha rostrata var. mononucleata Stiller, 1974 (syn.)
Pseudokeronopsis retrovacuolata (Tucoleso, 1962) Borror and Wicklow, 1983 (syn.)
Holosticha pullaster (Müller, 1773) Foissner, Blatterer, Berger and Kohmann, 1991 (reclass.)
Homalogastra setosa Kahl, 1926
Kahlilembus attenuatus (Smith, 1897)
Lembus attenuata Smith, 1897 (orig.)
Lembus fusiformis Kahl, 1926 (syn.)
Cohnilembus fusiformis Kahl 1926 (syn.)
Kahlilembus attenuatus (Smith, 1897) Foissner, Berger and Kohmann, 1994 (reclass.)
Keronopsis helluo Penard, 1922
Lamtostyla perisincirra (Hemberger, 1985)
Tachysoma perisincirra Hemberger, 1985 (orig.)
Lamtostyla perisincirra (Hemberger 1985) Berger and Foissner 1987 (reclass.)
Lamtostylides edaphoni (Berger and Foissner, 1987)
Amphisiella edaphoni Berger and Foissner, 1987 (orig.)
Lamtostyla edaphoni Berger and Foissner, 1987 (syn.)
Lamtostylides edaphoni (Berger and Foissner, 1987) Berger, 2008 (reclass.)
Leptopharynx costatus Mermod, 1914
Leptopharynx sphagnetorum (Levander, 1900)
Trichopelma sphagnetorum Levander, 1900 (syn.)
Trichoderum sphagnetorum (Levander, 1900) Strand, 1942 (syn.)
Leptopharynx sphagnetorum (Levander, 1900) Corliss, 1960 (reclass.)
Microdiaphanosoma arcuatum (Grandori and Grandori, 1934)
Diaphanosoma arcuata Grandori and Grandori, 1934 (orig.)
Microthorax elegans Giraud, 1863
Microthorax simulans (Kahl, 1926)
Microthorax simulans (Kahl, 1926) Kahl, 1931
Nassula tuberculata Foissner, Agatha and Berger, 2002
Nivaliella plana Foissner, 1980
Odontochlamys wisconsinensis (Kahl, 1931)
Chilodonella wisconsinensis Kahl, 1931 (orig.)
Odontochlamys wisconsinensis (Kahl, 1931) Petz and Foissner, 1997 (reclass.)
Opercularia curvicaule (Penard, 1922)
Pyxidium curvicaule Penard, 1922 (orig.)
Pyxidium arboricolum Biegel, 1954 (syn.)
Pyxidium arboricola Biegel, 1954 (syn)
Opercularia arboricolum Biegel, 1954 (syn.)
Opercularia arboricola (Biegel, 1954) Foissner, 1981 (syn.)
Opercularia curvicaule (Penard, 1922) Foissner, 1998 (reclass.)
Orthamphisiella breviseries Foissner, Agatha, and Berger, 2002
Orthamphis breviseries Foissner, Agatha, and Berger, 2002: Fell, 2006 (misspelling)
Oxytricha fallax Stein, 1859
Oxytricha granulifera Foissner and Adam, 1983
Oxytricha opisthomuscorum Foissner, Blatterer, Berger and Kohmann, 1991
Oxytricha setigera Stokes, 1981
Paradileptus elephantinus (Svec, 1897)
Dileptus elephantinus Svec, 1897 (orig.)
Pelagodileptus elephantinus Svec, 1897 (syn.)
Paradileptus elephantinus (Svec, 1897) Kahl, 1931 (reclass.)
Amphileptus moniliger Ehrenberg, 1835 (syn.)
Amphileptus flagellatus Rousselet, 1890 (syn.)
Paradileptus flagellatus (Rousselet, 1890) Wenrich, 1929 (syn.)
Paradileptus robustus Wenrich, 1929 (syn.)
Paradileptus conicus Wenrich, 1929 (syn.)
Paradileptus ovalis Huber-Pestalozzi, 1945 (syn.)
Paradileptus estensis Canella, 1951 (syn.)
Paradileptus minutus Dragesco, 1972 (syn.)
Paraenchelys terricola Foissner, 1984
Paraholosticha muscicola (Kahl, 1932)
Keronopsis muscicola Kahl, 1932 (orig.)
Paraholosticha muscicola (Kahl, 1932) Wenzel, 1953 (reclass.)
Paramecium putrinum Claparède and Lachmann, 1858
Paramecium trichium Stokes, 1885 (syn.)
Paroxytricha longigranulosa (Berger and Foissner, 1989)
Oxytricha longigranulosa Berger and Foissner, 1989 (orig.)
Paroxytricha longigranulosa (Berger and Foissner, 1989) Foissner, 2016 (reclass.)
Plagiocampa difficilis Foissner, 1981
Platyophrya vorax Kahl, 1926
Pleuroplitoides smithi Foissner, 1996
Pleurotricha lanceolata (Ehrenberg, 1835)
Stylonychia lanceolata Ehrenberg, 1835 (orig.)
Pleurotricha lanceolata (Ehrenberg, 1835) Stein, 1859 (reclass.)
Protospathidium fraterculum Xu and Foissner, 2005
Protospathidium serpens (Kahl, 1930) Foissner, 1981 (syn., in part)
Protospathidium terricola Foissner, 1998
Pseudochilodonopsis mutabilis Foissner, 1981
Pseudocohnilembus pusillus (Quennerstadt, 1869)
Lembus pusillus Quennerstadt, 1869
Pseudocohnilembus pusillus (Quennerstadt, 1869) Foissner and Wilbert, 1981 (reclass.)
Pseudocyrtolophosis alpestris Foissner, 1980
Pseudoholophrya terricola Berger, Foissner, and Adam, 1984
Pseudonotohymena antarctica Park, Jung, Min and Kim 2016
Pseudoplatyophrya nana (Kahl, 1926)
Platyophrya nana Kahl, 1926 (orig.)
Pseudoplatyophrya nana (Kahl, 1926) Foissner, 1980 (reclass.)
Pseudoplatyophrya saltans Foissner, 1988
Rigidohymena quadrinucleata (Dragesco and Njiné, 1971)
Steinia quadrinucleata Dragesco and Njiné, 1971 (orig.)
Cyrtohymena quadrinucleata (Dragesco and Njiné, 1971) Foissner, 1989 (syn.)
Rigidohymena quadrinucleata (Dragesco and Njiné, 1971) Berger, 2011 (reclass.)
Rurikoplites alpinus (Kahl, 1932)
Dileptus alpinus Kahl, 1932 (orig.)
Rurikoplites alpinus (Kahl, 1932) Vd'ačný and Rajter, 2015 (reclass.)
Sathrophilus muscorum (Kahl, 1931)
Saprophilus muscorum Kahl, 1931 (orig.)
Sathrophilus muscorum (Kahl, 1931) Corliss, 1960 (reclass.)
Spathidium claviforme Kahl, 1930
Spathidium seppelti Foissner, 1997
Sphaerophrya terricola Foissner, 1986
Sterkiella histriomuscorum Foissner, Blatterer, Berger, and Kohmann, 1991
Oxytricha trifallax Hunter, Cartinhour, Williams and Herrick, 1989 (nomen nudum)
Parasterkiella thompsoni (Foissner, 1996)
Sterkiella thompsoni Foissner, 1996 (orig.)
Parasterkiella thompsoni (Foissner, 1996) Küppers et al., 2011 (reclass.)
Tachysoma pellionellum (Müller, 1773)
Oxytricha pellionella Stein, 1859 (syn.)
Tachysoma agilis Stokes, 1887 (syn.)
Tachysoma pellionellum (Müller, 1773) Borror, 1972 (reclass.)
Tetrahymena rostrata Kahl, 1926
Trochilia minuta (Roux, 1899)
Dysteropsis minuta Roux, 1899 (orig.)
Trochilia minuta (Roux, 1901) (error in year)
Trochilia minuta (Kahl, 1931) (error in authorship)
Trochilia minuta (Roux, 1901) Kahl, 1931 (reclass.)
Uroleptus (Caudiholosticha) antarctica Park, Min and Kim 2018
Uronema nigricans (Müller, 1786)
Cyclidium nigricans Müller, 1786 (orig.)
Cryptochilium nigricans (Müller, 1773) Maupas, 1883 (syn.)
Uronema nigricans (Müller, 1786) Florentin, 1901 (reclass.)
Uronema parduczi Foissner, 1971 (syn.)
Urosomoida antarctica Foissner, 1996
Urosomoida granulifera Foissner, 1996
Urotricha agilis (Stokes, 1886)
Balanitozoon agilis Stokes, 1886 (orig.)
Urotricha agilis (Stokes, 1886) Kahl, 1930 (reclass.)
Vorticella astyliformis Foissner, 1981
Vorticella companula Ehrenberg, 1831
Vorticella aperta Fromental, 1874 (syn.)
Vorticella infusionum Dujardin, 1841
Vorticella microstoma Ehrenberg, 1830
Vorticella striata Dujardin, 1841
SAR: Rhizaria (Cercozoa)
Allantion tachyploon Sandon, 1924
Assulina muscorum Greeff, 1888
Asculina muscora Greeff: Hada, 1967 (misspelling both species and genus)
Assulina seminulum Leidy, 1879 (syn., in part)
Assulina minor Penard, 1890 (syn.)
Assulina seminulum (Ehrenberg, 1848)
Difflugia seminulum Ehrenberg, 1848 (orig.)
Asculina seminulum (Ehrenberg) (misspelling)
Difflugia Assulina seminulum Ehrenberg, 1871 (syn.)
Difflugia semen Ehrenberg, 1871 (syn.)
Euglypha brunnea Leidy, 1874 (syn.)
Euglypha seminulum Ehrenberg, 1845 (syn., error in year)
Euglypha seminulum Leidy, 1878 (syn.)
Assulina seminulum (Ehrenberg, 1848) Leidy, 1879 (reclass.)
Biomyxa vagans Leidy, 1879
Cavernomonas stercoris Vickerman, 2009 in Bass et al., 2009
Cercomonas agilis (Moroff, 1904)
Dimastigamoeba agilis Moroff, 1904 (orig.)
Cercobodo agilis (Moroff, 1904) Lemmermann, 1914 (reclass.)
Cercobodo agilis Martin (error in authorship)
Cercomonas agilis (Moroff, 1904) Mylnikov and Karpov, 2004 (reclass.)
Cercomonas longicauda Dujardin, 1841
Dimorpha longicauda (Dujardin, 1841) Klebs, 1892(syn.)
Cercobodo longicauda (Dujardin, 1841) Lemmerman, 1913 (syn.)
Cercomonas longicauda Stein (error in authorship)
Cercomonas plasmodialis (Mylnikov, 1985)
Cercobodo plasmodialis Mylnikov, 1985 (orig.)
Cercomonas plasmodialis (Mylnikov, 1985) Mylnikov, 1992 (reclass.)
Cercomonas vibrans (Sandon, 1927)
Cercobodo vibrans (Sandon, 1927) (orig.)
Cercomonas vibrans (Sandon, 1927) Mylnikov and Karpov, 2004 (reclass.)
Clathrulina elegans Cienkowski, 1867
Podosphaera haeckeliana Archer, 1869 (syn.)
Elaster greeffi Grimm, 1872 (syn.)
Clathrulina cienkowskyi Mereshkowsky, 1877 (syn.)
Clathrulina cienkowskyi ssp. ovalis von Daday, 1885 (syn.)
Clathrulina stuhlmanni Schaudinn, 1897 (syn.)
Clathrulina cienkowskii Mereshkowsky, 1877: Penard, 1913 (misspelling)
Clathrulina ovalis (von Daday, 1885) Deflandre, 1926 (syn.)
Corythion aerophila (Decloitre, 1850)
Trinema enchelys aerophila Decloitre, 1950 (orig.)
Corythion constricta (Certes, 1889)
Trinema constricta Certes, 1889 (orig.)
Corythion constricta (Certes, 1889) Jung, 1942 (reclass.)
Corythion dubium Taránek, 1881
Arcella constricta Ehrenberg, 1841 (syn., in part)
Arcella disphaera Ehrenberg, 1841 (syn., in part)
Trinema acinus Leidy, 1879 (syn., in part)
Trinema constricta Certes, 1889 (syn.)
Euglypha bryophila Brown, 1911
Euglypha α Vejdovsky, 1882 (syn.)
Euglypha cristata Penard, 1890 (syn., in part)
Euglypha ciliata (Ehrenberg, 1848)
Difflugia ciliata Ehrenberg, 1848 (orig.)
Euglypha setigera Perty, 1852 (syn., in part)
Difflugia pilosa Ehrenberg, 1871 (syn.)
Difflugia ciliata Ehrenberg, 1871 (syn., error in year)
Euglypha ciliata (Ehrenberg, 1848) Leidy, 1878 (reclass.)
Euglypha ciliata f. glabra Wailes, 1915
Euglypha compressa Carter, 1864
Euglypha ampullacea Hertwig and Lesser, 1874 (syn.)
Euglypha ciliata Leidy, 1879 (syn., in part)
Euglypha α Vejdovsky, 1882 (syn., in part)
Euglypha compressa f. glabra Cash, 1915
Euglypha cristata Leidy, 1874
Euglypha denticulata Brown, 1912
Euglypha laevis (Ehrenberg, 1845)
Difflugia laevis Ehrenberg, 1845 (orig.)
Euglypha laevis (Ehrenberg, 1845) Perty, 1849 (reclass.)
Euglypha alveolata Leidy, 1879 (syn., in part)
Euglypha γ Vejdovsky, 1882 (syn.)
Euglypha rotunda Wailes and Penard, 1911
Euglypha rotunda Wailes (error in authorship)
Euglypha strigosa (Ehrenberg, 1871)
Difflugia strigosa Ehrenberg, 1871 (orig.)
Difflugia Setigerella strigosa Ehrenberg, 1871 (syn.)
Euglypha strigosa (Ehrenberg, 1871) Leidy, 1878 (reclass.)
Euglypha ciliata var. strigosa Leidy, 1879 (syn., in part)
Euglypha heterospina Penard, 1890 (syn.)
Euglypha strigosa f. glabra Wailes, 1898
Euglypha tuberculata Dujardin, 1841
Difflugia areolata Ehrenberg, 1841 (syn.)
Euglypha alveolata Dujardin, 1841 (syn., in part)
Euglypha tuberculosa Dujardin, 1841 (syn.)
Difflugia alveolata Pritchard, 1861 (syn.)
Euglypha pusilla Entz, 1877 (syn.)
Euglypha β Vejdovsky, 1882 (syn.)
Lecythium hyalinum Hertwig and Lesser, 1874
Paracercomonas crassicauda (Dujardin, 1836)
Cercomonas crassicauda Dujardin, 1836 (orig.)
Paracercomonas crassicauda (Dujardin, 1836) Bass and Cavalier-Smith, 2009 in Bass et al., 2009 (reclass.)
Cercomonas crassicauda Alexeieff (error in authorship)
Cercomonas crasicauda Lemmermann (error in authorship)
Pseudodifflugia gracilis Schlumberger, 1845
Pleurophrys sphaerica Claparède and Lachmann, 1858 (syn.)
Pleurophrys angulata Mereschkovsky, 1879 (syn.)
Pseudodifflugia gracilis var. terricola Bonnet and Thomas, 1960
Sainouron mikroteron Sandon, 1924
Spongomonas uvella Stein, 1878
Trachelocorythion pulchellum (Penard, 1890)
Corythion pulchellum Penard, 1890 (orig.)
Chorythion pulchellum Awerintzew, 1907 (syn.)
Trachelocorythion pulchellum (Penard, 1890) Bonnet, 1979 (reclass.)
Trinema contraria Decloitre, 1961
Trinema complanatum Penard, 1890
Trinema acinus Leidy, 1879 (syn., in part)
Trinema enchelys (Ehrenberg, 1838)
Difflugia enchelys Ehrenberg, 1838 (orig., in part)
Trinema acinus Dujardin, 1841 (syn.)
Arcella enchelys Ehrenberg, 1844 (syn.)
Arcela enchelys Ehrenberg, 1854 (misspelling, error in year)
Euglypha pleurostoma Carter, 1857 (syn.)
Euglypha enchelys Wallich, 1864 (syn.)
Trinema (Difflugia) enchelli Crevier, 1870 (syn.)
Trinema enchelys (Ehrenberg, 1838) Leidy, 1878 (reclass.)
Trinema enchelys (Ehrenberg, 1938) Leidy, 1878 (error in year)
Trinema enchelys (Ehrenberg, 1838) Leidy, 1879 (error in year)
Trinema enchelys Leidy (error in authorship)
Trinema lineare Penard, 1890
Difflugia enchelys Ehrenberg, 1838 (orig., in part)
Arcella hyalina Ehrenberg, 1841 (syn.)
Arcella enchelys Ehrenberg, 1847 (syn.)
Arcella enchelys Ehrenberg, 1854 (error in year)
Arcella enchelys alpha Ehrenberg, 1854 (syn.)
Trinema acinus Leidy, 1879 (syn., in part)
Trinema enchelys f. beta Awerintzew, 1906 (syn.)
Trinema lineare var. truncatum Chardez, 1964
Valkanovia elegans Schönborn, 1964
Excavata
Astasia inflata Dujardin, 1841
Bodo angustus (Dujardin, 1841)
Bodo angusta Dujardin, 1841 (orig.)
Bodo angustus (Dujardin, 1841) Bütschli 1883
Bodo globosus Stein, 1878
Bodo globose Stein, 1878 (orig.)
Bodo saltans Ehrenberg, 1831
Bodo jaculans Perty (syn.)
Naegleria gruberi (Schardinger, 1899)
Amoeba gruberi Schardinger, 1899 (orig.)
Naegleria gruberi (Schardinger, 1899) Wilson, 1916 (reclass.)
Naegleria neopolaris De Jonckheere, 2006
Parabodo caudatus (Dujardin, 1841)
Amphimonas caudatus Dujardin, 1841 (orig.)
Bodo caudatus (Dujardin, 1841) Stein, 1878 (reclass.)
Bodo alexeieffi Lemm. (syn., no year)
Bodo asiaticus Castellanii and Chalmers (syn., no year)
Bodo compressus Lemm. (syn., no year)
Bodo cruzi Hartm. and Chagas (syn., no year)
Bodo josephi Belar (syn., no year)
Bodo mutabilis Klebs 1892 (syn.)
Bodo obovatus Lemm. (syn., no year)
Bodo putrinus (Stokes) Lemm. (syn., no year)
Heteronema minima Form. (syn., no year)
Bodo caudatus Hollande (error in authorship)
Bodo cudatus (misspelling)
Parabodo caudatus (Dujardin 1841) Vickerman in Moreira, López-García and Vickerman 2004
Paratrimastix pyriformis (Klebs, 1893)
Tetramitus pyriformis Klebs, 1893 (orig.)
Coelotrichomastix convexa Hollande, 1939 (syn.)
Trimastix convexa (Hollande, 1939) Grassé, 1952 (syn.)
Percolomonas pyriformis (Klebs, 1893) Larsen and Patterson, 1990 (syn.)
Trimastix pyriformis (Klebs, 1893) Bernard et al. 2000 (reclass.)
Paratrimastix pyriformis (Klebs, 1893) Zhang, Táborsky, Silberman, Pánek, Čepička and Simpson, 2015 (reclass.)
Paravahlkampfia ustiana (Page, 1974)
Vahlkampfia ustiana Page, 1974 (orig.)
Paravahlkampfia ustiana (Page, 1974) Brown and De Jonckheere, 1999 (reclass.)
Peranemopsis trichophora (Ehrenberg, 1832)
Trachelius trichophorus Ehrenberg, 1832 (orig.)
Peranema trichophora Ehrenberg, 1838 (error in year)
Peranema trichophora (Ehrenberg, 1832) Dujardin, 1841 (reclass.)
Peranema trichophorum (Ehrenberg 1832) Stein, 1859 (syn.)
Paranema trichophorum (Ehrenberg 1832) Stein, 1878 (syn.)
Peranemopsis trichophora (Ehrenberg 1832) Péterfi, 1986 (reclass.)
Peranemopsis trichophora (Ehrenberg 1832) Péterfi, 1988 (error in year)
Petalomonas angusta (Klebs, 1893)
Petalomonas mediocanellata var. angusta Klebs, 1893 (orig.)
Petalomonas angusta (Klebs, 1893) Lemmermann, 1910 (reclass.)
Petalomonas augusta (Klebs, 1893) Lemmermann, 1910 (misspelling)
Petalomonas mediocanellata Stein, 1878
Tetramitus rostratus Perty, 1852
Vahlkampfia limax (Vahlkampf, 1905)
Amoeba limax Vahlkampf, 1905 (orig.)
Amoeba proteus Dujardin, 1841 (syn., in part)
Vahlkampfia limax (Vahlkampf, 1905) Chatton, 1912 (reclass.)
Amoebozoa
Acanthamoeba castellanii (Douglas, 1930)
Acanthamoeba castellanii (Douglas, 1930) Volkonsky, 1931 (reclass.)
Acanthamoeba castellani (Douglas, 1930) (misspelling)
Acanthamoeba polyphaga (Puschkarew, 1913)
Amoeba discoides Schaeffer, 1916
Amoeba discoides Greeff (error in authorship)
Amoeba limicola Rhumbler, 1894
Amoeba limicola Rhumbler, 1894 (orig.)
Pelomyxa limicola (Rhumbler, 1894) Bovee 1951 (syn.)
Pelomyxa limnicola (Rhumbler, 1894) (misspelling)
Arcella arenaria Greeff, 1866
Arcella aureola Maggi, 1883 (syn.)
Arcella microstoma Penard, 1890 (syn.)
Arcella arenaria var. compressa Chardez, 1965
Arcella arenaria var. sphagnicola Deflandre, 1928
Arcella vulgaris Ehrenberg, 1830
Arcella vulgaris Ehr. (abbrev. author)
Astramoeba radiosa (Ehrenberg, 1830)
Amoeba radiosa Ehrenberg, 1830 (orig.)
Calomyxa metallica (Berk., 1837)
Physarum metallicum Berk., 1837 (orig.)
Cornuvia metallica (Berk.) Rostafinsky, 1876 (reclass.)
Oligonema aeneum P. Karst., 1879 (syn.)
Perichaena krupii Racib., 1889 (syn.)
Perichaena plasmodiocarpa Blytt, Förh, 1892 (syn.)
Margarita metallica (Berk.) Lister, 1894 (reclass.)
Margarita pictoviana Moore, 1902 (syn.)
Margarita metallica var. intermedia Meylan, 1910 (syn.)
Margarita metallica var. plasmodiocarpa (Blytt) R.E. Fr., 1912 (reclass.)
Cornuvia metallica var. intermedia (Meylan, 1910) Sacc. and Trotter, 1913 (reclass.)
Calomyxa metallica (Berk., 1837) Nieuwl., 1916 (reclass.)
Calomyxa metallica var. megaspora Yamamoto and Nannenga-Bremekamp 1990, in Nannenga-Bremekamp and Yamamoto, 1990 (syn.)
Centropyxis aculeata (Ehrenberg, 1832)
Arcella aculeata Ehrenberg, 1832 (orig.)
Difflugia aculeata Perty, 1852 (syn.)
Echinopyxis aculeata Claparède et Lachmann, 1859 (syn.)
Centropyxis aculeata (Ehrenberg, 1832) Stein, 1859 (reclass.)
Centropyxis aculeata Stein, 1857 (error in authorship, error in year)
Centropyxis aerophila Deflandre, 1929
Difflugia constricta Ehrenberg, 1838 (syn., in part)
Arcella arctiscon Ehrenberg, 1854 (syn.)
Centropyxis aerophila var. sphagnicola Deflandre, 1929
Centropyxis cassis (Wallich, 1864)
Centropyxis cassis (Wallich, 1864) Deflandre, 1929 (reclass.)
Centropyxis constricta (Ehrenberg, 1838)
Difflugia constricta Ehrenberg, 1838 (orig.)
Arcella consricta Ehrenberg, 1841 (syn.)
Centropyxis constricta (Ehrenberg, 1838) Deflandre, 1929 (reclass.)
Centropyxis elongata (Penard, 1890)
Difflugia constricta var. elongata Penard, 1890 (orig.)
Centropyxis elongata (Penard, 1890) Thomas, 1959 (reclass.)
Centropyxis minuta Deflandre, 1929
Difflugia constricta Leidy, 1879 (syn.)
Difflugia constricta Penard, 1902 (syn.)
Centropyxis sylvatica (Deflandre, 1929)
Centropyxis aerophila var. sylvatica Deflandre, 1929 (orig.)
Centropyxis sylvatica (Deflandre, 1929) Bonnet and Thomas, 1955 (reclass.)
Cryptodifflugia compressa Penard, 1902
Cryptodifflugia sacculus (Penard, 1902)
Difflugiella sacculus Penard, 1902 (orig.)
Cryptodifflugia sacculus (Penard, 1902) Deflandre, 1953 (reclass.)
Cryptodifflugia oviformis Penard, 1890
Difflugiella oviformis Bonnet and Thomas, 1955 (syn.)
Cryptodifflugia operculata Page, 1966 (syn.)
Cyclopyxis eurystoma Deflandre, 1929
Centropyxis (Cyclopyxis) eurystoma Deflandre, 1929
Diderma antarcticolum Horak, 1966
Diderma crustaceum (Peck, 1873)
Diderma crustaceum Peck, 1873 (orig.)
Chondrioderma crustaceum (Peck, 1873) Peck., 1878 ['1879'] (syn.)
Chondrioderma crustaceum (Peck, 1873) Berl., 1888 [Comb. Superfl., previously proposed by Peck, 1878]
Diderma niveum (Rostafinsky, 1874)
Chondrioderma niveum Rostafinsky, 1874 (orig.)
Chondrioderma physaroides Rostafinsky, 1874 (syn.)
Diderma albescens Phillips, 1877 (syn.)
Chondrioderma albescens (Phillips, 1877) Massee, 1892 (reclass.)
Diderma niveum (Rostafinsky, 1874) Sheldon 1895 (reclass.)
Diderma niveum (Rostafinsky, 1874) Kuntze, Revis., 1898 (reclass.) [Comb. Superfl., previously proposed by Sheldon, 1895]
Diderma niveum (Rostafinsky, 1874) Macbride, 1899 [Comb. Superfl., previously proposed by Sheldon, 1895] (reclass.)
Diderma niveum f. pulverulentum Meylan, 1922 (syn.)
Diderma niveum f. endoleucum Meylan, 1924 (syn.)
Diderma niveum var. ferrugineum Meylan, 1924 (syn.)
Diderma niveum var. ferruginea Meylan, 1924 (misspelling)
Diderma subcaeruleum Kowalski, 1968 (syn.)
Diderma cristatosporum Sánchez, Moreno and Illana, 2002 (syn.)
Diderma niveum var. cristatosporum (Sánchez, Moreno and Illana, 2002) Singer, Moreno, Illana and Sánchez, 2003 in Moreno, Singer, Illana and Sánchez, 2003 (reclass.)
Difflugia ampullula Playfair, 1918
Difflugia bryophila (Penard, 1902)
Difflugia piriformis var. bryophila Penard, 1902 (orig.)
Difflugia oblonga var. longicollis Gassowsky, 1936 (syn.)
Difflugia bryophila (Penard, 1902) Jung, 1942 (reclass.)
Difflugia longicollis (Gassowsky, 1936) Ogden and Hedley, 1980 (syn.)
Difflugia gassowskii Ogden, 1983 (syn.)
Difflugia globulosa Dujardin, 1837
Difflugia proteiformis globularis Wallich, 1864 (syn.)
Difflugia globularis (Wallich, 1864) Leidy, 1877 (syn.)
Difflugia chardezi Godeanu, 1972 (syn.)
Difflugia lanceolata Penard, 1890
Difflugia lucida Penard, 1890
Difflugia manicata var. langhovdensis Sudzuki, 1964
Difflugia mica Frenzel, 1892
Difflugia pristis Penard, 1902
Difflugia pulex Penard, 1890
Difflugia minuta var. minor Godeanu, 1972 (syn.)
Difflugia ovalisina Beyens et Chardez, 1994 (syn.)
Certesella certesi (Penard, 1911)
Nebela certesi Penard, 1911 (orig.)
Certesella certesi (Penard, 1911) Loeblich and Tappan, 1961 (reclass.)
Cryptodifflugia apiculata (Cash, 1904)
Difflugiella apiculata Cash, 1904 (orig.)
Cryptodifflugia apiculata (Cash, 1904) Page, 1966 (reclass.)
Diplochlamys gruberi Penard, 1909
Diplochlamys timida Penard, 1909
Diplochlamys vestita Penard, 1909
Echinamoeba silvestris Page, 1975
Pyxidicula operculata (Agardh, 1827)
Frustulia operculata Agardh, 1827 (orig.)
Cymbella operculata (Agardh, 1827) Agardh, 1830 (reclass.)
Galionella operculata (Agardh, 1827) Ehrenberg, 1834 (reclass.)
Pyxidicula operculata (Agardh, 1827) Ehrenberg, 1838 (reclass.)
Pyxidicula operculata Ehrenberg (error in authorship)
Heleopera petricola Leidy, 1879
Heleopera sylvatica Penard, 1890
Hyalosphenia elegans (Leidy, 1874)
Difflugia elegans Leidy, 1874 (orig.)
Hyalosphenia elegans (Leidy, 1874) Leidy, 1879 (reclass.)
Hyalosphenia turfacea Taránek, 1881 (syn.)
Hyalosphenia elegans Leidy var. major Decloitre, 1964
Hyalosphenia minuta Cash, 1891
Hyalosphenia subflava Cash, 1909
Hyalosphenia subflava Cash and Hopkinson (error in authorship)
Hyalosphenia subflava Hopkinson (error in authorship)
Leptoderma megaspora Arambarri and Spinedi, 1989
Mayorella clavabellans Bovee, 1970
Mayorella vespertilio (Penard, 1902)
Amoeba vespertilio Penard, 1902 (orig.)
Mayorella vespertilio (Penard, 1902) LaPage, 1922 (reclass.)
Microchlamys patella (Claparède and Lachmann, 1859)
Pseudochlamys patella Claparède and Lachmann, 1859 (orig.)
Microchlamys patella (Claparède and Lachmann, 1859) Cockerell, 1911 (reclass.)
Microcorycia tessellata (Penard, 1917)
Corycia tessellata Penard, 1917 (orig.)
Microcorycia tessellata (Penard, 1917) Chardez, 1965 (reclass.)
Microcorycia bryophila Decloitre, 1974 (syn.)
Microcorycia flava (Greeff, 1866)
Amphizonella flava Greeff, 1866 (orig.)
Corycia flava (Greeff, 1866) Penard, 1902 (syn.)
Microcorycia flava (Greeff, 1866) Cockerell, 1911 (reclass.)
Microcorycia radiata (Brown, 1912)
Corycia radiata Brown, 1912 (orig.)
Microcorycia radiata (Brown, 1912) Hopkinson, 1919 (reclass.)
Nebela bohemica Taránek 1882 var. adelia Decloitre, 1964
Nebela collaris (Ehrenberg, 1848)
Difflugia collaris Ehrenberg, 1848 (orig.)
Diffluga cancellata Ehrenberg, 1848 (syn.)
Difflugia reticulata Ehrenberg, 1848 (syn.)
Difflugia carpio Ehrenberg, 1854 (syn.)
Difflugia laxa Ehrenberg, 1871 (syn.)
Difflugia cellulifera Ehrenberg, 1874 (syn.)
Nebela numata Leidy 1874 (syn.)
Nebela collaris (Ehrenberg 1848) Leidy, 1879 (reclass.)
Nebela bohemica Taranek, 1882 (syn.)
Nebela sphagnophila (Steinecke) Van Oye, 1933 (syn., no year)
Nebela tincta var. major Deflandre 1936 (syn.)
Nebela tincta f. stenostoma Jung 1936 (syn.)
Nebela tincta (Leidy, 1879)
Hyalosphenia tincta Leidy, 1879 (orig.)
Euglypha bursella Veidowsky (syn., no year)
Nebela bursella Vejdovsky, 1882 (syn.)
Nebela minor Penard, 1902 (syn.)
Nebela tincta (Leidy, 1879) Awerintzew, 1906 (reclass.)
Nebela parvula Cash, 1909 (syn.)
Oligonema dancoii Arambarri and Spinedi, 1989
Padaungiella lageniformis (Penard, 1890)
Nebela lageniformis Penard, 1890 (orig.)
Nebela lageniformes Penard, 1890 (misspelling)
Padaungiella lageniformis (Penard, 1890) Lara and Todorov 2012 (reclass.)
Padaungiella wailesi (Deflandre, 1936)
Nebela wailesi Deflandre, 1936 (orig.)
Padaungiella wailesi (Deflandre, 1936) Lara and Todorov, 2012 (reclass.)
Parmulina cyathus Penard, 1902
Phalansterium solitarium Sandon, 1924
Phryganella acropodia (Hertwig and Lesser, 1874)
Difflugia acropodia Hertwig and Lesser, 1874 (orig.)
Phryganella acropodia (Hertwig and Lesser, 1874) Hopkinson, 1909 (reclass.)
Phryganella acropodia Penard (error in authorship)
Phryganella hemisphaerica (Penard, 1890)
Pseudodifflugia hemisphaerica Penard, 1890 (orig.)
Difflugia globulosa Leidy, 1879 (syn., in part)
Phryganella hemisphaerica (Penard, 1890) Penard, 1902 (reclass.)
Plagiopyxis callida var. grandis Thomas, 1958
Plagiopyxis declivis Thomas, 1955
Plagiopyxis labiata Penard, 1910
Centropyxia labiata Bartoš, 1947
Stenamoeba stenopodia (Page, 1969)
Platyamoeba stenopodia Page, 1969 (orig.)
Stenamoeba stenopodia (Page, 1969) Smirnov, Nassonova, Chao and Cavalier-Smith, 2007 (reclass.)
Saccamoeba limax (Dujardin, 1841)
Amoeba limax Dujardin, 1841 (orig.)
Saccamoeba limax (Penard, 1902) (error in authorship)
Saccamoeba stagnicola Page, 1974
Schoenbornia viscicula Schönborn, 1964
Thecamoeba striata (Penard, 1890)
Thecamoeba striata (Penard, 1890) Schaeffer, 1926 (reclass.)
Thecamoeba terricola (Greeff, 1866)
Amoeba terricola Greeff, 1866 (orig.)
Thecamoeba terricola (Greeff, 1866) Lepşi, 1960 (reclass.)
Thecamoeba verrucosa (Ehrenberg, 1838)
Thecamoeba verrucosa (Ehrenberg, 1838) Schaeffer, 1926 (reclass.)
Trichamoeba osseosaccus Schaeffer, 1926
Trichamoeba osseocuccus Schaeffer (misspelling)
Trichia antarctica Arambarri and Spinedi, 1989
Trichia varia (Pers., 1792)
Stemonitis varia Pers., 1792 (orig.)
Trichia varia (Pers., 1792) Pers., 1794 (reclass.)
Trichia olivacea Pers., 1796 (syn.)
Trichia cordata Pers., 1800 (syn.)
Trichia nigripes var. cordata (Pers., 1800) Pers., 1801 (syn.)
Trichia nigripes var. cordata (Pers., 1800) Alb. and Schwein., 1805 (syn.)
Trichia cylindrica Pers., 1800 (syn.)
Trichia nigripes var. cylindrica (Pers., 1800) Pers., 1801 (syn.)
Trichia nigripes Pers., 1801 (syn.)
Trichia varia var. diluta Pers., 1801 (syn.)
Trichia varia var. subrufescens Pers., 1801 (syn.)
Trichia varia var. nigripes (Pers., 1792) Rostafinsky, 1875 (syn.)
Lycoperdon luridum Hedw., 1802 (syn.)
Trichia varia var. sessilis Rostafinsky, 1875 (syn.)
Trichia aculeata Celak., 1893 (syn.)
Trichia varia var. aurata Meylan, 1908 (syn.)
Trichia varia var. irregularis Meylan, 1908 (syn.)
Trichia varia var. olivacea Brândza, 1928 (syn.)
Trichia synspora Kowalski and McNichols in Kowalski, 1974 (syn.)
Trigonopyxis arcula (Leidy, 1879)
Difflugia arcula Leidy, 1879 (orig.)
Trigonopyxis arcula (Leidy, 1879) Penard, 1912 (reclass.)
Cystidina arcula (Leidy, 1879) Volz, 1929 (syn.)
Vannella contorta (Moran and Anderson 2007)
Platyamoeba contorta Moran and Anderson 2007 (orig.)
Vannella contorta (Moran and Anderson 2007) Smirnov, Nassonova, Chao and Cavalier-Smith, 2007 (reclass.)
Vannella mira (Schaeffer, 1926)
Flabellula mira Schaeffer, 1926 (orig.)
Vannella mira (Schaeffer, 1926) Bovee, 1965 (reclass.)
Vannella simplex (Wohlfarth-Bottermann, 1960)
Hyalodiscus simplex Wohlfarth-Bottermann, 1960 (orig.)
Vannella simplex (Wohlfarth-Bottermann, 1960) Bovee, 1965 (reclass.)
Vermamoeba vermiformis (Page, 1967)
Hartmannella vermiformis Page, 1967 (orig.)
Hartmannella vermiformes Page, 1967 (misspelling)
Hartmanella vermiformes Page, 1967 (misspelling)
Vermamoeba vermiformis (Page, 1967) Smirnov and Cavalier-Smith, 2011 (reclass.)
Incertae sedis
Polypseudopodius bacterioides Puschkarew, 1913
Incomplete records
Cochliopodium tentaculatus
Stylonychia mytilus complex
Bodo terricolus Martin
Heteromita globosa (Stein, 1878)
Heteromita globosa (Stein, 1878) Kent, 1881 (reclass.)
Discussion
Taxonomic changes and discrepancies
A number of taxonomic designations for the taxa recovered have changed since the original record was published or were ambiguous. Dillon et al. (1968) reported Pelomyxa (or Amoeba) limnicola (a probable misspelling), though a search of the literature failed to find this species. Bovee (Reference Bovee1951) proposed to move Amoeba limicola to Pelomyxa limicola, and the latter designation was used in several ecological papers in subsequent decades (Bovee Reference Bovee1965, Dillon et al. Reference Dillon, Walsh and Bierle1968); however, A. limicola is still considered accepted in online databases (www.itis.gov). The numerous species added to the genus Pelomyxa in the nineteenth and twentieth centuries were later reduced to a single valid species (Griffin Reference Griffin1988, Whatley & Chapman Reference Whatley, Chapman, Corliss, Melkonian and Chapman1990), Pelomyxa palustris, although no mention of Pelomyxa limicola was made in this move (Goodkov et al. Reference Goodkov, Chistyakova, Seravin and Frolov2004). Thus, we retain A. limicola and its associated synonyms in this checklist. Due to the difficulty in distinguishing between some Stylonychia species (Haentzsch et al. Reference Haentzsch, Schmidt, Bernhard, Ammermann, Berendonk and Schlegel2006), Mieczan and Tarkowska-Kukuryk (Reference Mieczan and Tarkowska-Kukuryk2014) reported a Stylonychia species as Stylonychia mytilus complex, which includes Stylonychia lemnae, Stylonychia mytilus, Stylonychia ammermanni and Stylonychia harbinensis. We include this record due to its ecological significance, even though it is taxonomically incomplete. We placed Euglypha bursella Veidowsky under Nebela bursella Vejdovsky, 1882, as the authors are similar and no occurrence of E. bursella was found in database searches beyond the ecological paper we reviewed (Richters Reference Richters1908). No further taxonomic information could be found than what was given for Cochliopodium tentaculatus from Sudzuki (Reference Sudzuki1979) and Bodo terricolus Martin from Smith (Reference Smith1972), and these are included as incomplete taxonomic records. B. terricolus may be an erroneous entry, as it was not included by the same author in a later, more comprehensive publication (Smith Reference Smith1978). Centropyxis aerophila var. sphagnicola from Golemansky and Todorov (Reference Golemansky and Todorov2004) is now treated as part of the C. aerophila complex (Foissner & Korganova Reference Foissner and Korganova2000), but as this would have resulted in a loss of potentially valuable ecological information, we retain its original nomenclature in this checklist. Howe et al. (Reference Howe, Bass, Vickerman, Chao and Cavalier-Smith2009) split Heteromita globosa, a very common soil flagellate, into 5 new genera and 29 new species, rendering the original name invalid. However, as the records of H. globosa from the Antarctic literature predated this change and provided no taxonomic diagnoses, pictures or sequence information for their identifications of their organisms, we retain H. globosa in our checklist in order to avoid confusion (Sandon & Cutler Reference Sandon and Cutler1924, Lawley et al. Reference Lawley, Ripley, Bridge and Convey2004, Bamforth et al. Reference Bamforth, Wall and Virginia2005). Microcorycia bryophila from Sudzuki (Reference Sudzuki1979), synonymized with Microcorycia tessellata in Badewitz (Reference Badewitz2004), was considered by the latter author as a suspicious record because the species was listed with a ‘?' in the paper's checklist. We retain it here because there are in fact two records of it in that paper (Sudzuki Reference Sudzuki1979), one of which was not considered ambiguous by Sudzuki. Mayorella clavabellans and Mayorella vespertilio may now be considered invalid (Smirnov & Brown Reference Smirnov and Brown2004, Glotova et al. Reference Glotova, Bondarenko and Smirnov2018); however, we were unable to find confirmation of this, so we retained these records in this list. Dumack et al. (Reference Dumack, Mausbach, Hegmann and Bonkowski2017) split the genus Lecythium into two, but retained Lecythium hyalinum, reported in Smith (Reference Smith1972), as a valid species. As no taxonomic information was reported in the latter paper, we cannot determine whether L. hyalinum sensu Smith, Reference Smith1972 belongs to the new genus, Fisculla, and thus we retain it as it was originally reported. Foissner et al. (Reference Foissner, Agatha and Berger2002) retroactively reassigned the Paruroleptus notabilis Foissner, 1982 and Nassula picta Greeff, 1888 reported in Foissner (Reference Foissner1996) as Uroleptus paranotabilis (now Acuholosticha paranotabilis) and Nassula tuberculate, respectively, on the grounds that the original isolates had been misidentified. Finally, Hada (Reference Hada1966) reported a total of 37 protists, yet due to ambiguity over the source of the moss used for analysis (freshwater or terrestrial), we did not include these species in our checklist. Sudzuki (Reference Sudzuki1979) attributes some of the species from Hada's Reference Hada1966 study to ‘Antarctic moss’, potentially implying their terrestrial origin; however, it is still not clear from this latter study whether these species were in fact terrestrial or aquatic in origin.
Estimate of taxonomic diversity
The numbers presented here reflect the most comprehensive taxonomic summary of HPs in continental and peninsular Antarctica to date. Climate change has probably already impacted this diversity, especially as recorded in the earliest studies from the peninsular zone (Richters Reference Richters1907, Reference Richters1908, Penard Reference Penard1911, Sandon & Cutler Reference Sandon and Cutler1924, Smith Reference Smith1972, Reference Smith1974, Reference Smith1978, Sudzuki Reference Sudzuki1979). Early surveys probably represent different communities from those that exist at the same sites today (Royles et al. Reference Royles, Amesbury, Roland, Jones, Convey and Griffiths2016) due to invasions (Hughes et al. Reference Hughes, Pertierra, Molina-Montenegro and Convey2015) or warming (Nielsen & Wall Reference Nielsen and Wall2013).
How many species of terrestrial protists, if any, in Antarctica remain to be discovered is difficult to estimate. Foissner (Reference Foissner1996) estimated an order of magnitude difference between soil ciliate diversity in the Antarctic and in alpine and temperate zones. Chao et al. (Reference Chao, Li, Agatha and Foissner2006) reported 644 described and 320 undescribed soil ciliate species from five continents (not including Antarctica or North America), with no less than 400 and no more than 1000 species from any single continent. Additionally, they estimated global soil ciliate diversity at a minimum of 1900 species. Our review of the literature found 208 terrestrial ciliate taxa (95 identified to species and 113 additional records), which suggests that a significant proportion of terrestrial Antarctic ciliate species may have been recovered, although an unknown degree of overlap between described and undescribed species confounds this conclusion. Specific estimates for the diversity of other HP groups in soils are scarce, but Adl et al. (Reference Adl, Leander, Simpson, Archibald, Anderson and Bass2007) predicted total richness by group (not only from soils) at c. 17 000 Amoebozoa, 5000 Cercozoa, 30 000 Ciliophora and 3000 Excavata species. The relative proportion of protist groups in these global estimates is mirrored by that of our list of Antarctic protists - ciliates (55% of the total of these groups globally vs 41% in Antarctica), Amoebozoa (31% vs 36%), Cercozoa (9% vs 17%) and Excavata (5% vs 6%). However, this pattern might only reflect the past sampling bias towards ciliates and testate amoeba (an unofficial term that includes members of the Amoebozoa and Cercozoa) and misrepresents the potential diversity of underexplored flagellate groups (e.g. other Cercozoa). Additionally, of the 180 total genera found, 42 were recorded without being identified to species, indicating that there may be at least as many additional species not included in this checklist. Additional ciliate genera account for the majority of these genera (28), but Amoebozoa (7), Cercozoa (3), Excavata (2), an opisthokont and a stramenopile are also represented. Moreover, of the 147 remaining genera, 48 were reported without an associated species identification at least once in addition to being reported elsewhere to species. Therefore, this current list greatly underestimates the total diversity of terrestrial Antarctic protists, highlighting the fact that establishing an appropriate baseline for conservation management requires additional effort.
Endemicity of Antarctic heterotrophic protists
There seems to be a trend among early studies to declare a complete lack of endemicity among Antarctic fauna after finding that most communities were similar to those found elsewhere (Sandon & Cutler Reference Sandon and Cutler1924, Janetschek Reference Janetschek1963, Sudzuki Reference Sudzuki1964, Todorov & Golemansky Reference Todorov and Golemansky1996). In fact, the majority of taxa found by morphological studies have been described as non-endemic (Todorov & Golemansky Reference Todorov and Golemansky1996, Petz Reference Petz1997, Petz & Foissner Reference Petz and Foissner1997) and include such widespread species as Colpoda cucullus, Colpoda inflata, Colpoda steinii, Centropyxis aerophila, Assulina muscorum, Euglypha rotunda, Euglypha laevis and Heteromita globosa. Possible explanations for this pattern could be: 1) that culturing techniques are biased towards generalist, r-selected taxa that are indeed more cosmopolitan, 2) that examination of samples involved accidental inoculation with local species (as many of these studies were undertaken at their authors' home institutions), or 3) that the observations reflected reality. The latter undermines the assumption that Antarctic protists are specially adapted to uniquely harsh environmental conditions. Conversely, mounting evidence suggests that many Antarctic microbial species are not recent transplants, but are instead native fauna that arrived long before the most recent glacial maxima (Chown & Convey Reference Chown and Convey2007, Vyverman et al. Reference Vyverman, Verleyen, Wilmotte, Hodgson, Willems and Peeters2010) or are demonstrably distinct from their non-Antarctic relatives (Boenigk et al. Reference Boenigk, Pfandl, Garstecki, Harms, Novarino and Chatzinotas2006). Moreover, cryptic species are common in protists (Adl et al. Reference Adl, Leander, Simpson, Archibald, Anderson and Bass2007, Venter et al. Reference Venter, Nitsche and Arndt2018), and distinguishing species in some groups (i.e. naked amoeba (Amoebozoa) and flagellates (e.g. Cercozoa, Amoebozoa and Excavata)) is notoriously difficult using morphological analysis alone (Smirnov & Brown Reference Smirnov and Brown2004, Venter et al. Reference Venter, Nitsche and Arndt2018).
Thus far, sampling appears to be skewed towards areas that are more likely to experience invasion and to host cosmopolitan taxa due to their higher latitudes and milder climates, such as peninsular and coastal Antarctic sites. Additional sampling of more extreme intra-continental sites (e.g. Ellsworth Land and the Ellsworth, Transantarctic and Prince Charles Mountains) could yield a greater number of uniquely Antarctic species. There have been species found that appear to be restricted to the Antarctic, including three of the reported slime moulds: Leptoderma megaspora, Oligonema dancoii and Trichia antarctica (Stephenson et al. Reference Stephenson, Laursen and Seppelt2007). Urosomoida antarctica possesses numerous unique characteristics (Foissner Reference Foissner1996), while Pseudonotohymena antarctica, Spathidium seppelti and Urosomoida granulifera have yet to be found outside Antarctica (Petz et al. Reference Petz, Valbonesi, Schiftner, Quesada and Cynan Ellis-Evans2007, Park et al. Reference Park, Jung, Min and Kim2017). Tyml et al. (Reference Tyml, Skulinova, Kavan, Ditrich, Kostka and Dykova2016) reported two strains of Naegleria neopolaris that matched Arctic 18S sequences exactly (from Greenland and Svalbard), a taxon apparently exclusive to the poles. Moreover, certain populations of Antarctic species otherwise indistinguishable from their more temperate counterparts exhibit different growth preferences (Bamforth et al. Reference Bamforth, Wall and Virginia2005) and body sizes (Roland et al. Reference Roland, Amesbury, Wilkinson, Charman, Convey and Hodgson2017). Whether these differences are indicative of cryptic species or are only physiological responses to the extremes of the environment remains unexplored. Thus, Antarctica appears to host both cosmopolitan and endemic species of terrestrial HPs, although the relative amounts may differ by geographical region.
Conclusions
The checklist provided here is a first step towards better management of HP biodiversity in the Antarctic, and it provides a baseline for future efforts. Additional research assessing HP diversity in the Antarctic is clearly needed, as many species remain to be incorporated into a comprehensive checklist. Future research should focus especially on regions most sensitive to anthropogenic climate change, on regions that are most likely to host species with unique physiologies (e.g. endemic extremophiles) and on accurate taxonomic identification. Beyond benefitting conservation efforts, an improved understanding of HP biodiversity will also contribute to our understanding of ecosystem-level processes in Antarctica and protistology generally.
Acknowledgements
This research was funded by the National Science Foundation Grant #OPP-1637708 and is a contribution to the McMurdo Dry Valleys Long Term Ecological Research (LTER) programme. We would like to thank our reviewers, David M. Wilkinson and Alex Whittle, whose comments and suggestions were insightful and greatly improved this manuscript.
Author contributions
ART and BJA conceived the project. ART performed the literature search and recovery of taxonomic records and verified the taxonomic history for the Cercozoa, Excavata and Amoebozoa. GSP verified the taxonomic history for the Ciliophora and constructed the checklist, including the taxonomic history for all groups. ART drafted the manuscript. All authors participated in revising the draft manuscript.
Supplemental material
Resources used to construct the checklist will be found at https://doi.org/10.1017/S0954102019000361.



