Introduction
Lakes and ponds are common features in the ice-free areas of the Antarctic continent. Often influenced by marine aerosols these limnetic systems possess a wide range of salinities and ionic compositions. Nutrients are typically supplied by local snow or glacial meltwaters, but highly enriched lakes and ponds can also be found in the vicinity of bird or seal colonies. The planktonic community in these lakes is dominated by microorganisms, including bacteria, phytoplankton and protozoa, with little or no metazoans typically present (Perriss & Laybourn-Parry Reference Perriss and Laybourn-Parry1997, Bell & Laybourn-Parry Reference Bell and Laybourn-Parry1999, Laybourn-Parry et al. Reference Laybourn-Parry, Quayle and Henshaw2002). While most Antarctic lakes are perennially or seasonally ice-covered with liquid water present beneath the ice, shallow lakes and ponds freeze solid to the base during winter. The exclusion of salts and solutes from the expanding ice develops a saline, basal brine solution during freezing (Healy et al. Reference Healy, Webster-Brown, Brown and Lane2006, Hawes et al. Reference Hawes, Safi, Sorrell, Webster-Brown and Arscott2011). During the summer, these shallow lakes and ponds can become partially or completely ice-free, thereby creating a unique and rapidly changing environment. The chemical stratification present within the ice can persist in the summer lakewater in the absence of strong winds to disrupt the salinity gradient. Thus, whether the pond remains chemically stratified or not after thawing will play an important role in structuring the microbial community. Organisms that persist throughout the course of the year in such an environment must be capable of surviving severe seasonal variations in chemical, redox and temperature conditions (Schmidt et al. Reference Schmidt, Moskal, De Moraz, Howard-Williams and Vincent1991, Hawes et al. Reference Hawes, Safi, Sorrell, Webster-Brown and Arscott2011, Webster-Brown et al. Reference Webster-Brown, Hawes, Safi, Sorrell and Wilson2012). Most importantly, outlasting the hostile, frozen entrapment or the hypersaline basal liquids is essential for re-colonizing the lake when melt is initiated. This study describes the biological, physical, and chemical parameters of a shallow Antarctic lake during the annual transition from frozen solid to an open lake system.
Pony Lake is a coastal, eutrophic lake located on Cape Royds (77°33′S, 166°00′E), Ross Island, Antarctica (Fig. 1). The lake is c. 120 m long, 70 m wide, and 1–2 m deep. Except for midsummer, when warmer temperatures melt the ice-cover and strong winds cause thorough mixing of the water column, the lake is frozen solid. Ice melt typically begins in mid-December, however, during years with heavy snowfall (e.g. 2005–06) the ice cover may persist throughout the summer season. The source of water to the lake is the accumulated snowpack, while water is lost by both sublimation of ice and evaporation. As a result of its proximity to the sea Pony Lake is brackish (5.5 ppt). Previous studies have shown that Pony Lake may support very high dissolved organic carbon (DOC) concentrations (up to 100 mg C l-1 in some seasons) (McKnight et al. Reference McKnight, Andrews, Spaulding and Aiken1994, Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004). There are no higher plants in the basin, but algal populations in the lake are abundant. Chlamydomonas intermedia Chodat has been reported as being the dominant chlorophyte algal species (McKnight et al. Reference McKnight, Andrews, Spaulding and Aiken1994), the same as found during Sir Ernest Shackleton's 1908 expedition (West & West Reference West and West1911). Brown et al. (Reference Brown, McKnight, Chin, Roberts and Uhle2004) reported that during the transition from ice-covered to ice-free conditions, the chlorophyte bloom in the lake was displaced by a cryptophyte bloom. Along the western shore of the lake lies an Adélie penguin rookery. However, runoff from the rookery into Pony Lake is believed to be minimal due to highly evaporative conditions and Brown et al. (Reference Brown, McKnight, Chin, Roberts and Uhle2004) indicated that the organic matter in the lake is primarily derived from microbial sources. In a lacustrine system such as Pony Lake that exhibits low levels of proto- and metazooplankton, bacterioplankton will play a significant role in the flow of energy and nutrients.

Fig. 1 Map of Cape Royds showing the location of Pony Lake (modified from www.scar.org, accessed October 2012). The inset image was taken in late December 2004 and shows Pony Lake partially ice-free.
Materials and methods
Sample collection
Water samples were collected during the summer season of 2004/05. Four time points were chosen to measure basic limnological parameters (biological, physical, and chemical) in Pony Lake during the transition from a frozen to ice-free lake. During our studies melting began along the edges of the lake in early December and continued into January. Strong winds allowed for frequent mixing in this shallow lake, which was reflected in a homogeneous conductivity profile of the water column. Thus, one representative depth was chosen after preliminary analyses, and instrument data and water samples were subsequently collected c. 30 cm beneath the water surface. Water samples (c. 5 l) were collected in acid washed, deionized water rinsed (x 6) Nalgene bottles and stored in coolers for transport back to Crary laboratory in McMurdo Station. Samples were processed within four hours of collection.
Physical parameters and chemical analyses
Conductivity, salinity, and temperature were measured with a portable multi-meter (Hydrolab minisonde). Dissolved oxygen (YSI) and pH (Beckman) were recorded for each sample. Samples were prepared for chemical analysis according to the protocols of the McMurdo Dry Valleys Long-term Ecological Research Group (Priscu & Wolf Reference Priscu and Wolf2000). Samples for DOC and total nitrogen (TN) analyses were filtered under low vacuum (< 7 psi) in the dark through 25 mm pre-combusted GF/F filters, acidified with 6 N HCl to pH2 and analysed on a Shimadzu TOC-V and Shimadzu TNM-1 analyser, respectively. Filters from the above analyses were wrapped in aluminum foil and kept frozen until extraction for chlorophyll a (chl a) analysis. Chlorophyll a was extracted in a 1:1 solution (90% acetone and dimethyl sulfoxide) for 12 hours under dark conditions at -20°C. Extracted chl a was analysed on a Turner 10-AU fluorometer. Samples for macronutrients were filtered through 25 mm pre-combusted GF/F filters and stored frozen until analysis (within a month of collection) on a Lachatt autoanalyser. Samples for anion and cation determination were filtered through 0.4 μm 47 mm nucleopore filters. Deionized water was used as a filtration blank and samples were analysed on a Dionex DX-300 ion chromatography system. Dissolved inorganic carbon (DIC) was measured using infrared absorption following acidification and sparging of the sample with high purity N2 gas. Peak areas were integrated and converted to mg l-1 using a standard curve based upon a freshly prepared standard of NaHCO3.
Productivity measurements
Bacterial productivity (BP) was measured via 3H-thymidine incorporation (20 nM final concentration) as described in Takacs & Priscu (Reference Takacs and Priscu1998). Five 3H-thymidine assays and triplicate formalin killed controls (5% final concentration, 30 min prior to 3H-thymidine addition) were incubated at 4°C for 20 hours. Samples were analysed using a liquid scintillation counter (Beckman LS 7200). Thymidine incorporation rates were converted to bacterial production rates using a conversion factor of 2.0 x l018 cells mol-l TdR and a cell-to-carbon conversion factor of 11 fg C cell-l as outlined by Takacs & Priscu (Reference Takacs and Priscu1998).
Primary production (PPR) was measured via 14C-carbonate/bicarbonate incorporation (114.4 μCi ml-1, pH ∼ 9.5; ICN/MP Biomedicals) as described in Lizotte et al. (Reference Lizotte, Sharp and Priscu1996). Quadruple light assays and duplicate dark controls were incubated at 4°C in an illuminated chamber for 24 hours. Following incubation, samples were filtered through pre-combusted 25 mm GF/F filters in the dark. Filters were transferred into 20 ml scintillation vials, acidified with 500 μl 3 M HCL and dried before liquid scintillation counting (Beckman LS 7200).
Plankton analyses
Water samples for the determination of bacterial abundances were fixed with formalin (2% final concentration) and stained with a 25X solution of the fluorochrome SYBR® Gold (Invitrogen Inc) for 15 min following Lisle & Priscu (Reference Lisle and Priscu2004). Samples were filtered onto 25 mm 0.2 μm black polycarbonate filters with a 0.45 μm nitrocellulose backing filter under gentle vacuum. To reduce the possibility of contamination, filter towers were pre-combusted and all reagents used for the staining were passed through 0.2 μm sterile filters to remove extraneous particles and cells. Bacterial cells were enumerated using a Zeiss Axioscop epifluorescence microscope with a final magnification of 1000x.
Samples for bacteriophage or virus like particle (VLP) enumeration were collected in sterile 125 ml screw cap flasks. All flasks were immediately flash frozen in liquid nitrogen and stored at -80°C. Prior to sample processing all flasks were removed from -80°C storage and allowed to thaw in the dark at room temperature overnight. Samples were pre-filtered through a 0.20 μm pore size filter to remove bacteria. Filtrate from each sample was aseptically collected and filtered through a 25 mm diameter, 0.02 μm pore size filter to retain the VLP. Virus like particles were stained with SYBR® Gold as described by Lisle & Priscu (Reference Lisle and Priscu2004) and counted using an Olympus BX51 epifluorescent microscope.
Samples (1 l) for phytoplankton (algae, diatoms, Cyanobacteria) and zooplankton analyses were fixed with Lugol's iodine (10 ml Lugol's) and concentrated by settling for one week in amber Nalgene bottles. After settling, the upper solution was gently siphoned off, leaving c. 60 ml of sample, which was then transferred to a clean 60 ml Nalgene bottle for transport to the UK. Subsamples were counted in a Sedgewick-Rafter counting chamber using phase microscopy at 320x magnification (Laybourn-Parry & Marshall Reference Laybourn-Parry and Marshall2003).
Environmental DNA extraction and DGGE
Water samples (70–100 ml) were filtered onto 47 mm Supor®-200 0.2 μm pore size, sterile membrane filters under low pressure (< 7 psi). Filters were placed into 5 ml cryovials filled with TES (100 mM Tris, 100 mM EDTA and 2% SDS) buffer, flash-frozen in liquid nitrogen, and stored at -80°C.
DNA was extracted from the Supor®-200 membrane filters using an Ultra Clean Soil DNA Kit (MoBio). Denaturing gradient gel electrophoresis (DGGE) was used as a molecular fingerprinting tool to characterize the microbial population structure and diversity. A portion of the 16S rRNA gene was amplified with primers 341F (5′-CCTACGGGAGGCAGCAG-3′) and 534R (5′-AATACCGCGGCTGCTGG-3′) (Muyzer et al. Reference Muyzer, Hottentrager, Teske and Wawer1996). A 40 base pair GC clamp was added to the 5′ end of the 341F primer (CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGG). The amplification protocol included a hot start (94°C for 4 min) and a touchdown programme that consisted of an initial annealing temperature of 65°C followed by a 1°C decrease for eight cycles down to 58°C, and 17 cycles with an annealing temperature of 58°C. A final elongation step occurred for 10 min at 72°C. Each 50 μl reaction mixture contained 1.5 μl of environmental DNA extract, MgCl2 buffer (final concentration 1X), Taq Master (final concentration 1X), PCR nucleotide mix (final concentration 800 μM), and Taq DNA polymerase (final concentration 0.025 u μl-1) (all components from 5 Prime, Eppendorf), upstream and downstream primers (final concentration 0.5 μM), and nuclease free water (Promega). Polymerase Chain Reaction (PCR) amplifications were carried out in an automated thermal cycler (Mastercycler ep, Eppendorf). DGGE was performed with a BioRad D Code™ system as described by Murray et al. (Reference Murray, Hollibaugh and Orrego1996). PCR products were loaded onto 8–12% polyacrylamide gels. The denaturing gradient contained 40–70% denaturant. The gels ran in 1X TAE at 60 V for 17 hours. Gels were stained with SYBR® Gold (Invitrogen) for 15 min and viewed with an Alpha Innotech FluorChem™ 8800 system. Gel images were analysed using GelComparII software (Applied Math).
16S rRNA gene clone library
We constructed clone libraries for three different dates (early December = 11 December 2004 (ED), late December = 29 December 2004 (LD), and mid-January = 14 January 2005 (MJ)) during the transition period from ice-covered to ice-free conditions by amplifying the 16S rRNA gene with primers 9F (5′-GAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) (Stackebrandt & Liesack Reference Stackebrandt and Liesack1993). PCR products were cloned into pCR®2.1-TOPO vectors (TOPO TA cloning kit, Invitrogen) following the manufacturers guidelines. From each sample 70 clones containing inserts were picked for further analyses. Bacterial clones were sent to Functional Bioscience Inc on LB agar plates for high throughput DNA preparation and DNA sequencing using primer M13F (20). Nucleotide sequences were edited using Sequencher 4.5 (Gene Code Corporation). For each sequence a National Centre for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST) search was performed in order to determine the closest relatives (BLASTN 2.2.21, ncbi.nlm.nih.gov/BLAST/, accessed May 2009; Zhang et al. Reference Zhang, Schwartz, Wagner and Miller2000).
Statistical analyses
Monothetic cluster analysis was applied as outlined by Foreman et al. (Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011) and Greenwood (Reference Greenwood2012). This approach aimed to describe differences in the microbial community composition throughout the summer based on the binary presence/absence patterns of bacterial clones.
Results
Physical parameters and chemical analyses
Pony Lake was frozen solid to its base until late November 2004. By the end of November, peripheral moating occurred at the west end of the lake and by 14 January 2005 c. 80% of the lake was ice-free. The remaining 20% of the ice layer was multi-year ice, but did not impede mixing of the main water column. Surface inflow or runoff from the surrounding hills was not observed. During the open water period the water column was fully mixed by strong winds.
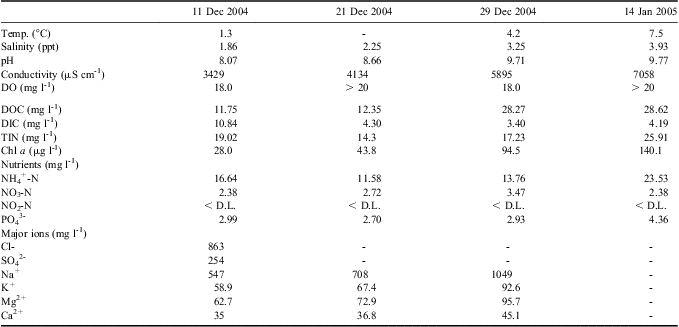
Pony Lake was sampled on four different time points during summer 2004/05, corresponding to changes in the lake ice cover (Table I). During mid-December 2004 to mid-January 2005 Pony Lake was supersaturated with dissolved oxygen (DO). The water temperature increased gradually throughout the sampling period from 1.3°–7.5°C. Lake water was moderately to highly alkaline, ranging from a pH of 8.07–9.77. Pony Lake was Cl- dominated, although Na+ and SO42- concentrations remained high. The suite of major ion concentrations increased between the initial melt period and mid-January. Dissolved inorganic carbon and DOC concentrations ranged between 3.40–10.84 mg l-1 and 11.75–28.62 mg C l-1, respectively. Characteristically for a eutrophic lake, inorganic nitrogen and phosphorous concentrations were elevated, with the exception of nitrite, which was below the detection limit (3 μg l-1) throughout this study (Table I).
Table I General site characteristics, dissolved organic carbon (DOC), dissolved inorganic carbon (DIC), total inorganic nitrogen (TIN), nutrient and major ion concentrations for Pony Lake measured during the summer 2004/05.
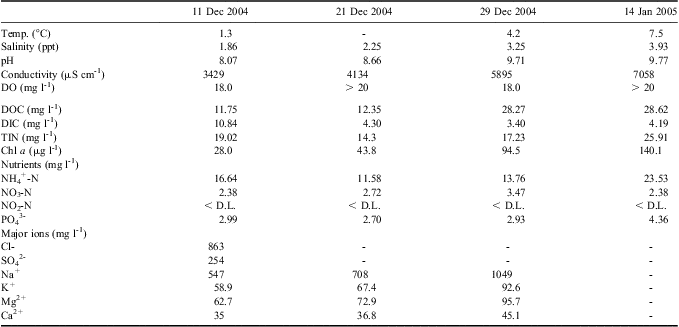
DO = dissolved oxygen, - = not analysed, D.L. = detection limit.
Productivity measurements and plankton analyses
Pony Lake was populated by bacteria, viruses, phytoflagellates and protozooplankton. Numbers of planktonic organisms are presented in Table II. Bacterial abundances and productivity ranged between 2.15 x 105 and 1.36 x 106 cells ml-1 and from 19.7–30.5 μg C l-1 d-1. Virus like particles peaked by the end of December and abundances ranged from 3.72 x 104–4.5 x 105 cell ml-1. Within Pony Lake the phototrophic nanoflagellate (PNAN) assemblage consisted of chlorophytes and cryptophytes (Fig. 2). These phyla were at their maximum of 2.48 x 103 and 7.60 x 102 cells ml-1, respectively, in mid-January. In contrast, heterotrophic nanoflagellates (HNAN) were not detected. Maximum primary production did not coincide with highest phytoplankton numbers. Rather, rates were at their highest in mid-December and ranged from 0.67–2.66 mg C l-1 d-1. Ciliated protozoans were poorly represented in Pony Lake and comprised only one dominant morphotype, Plagiocampa sp. (Fig. 2). About 30% of the total number of ciliates remained unidentified (equivalent to ∼1 cell ml-1). Ciliated cysts were present during the initial melting stage. Numerically, Cyanobacteria and diatoms accounted for < 0.5% of the total phytoplankton population in Pony Lake. Planktonic Cyanobacteria were rare (∼3 cell ml-1) throughout December 2004 and undetectable in mid-January 2005 (Fig. 2). The dominant Cyanobacteria found in Pony Lake belonged to the Oscillatoria and Phormidium genera. Diatoms were only present on one sampling date. Metazooplankton were sparse with rotifers (one organism l-1; Fig. 2) present only in mid-December.
Table II Planktonic community abundance and productivity in Pony Lake during the summer 2004/05.

*including cysts.
BP = bacterial production, VLP = virus like particles, PPL = phytoplankton, PPR = primary production.

Fig. 2 Planktonic composition of Pony Lake during the 2004/05 summer. Changes in the planktonic composition are displayed by the gradual increase of algal species (chlorophytes and cryptophytes), the increasing abundance of ciliates (e.g. Plagiocampa) and the short-term appearance of Cyanobacteria and diatoms during the observation period.
Community structure analysis
DGGE banding patterns (Fig. 3), analysed with GelCompar II software, were used to compare the complex microbial community composition between sampling dates. We further compared the DGGE patterns from these Pony Lake water samples to the DGGE patterns from Pony Lake ice core samples, which were collected earlier in November 2004 when the lake was frozen solid to its base (for GelCompar II dendrogram see Dieser Reference Dieser2009). DGGE image analysis demonstrated that the dominant bacterial community structure in Pony Lake changed during the transition from ice-covered to ice-free conditions. Several different clusters were distinguishable. The water sample collected on 11 December 2004 was most closely related to the community found in the bottom half of the ice core, while samples collected later in the season clustered with DGGE banding patterns obtained from the top ice core section. The samples collected on 21 December and 29 December grouped together, but were different from the samples collected from Pony Lake in January.

Fig. 3 Denaturing gradient gel electrophoresis (DGGE) profiles of microbial communities from different Pony Lake water samples collected between 11 December 2004 and 14 January 2005 show a shift in community structure. From left to right: 11 December 2004, 21 December 2004, 29 December 2004, 14 January 2005. Image colours were inverted on the camera, but not manipulated.
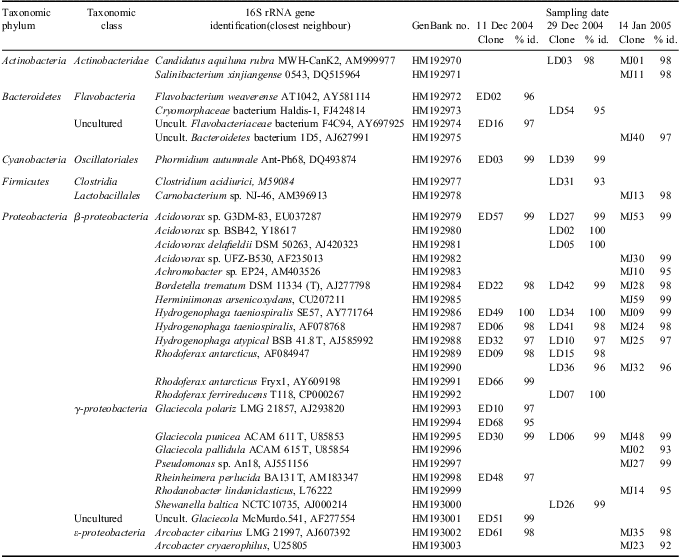
The relative distribution of major phylogenetic groups found within each clone library from the individual sampling dates is shown in Fig. 4. The phylogenetic relationship of Pony Lake clones to their closest neighbours, according to a BLAST search, is summarized in Table III. All clone sequences were submitted to GenBank and bear the prefix ANTPL_, for Antarctic Pony Lake clones. Accession numbers for the Pony Lake clones are from HM192934–HM193003. A total of 34 unique clones were identified from the three sampling dates, with β and γ-proteobacteria comprising the dominant fractions of the clone libraries. These two groups also showed the most compositional overlap between the three sampling dates. For example, Acidovorax sp., Bordetella sp., Hydrogenophaga sp., and Glaciecola sp. were the closest reported relatives to clone sequences found throughout the summer season. Actinobacteria, Bacteriodetes, Cyanobacteria, Firmicutes, and ε-proteobacteria were described by only a small number of sequences. A statistical comparison based on the presence or absence of sequence types was used to discriminate differences between clone libraries for the individual sampling dates (Fig. 5). Distinct clusters, as well as overlap, can be seen for each individual clone library indicating that a shift in the bacterioplankton community did occur during the summer of 2004/05. Clone libraries from early December and mid-January consisted of large numbers of clones (∼44% and 53% respectively) that were restricted to these individual sampling dates. Compositional overlap between clone libraries was small and more likely to occur between consecutive sampling dates. Only six sequence types were present on all three sampling dates. Based upon the small number of clones sequenced we cannot account for all microbes, however our data suggest that a transition occurred among the dominant members of the community that we were able to identify.

Fig. 4 Distribution of taxonomic classes within the Pony Lake water sample clone libraries from three sampling dates. ED (11 December 2004), LD (29 December 2004), and MJ (14 January 2005).
Table III Affiliation of 16S rRNA gene phylotypes found in Pony Lake water during 2004/05. Clones labelled ED were from early December, LD from late December and MJ from mid-January in Pony Lake. All Pony Lake clones submitted to GenBank have the prefix ANTPL_.


Fig. 5 Banner plot of the monothetic cluster analysis showing closely related taxonomic classes and phyla assigned to Pony Lake clones isolated from three different sampling dates. Orange indicates the presence and black the absence of a clone. Gray represents all of the clones before any clustering steps. The order of the sampling dates was chosen by the clustering algorithm. Thereby, each individual column best explains the responses in the column to its right. The plot is read from top to bottom. Two observations with the same response (absent or present) lead to a coloured bar between their lines. White cells indicate the transition between presence and absence, with the colour of the text indicating the presence or absence of the clone at the bottom of a cell. The plot highlights clusters of clones only found on a single date as well as the small degree of compositional overlap between dates.
Discussion
Physical parameters and chemical analyses
Evolution of the summer meltwaters of Antarctic ponds greatly depends on the extent of melt, wind derived mixing of the water column, and the characteristics of the density gradient after thaw. Typically basal brines, derived from ion exclusion during the freezing process, establish a stratification of the meltwater pond with freshwater floating on top of layers of high salinity (Healy et al. Reference Healy, Webster-Brown, Brown and Lane2006). Physical mixing of the water column can gradually introduce salts and nutrients into the top layers and oxygen into the reduced bottom waters. Consequently, the fate of the limnological structure of the ponds during summer will depend on whether or not this chemical stratification is maintained.
Pony Lake exhibits a steep chemical gradient within its ice column (Dieser Reference Dieser2009). During the summer of 2004/05 strong winds disrupted this chemical stratification after ice break-up and major ion concentrations along with dissolved organic matter and inorganic nutrients increased between the initial melt and late summer lakewater. Although previously attributed to evaporative concentration processes (Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004), wind induced mixing of the highly concentrated bottom waters as well as the area of the lake that becomes ice-free during summer more likely determined the chemical composition of the water column. Pony Lake was revisited during the 2005/06 season, however, due to extensive snow accumulation, there was only a small, peripheral ice-free area and mixing was insufficient to break down the salinity gradient. Thus, Pony Lake's water column remained chemically stable (Dieser Reference Dieser2009). The chemical composition of Pony Lake was similar to that found in other coastal Antarctic ponds (Torii et al. Reference Torii, Matsumoto and Nakaya1988, Schmidt et al. Reference Schmidt, Moskal, De Moraz, Howard-Williams and Vincent1991). The brackish character of the lakewater is indicative of a legacy of evaporation and sublimation, the lack of inflows, and the accumulation of marine aerosols. It is important to note that the dissolution of soil salts could have potentially contributed to the increased ion concentrations.
Considerably lower (∼three orders of magnitude) phytoplankton counts were measured in our study than those found in the mid 1990s by McKnight et al. (Reference McKnight, Andrews, Spaulding and Aiken1994), which corresponds with a substantial decrease in DOC concentrations found in Pony Lake during this study (11.75–28.62 mg C l-1) than previously reported (95–110 mg C l-1, McKnight et al. Reference McKnight, Andrews, Spaulding and Aiken1994; 32.4–92.4 mg C l-1, Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004). Several processes such as changes in phytoplankton blooms, progressive exploitation of the carbon pool by bacterioplankton (Bell & Laybourn-Parry Reference Bell and Laybourn-Parry1999, Laybourn-Parry et al. Reference Laybourn-Parry, Quayle and Henshaw2002, Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004) or variable rates of dissolved organic matter (DOM) production by algal populations (Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004) may alter DOC concentrations. Our seasonal dataset suggests that a progressive exploitation of the DOC pool by bacteria seems unlikely in this instance as bacterial cell numbers and DOC concentrations peaked in January when production rates were markedly reduced compared to early and mid-December (Table II). Moreover, the decoupling of bacterial production and DOC maxima suggests changes in the DOM composition over the course of the summer. Generally, organic matter can undergo photochemically induced transformations that may render it more refractory and hence less bioavailable for microbial uptake (Benner & Biddanda Reference Benner and Biddanda1998). Thus, the observed increase in DOC concentration and the simultaneous decrease in bacterial production between early December and mid-January may be due to the accumulation of recalcitrant photolytic products. Mao et al. (Reference Mao, Cory, McKnight and Schmidt-Rohr2007) demonstrated that the fulvic acid fraction in Pony Lake had undergone significant humification from the original algal precursor material that generated highly cross-linked structures. While algal derived DOC is considered to be of young age, Mao et al. (Reference Mao, Cory, McKnight and Schmidt-Rohr2007) suggested that the organic matter in Pony Lake has been structurally altered (‘aged’) and would be of comparably poor substrate quality. Nonetheless, a steady exploitation of the rich DOC pool detected during previous studies over the past ten years seems evident (McKnight et al. Reference McKnight, Andrews, Spaulding and Aiken1994, Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004). Further, different phytoplankton blooms associated with changes in DOM production rates may have accounted for shifts in the DOC levels and the overall decrease in DOC concentrations (McKnight et al. Reference McKnight, Andrews, Spaulding and Aiken1994, Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004, this study). In past studies, algal blooms have correlated with DOC concentrations in Pony Lake. McKnight et al. (Reference McKnight, Andrews, Spaulding and Aiken1994) reported high DOC concentrations during the chlorophyte bloom, and Brown et al. (Reference Brown, McKnight, Chin, Roberts and Uhle2004) observed a decrease in DOC concentrations concurrent with a declining chlorophyte population and a shift in algal blooms, underscoring the argument that different algal species exhibit different DOC production rates.
Inorganic nitrogen and phosphorous concentrations in Pony Lake were elevated compared to those found in other eutrophic Antarctic lakes (Mataloni et al. Reference Mataloni, Tesolin and Tell1998, Bell & Laybourn-Parry Reference Bell and Laybourn-Parry1999, Butler Reference Butler1999). Although runoff from the adjacent penguin rookeries was not observed, the high nitrogen and phosphorus levels in Pony Lake could potentially be derived from bird droppings (Vincent & Vincent Reference Vincent and Vincent1982). Decreasing NH4-N concentrations at the beginning of the growth season suggest that ammonium was the preferred nitrogen source for phytoplankton, as previously shown in several other Antarctic lakes (Hawes Reference Hawes1983). Maximum primary production rates were measured on 21 December 2004 when ammonium concentrations dropped markedly (Tables I & II). In contrast, while primary production rates gradually decreased between 21 December 2004 and mid-January 2005, the NH4-N concentrations peaked during this same period. Furthermore, decomposition of organic matter by heterotrophic bacteria or photolytic reactions may have resulted in mineralization of the dissolved organic matter, thereby generating NH4.
Productivity measurements and plankton analyses
In common with other continental Antarctic lakes, Pony Lake is populated by bacteria, viruses, phytoflagellates and protozooplankton. The high levels of inorganic nutrients and DOC concentrations (Table I) available throughout the study period imply that these factors should not limit summer plankton growth and that other parameters such as temperature, ionic composition, and light intensity may be more important in triggering plankton blooms.
Bacterial cell abundances ranging from 2.15 x 105–1.36 x 106 cells ml-1 were comparable to those reported from maritime or continental Antarctic lakes across different trophic levels (Takacs & Priscu Reference Takacs and Priscu1998, Bell & Laybourn-Parry Reference Bell and Laybourn-Parry1999, Butler Reference Butler1999, Butler et al. Reference Butler, Edworthy and Ellis-Evans2000). Bacterial production measured in Pony Lake was considerably higher compared to oligotrophic Antarctic lakes (Takacs & Priscu Reference Takacs and Priscu1998, Butler et al. Reference Butler, Edworthy and Ellis-Evans2000, Laybourn-Parry et al. Reference Laybourn-Parry, Quayle and Henshaw2002), but lower than reported in other saline or eutrophic Antarctic lakes (Laybourn-Parry et al. Reference Laybourn-Parry, Quayle and Henshaw2002). Importantly, DOC concentrations in these lakes (Laybourn-Parry et al. Reference Laybourn-Parry, Quayle and Henshaw2002) were similar to those found in Pony Lake. Bacterial growth is regulated by temperature, and the availability of inorganic nutrients (N and P) and organic substrates (DOC). It is unlikely that either temperature or nutrients would suppress summer bacterial production in Pony Lake. After an early season maximum, productivity decreased while water temperatures increased and nutrients were plentiful throughout the season. Although one might hypothesize that higher DOC concentrations would precede enhanced bacterial growth, this process is reversed in Pony Lake. Highly bioavailable DOC at the beginning of the season may not only be generated from a proliferating planktonic community, but may also be released from the melting ice. In shallow meltwater ponds, photosynthesis can persist beneath the ice during freeze-up when sufficient levels of incident light prevail (Hawes et al. Reference Hawes, Safi, Sorrell, Webster-Brown and Arscott2011). Subsequently newly released DOC would become incorporated and preserved in the ice matrix, providing a rapidly available carbon pool when growth conditions turn more favourable. This mechanism has previously been suggested for Pony Lake due to the stimulated bacterial production in the mid-section of the winter ice column (Foreman et al. Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011). Conversely, increased DOC concentrations mixed in from basal brines later in the season did not enhance bacterial growth. This supports the view of a strong conservative behaviour of organic compounds and an accumulation of refractory organic material in lakewater (Howard-Williams & Hawes Reference Howard-Williams and Hawes2007, Hawes et al. Reference Hawes, Safi, Sorrell, Webster-Brown and Arscott2011).
Virus like particle abundances reported from Pony Lake were in the same range as has been shown in other Antarctic lakes, such as Lake Druzhby, Crooked Lake, and Beaver Lake in Vestfold Hills or Lake Hoare and east lobe Lake Bonney in the McMurdo Dry Valleys (see Säwström et al. Reference Säwström, Lisle, Anesio, Priscu and Laybourn-Parry2008 for a review). The virus to bacteria ratio (VBR) in polar inland lakes usually falls between 1 and 34, however exceptionally high ratios (> 120) have also been reported for the saline lakes in Vestfold Hills (Säwström et al. Reference Säwström, Lisle, Anesio, Priscu and Laybourn-Parry2008). The VBR in Pony Lake ranged from 0.03–1.32. Low ratios can be explained in part by the autochthonous nature of carbon substrates in Antarctic lakes (Säwström et al. Reference Säwström, Lisle, Anesio, Priscu and Laybourn-Parry2008). Bacterial abundances are typically lower than VLP numbers due to the infectious interactions of VLP with the host cell causing viral induced cell lysis. However, low bacterial growth rates in polar waters do not sustain high infection rates. On average, only four viruses are released from each bacterial cell (Laybourn-Parry Reference Laybourn-Parry2009). Viral processes and infectivity have also been related to a range of abiotic factors. Madan et al. (Reference Madan, Marshall and Laybourn-Parry2005) reported a negative correlation between VLP and temperature, indicating lower decay rates under lower temperatures. Nutrient limitation can reduce bacterial proliferation, thereby indirectly affecting VLP numbers (Weinbauer Reference Weinbauer2004). Although the VBR was low in Pony Lake, the interaction between viruses and bacteria may play an important role in carbon and nutrient cycles. For instance, virus induced release of organic carbon from bacteria can provide a significant portion of the DOC pool and importantly short-circuit the carbon cycle before bacterial produced carbon is removed by protozoan grazing.
The dominance of chlorophytes and cryptophytes in Pony Lake has been previously reported (McKnight et al. Reference McKnight, Andrews, Spaulding and Aiken1994, Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004), and was also found during this study. It has been shown that these phyla dominate numerous Antarctic lakes (Butler et al. Reference Butler, Edworthy and Ellis-Evans2000, Roberts et al. Reference Roberts, Priscu and Laybourn-Parry2004), while Chlamydomonas species are typically found in eutrophic Antarctic lakes (Mataloni et al. Reference Mataloni, Tesolin and Tell1998, Butler Reference Butler1999). While the dominant organisms have remained consistent across the years there is variability in the abundance of individual species and the timing of phytoplankton blooms (McKnight et al. Reference McKnight, Andrews, Spaulding and Aiken1994, Brown et al. Reference Brown, McKnight, Chin, Roberts and Uhle2004, this study). For example, Brown et al. (Reference Brown, McKnight, Chin, Roberts and Uhle2004) showed that following the loss of the ice cover in mid-December 1997 the chlorophyte bloom was displaced by a bloom of cryptophytes, while the study by McKnight et al. (Reference McKnight, Andrews, Spaulding and Aiken1994) reported the dominance of chlorophytes in Pony Lake at the end of January 1994. Dominance of a particular phylum or a distinct algal bloom was not observed in the current study, where both chlorophytes and cryptophytes increased gradually during the transition from an ice-covered to an ice-free lake. The dynamic nature of Antarctic phytoplankton populations has also been observed by Mataloni et al. (Reference Mataloni, Tesolin and Tell1998) in the highly eutrophic Otero Lake (unofficial name). Overall, phytoplankton was two to three orders of magnitude less abundant in Pony Lake during this study along with a 1.4 to 3 fold decrease in salinity. Laybourn-Parry et al. (Reference Laybourn-Parry, Quayle and Henshaw2002) demonstrated a strong correlation between the productivity of the planktonic community and salinity in numerous lakes in Vestfold Hills, whereby productivity increased across the salinity spectrum (from brackish to hyper-saline).
Although phytoplankton numbers and chl a concentrations still increased throughout the season, primary production in Pony Lake peaked in mid-December. Chlorophyll a concentrations correlated with the phytoplankton maxima (r = 0.97), but were only weakly negatively correlated with primary production rates (r = -0.12). This temporal decoupling of production and plankton maxima was also observed in several lakes in Vestfold Hills (Laybourn-Parry et al. Reference Laybourn-Parry, Quayle and Henshaw2002). In general, primary productivity in Pony Lake was higher than found in other more oligotrophic Antarctic lakes (Butler et al. Reference Butler, Edworthy and Ellis-Evans2000, Laybourn-Parry et al. Reference Laybourn-Parry, Quayle and Henshaw2002). However, similar or even larger primary productivity rates have been reported in several Antarctic lakes across trophic levels (Butler Reference Butler1999, Laybourn-Parry et al. Reference Laybourn-Parry, Quayle and Henshaw2002). Variations in the photosynthetic communities or the underwater light climate between lakes (e.g. photosynthetically active radiation in an ice-covered vs ice-free system) may account for the differences in primary production.
Proto- and metazooplankton numbers in Pony Lake during this study were generally low with low species diversity. The dominant species, Plagiocampa, accounted for 70% of the total ciliate population. This differs from previous reports where Armitage & House (Reference Armitage and House1962) primarily found Euplotes in Pony Lake. Ciliate cysts, also detected in Pony Lake ice cores (Foreman et al. Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011), were only present at the beginning of the transition period from ice-covered to ice-free. Since the growth season during the summer is typically short, cysts are produced as a strategy to survive unfavourable conditions during the winter (Mataloni et al. Reference Mataloni, Tesolin and Tell1998, Bell & Laybourn-Parry Reference Bell and Laybourn-Parry1999). Cysts will then lie dormant until conditions become suitable for vegetative growth. Similar to Armitage & House (Reference Armitage and House1962) who identified Philodina as the dominant species, rotifers were rare and only detected in mid-December.
Although Pony Lake shows indications of a complex microbial food web, it seems unlikely that the small number of metazoan predators and the low abundance of raptorial ciliates (e.g. Plagiocampa) strongly control PNAN or bacteria by grazing. Mixotrophic cryptophytes could exert grazing pressure on bacteria, however, phagotrophy would seem to be more beneficial to support or supplement autotrophy during periods of light and nutrient limitation. As with other Antarctic lakes (Priscu et al. Reference Priscu, Wolf, Takacs, Fritsens, Laybourn-Parry, Roberts and Lynos1999, Roberts et al. Reference Roberts, Priscu and Laybourn-Parry2004) there appears to be little top-down control over the microbial food web.
Community structure analysis
Overall, c. 68% of the Pony Lake clones closely matched sequences reported from geographically diverse Antarctic lake and marine environments (e.g. Van Trappen et al. Reference Van Trappen, Mergaert, van Eygen, Dawyndt, Cnockaert and Swings2002, Glatz et al. Reference Glatz, Lepp, Ward and Francis2006) which indicates a high level of similarity for microorganisms inhabiting these cold ecosystems. An important goal of the phylogenetic analysis was to characterize any changes in the microbial community composition over the course of the summer in Pony Lake. Despite the inherent limitations of this technique, DGGE has been successfully employed to describe the minimum number of dominant phylotypes present (Pearce Reference Pearce2005, Villaescusa et al. Reference Villaescusa, Casamayor, Rochera, Velázquez, Chicote, Quesada and Camacho2010). Both gel comparison of DGGE banding patterns and cluster analysis of representative clones from Pony Lake indicate a shift in the microbial community during the early and late summer open water period. Differences in the microbial composition were also apparent when comparing the open water to the lake ice community (Foreman et al. Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011). From the sequences found in the Pony Lake ice cores only eight of these sequence types could be identified in the Pony Lake water column as well. The highest degree of sequence overlap was found among members of the β-proteobacteria lineage. When assigned putative identifications these Pony Lake clones were shown to be most closely related to Bordetella sp. and Hydrogenophaga sp. Representatives from the anaerobic δ-proteobacteria, Spirochaetes, and Verrucomicrobia lineages were solely present in the ice core samples (Foreman et al. Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011). In contrast, the phylum Actinobacteria was only found in the Pony Lake water samples. Obvious differences were observed between taxonomic classes. Within the ice, the most dominant classes were Bacteroidetes and ε-proteobacteria (Foreman et al. Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011). Over the course of the summer sequence types diverged widely from those found in the initial ice community and members of the β- and γ-proteobacteria prevailed (Fig. 4). Physicochemical conditions in the Pony Lake ice were distinctly different (oxygen depletion and severe chemical stratification of nutrients, DOC and ions; Dieser Reference Dieser2009) from those in the summer lakewater. It seems plausible that the chemical stratification of the ice column and the oxygen depletion prior to complete freeze-up created an environment favouring microbes with high salt and low temperature tolerance, as well as anaerobes, as suggested by Foreman et al. (Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011). Conversely, Pony Lake summer lakewater was well mixed and supersaturated with oxygen. Interestingly, despite these obvious differences in environmental conditions, the microbial community trapped within the ice column during winter is responsible for re-colonizing Pony Lake when melt is initiated. However, this process largely depends on the timing and extent of the ice cover loss. In mid-December 2004 the melt proceeded rapidly and physicochemical changes occurred in the water column (Table I), driven by higher air temperatures, strong winds, and proliferating algal communities. Pearce (Reference Pearce2005) found that in three lakes on Signy Island, South Orkney Islands, physical factors affected the development of the microbial community during the seasonal transition from ice-covered to the summer ice-free, whereas biotic factors were believed to become more important later in the season. The establishment or disruption of a vertical stratification and, in particular, temperature critically affected community structure. Temperature differences of as little as 0.1°C have been shown to cause significant variations in the community composition (Pearce Reference Pearce2005). Although we believe that grazing on bacterioplankton by bacteriophagous protists, as well as mortality rates by virioplankton in Pony Lake, were insufficient to offset bacterial abundance throughout the season, negative effects on individual group members of the community are still possible. Further, there is indication that the structuring of the microbial community is strongly correlated to the chemical progression of the water column. A distinct change in DGGE profiles was observed as thorough mixing gradually increased the salinity of Pony Lake. Conversely, when the water column of Pony Lake remained stable, the development of a different community throughout the open water period appeared to be minimal (Dieser Reference Dieser2009). In general, the introduction of new microorganisms from the deeper parts of the lake may also be considered. For instance, Firmicutes were confined to the bottom layer of the ice core (Foreman et al. Reference Foreman, Dieser, Greenwood, Cory, Laybourn-Parry, Lisle, Jaros, Miller, Chin and McKnight2011). Although absent at the beginning of the melt season, Firmicutes were present when Pony Lake became largely ice-free and fully mixed. Considering the extreme intra-seasonal alterations in environmental conditions in Pony Lake it does not seem surprising that we detected different, dominant members of the microbial community between winter ice, early, and late-summer lakewater. Depending on the lake area that becomes ice-free during summer it can be hypothesized that the microbial community within the ice initially seeds the development of the lakewater community. However, it is unclear whether the distribution of microbes within the ice is the effect of physical processes during freezing or other environmental factors. Hawes et al. (Reference Hawes, Safi, Sorrell, Webster-Brown and Arscott2011) showed that most of the lake/sediment area was frozen prior to the onset of more severe conditions, protecting planktonic and benthic organisms from stresses imposed by concentrated liquid brines. In this context it is noteworthy that while the presence of many community members is coupled with the physicochemical state of Pony Lake (e.g. redox conditions), more versatile species (e.g. clones that were related to Bordetella sp. and Hydrogenophaga sp.) find a perennial niche in this lake as well.
Conclusions
Pony Lake is a small, brackish, eutrophic lake at Cape Royds, Ross Island. Lake chemistry and nutrient levels are affected by the input of marine aerosols and the adjacent penguin rookery. The evolution of its microbial community appears to be a complex process, driven by seasonal physichemical changes. Over the course of the summer progressive melt of the ice column, wind mixing, the lack of inflows, increases in water temperature, and changes in salt and nutrient concentrations stimulated a compositional shift in the dominant microbial community in Pony Lake. Aside from intra-seasonal changes, a clear decrease in the phytoplankton numbers was apparent in Pony Lake when compared to previous studies. In Pony Lake, where microorganisms form the basis of the food web and control biogeochemical cycles, such a prominent drop in phytoplankton numbers will alter the carbon pool, its fluxes and transformation, and may compromise the entire lake ecosystem. Primary producers are the fundamental source of inorganic carbon fixation and autotrophic energy production, providing organic carbon and nutrients for subsequent trophic levels. Closely linked to primary production is the exudation of photosynthate as DOC, a major substrate for heterotrophic bacteria. It appears that over the past decade Pony Lake became less productive and its once rich DOC pool has been steadily exploited by the bacterioplankton community. Although bacteria are an essential link in the recycling of nutrients and transformation of organic carbon back into the food web, these organisms acted as a large carbon sink. The more labile DOC fractions would have been preferentially hydrolysed or re-mineralized by bacterioplankton while abiotic processes (e.g. solar radiation induced polymerization and condensation) can transfer the remaining DOC into more recalcitrant forms. Eventually what would emerge is a dissolved organic carbon pool, exhausted in rapidly, bioavailable substrates, but enriched in components that are less susceptible to enzymatic cleavage. Nonetheless, what triggered the decline in phytoplankton numbers in Pony Lake remains unclear. End member ecosystems, such as Pony Lake, can exhibit a rapid response to changes in their physicochemical environment. Highly enriched in nutrients, the microbial community in Pony Lake is probably controlled by factors such as air and water temperature, wind and salinity that determine the length of ice or open water period, the establishment of a stratified or mixed water column, and the ionic composition, respectively. Our seasonal dataset highlights the interaction between environmental parameters and changes in the microbial community composition. Thus, organisms which persist throughout the year in Pony Lake must be capable of surviving rapidly changing conditions.
Acknowledgements
Logistical support for this project was provided by Raytheon Polar Services, including several volunteer field assistants that greatly enabled our field efforts. Skilled helicopter transport came from the pilots of Petroleum Helicopters Inc and Helicopters New Zealand. K. Welch, A. Chiuchiolo, C. Gardner and R. van Treese of the McMurdo Dry Valleys LTER program provided analytical support. Daniel Horn, a summer intern with the American Indian Research Opportunities Program at MSU, aided in image analysis. We sincerely appreciate the valuable comments and suggestions from the reviewers, especially those of Dr Ian Hawes. Funding for this project came from NSF OPP-0338260 to YPC, OPP-0338299 to DMM, OPP-0338121 to PM, and OPP-0338342 to CMF. Any opinions, findings, or conclusions expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.