Introduction
Elasmosaurids are the most diverse Late Cretaceous plesiosaur group achieving a worldwide distribution (Welles Reference Welles1962, Vincent et al. Reference Vincent, Bardet, Suberbiola, Bouya, Amaghzaz and Meslouh2011, Kubo et al. Reference Kubo, Mitchell and Henderson2012). The record of elasmosaurids from Antarctica is restricted to the James Ross Archipelago (Fig. 1), where they have been collected from the Santa Marta Snow Hill Island and López de Bertodano formations (O’Gorman Reference O’Gorman2012, table II). The elasmosaurid fauna from the Weddellian Province sensu Zinsmeister (Reference Zinsmeister1979), i.e. Patagonia, western Antarctica and New Zealand, comprises aristonectine and non-aristonectine elasmosaurids (Gasparini et al. Reference Gasparini, Bardet, Martin and Fernández2003, O’Gorman et al. Reference O’Gorman, Salgado, Olivero and Marenssi2015). Despite the initial controversy about the elasmosaurid affinities of aristonectines (Welles Reference Welles1962, O’Keefe & Hiller 2009), the interpretation is now widely accepted (Gasparini et al. Reference Gasparini, Bardet, Martin and Fernández2003, Otero et al. Reference Otero, Soto-Acuña, O’Keefe, O’Gorman, Stinnesbeck, Suárez, Rubilar-Rogers, Salazar and Quinzio-Sinn2014, O’Gorman et al. Reference O’Gorman, Salgado, Olivero and Marenssi2015). Another contentious issue surrounding the aristonectines is the explanation for the large quantity of specimens that show juvenile morpho-osteological features (O’Gorman et al. Reference O’Gorman, Gasparini and Salgado2014a) but some show relatively large sized elements compared to other juvenile elasmosaurids (Otero et al. Reference Otero, Soto-Acuña and Rubilar-Rogers2012, O’Gorman et al. Reference O’Gorman, Gasparini and Salgado2013). One explanation for this pattern is that the aristonectines retain juvenile features until they reach larger sizes relative to other elasmosaurids (O’Gorman Reference O’Gorman2013, O’Gorman et al. Reference O’Gorman, Gasparini and Salgado2014a). To test this, Araújo et al. (Reference Araújo, Polcyn, Lindgren, Jacobs, Schulp, Mateus, Gonçalves and Morais2015) described the palaeohistology of a juvenile aristonectine from Angola and recorded features which are more compatible with an adult specimen. The present contribution includes a description of MLP 14-I-20-8 (specimen from the Museo de La Plata, Buenos Aires Province, Argentina), the most complete and smallest aristonectine juvenile postcranial specimen from Antarctica, the first palaeohistological description of an Antarctic aristonectine and some insights into the probable morphological changes that occurred during the ontogeny of the Aristonectinae.

Fig. 1 Aristonectinae indet. MLP 14-I-20-8 preserved material, locality and horizon. a. Materials preserved. b. Quarry diagram, squares=200 × 200 mm. c. Map showing the locality of MLP 14-I-20-8 discovery. d. Upper section of the López de Bertodano Formation indicating the level where the MLP 14-I-20-8 was positioned, modified from Montes et al. (Reference Montes, Nozal, Santillana, Marenssi and Olivero2013).
Geological setting
The López de Bertodano Formation crops out both in Seymour Island (Isla Marambio) and Vega Island (Sandwich Bluff Member), James Ross Archipelago, Antarctic Peninsula (Fig. 1c). The Maastrichtian López de Bertodano Formation comprises approximately 1150 m of sandy shales and sandstones with interbedded concretions and carbonate subordinate facies (Macellari Reference Macellari1988). The outcrops of the López de Bertodano Formation at Seymour Island were originally divided into ten units (Klb 1–10), but recently unit Klb 1 has been considered to be part of the Haslum Crag Sandstone (Macellari Reference Macellari1988, Olivero et al. Reference Olivero, Ponce and Martinioni2008).
The lower units, Klb 2–6 (the informal ‘rotularia units’), were deposited in a shallow marine environment near an estuary (Macellari Reference Macellari1988, Olivero et al. Reference Olivero, Ponce and Martinioni2008). While the upper units, Klb 7–10 (‘molluscan units’), where the specimen described in this contribution was collected (between Klb 8 and 9), were deposited in a middle to outer platform environment (Macellari Reference Macellari1988). The López de Bertodano is Maastrichtian–Danian in age (Olivero et al. 2000). Specifically, it is interesting that the Maastrichtian–Danian boundary is approximately between Klb 9 and 10 (top of the ‘molluscan units’) (Macellari Reference Macellari1988).
Methods
Description and comparison of external morphology
The ontogenetic categories proposed by Brown (Reference Brown1981), which are based on the fusion of the neural arch to the vertebral centrum, are considered. For descriptive purposes, Welles’ (Reference Welles1952) indices have been used. These indices considered vertebral centrum proportions, specifically the height (H)/length (L) ratio (HI=100 x H/L) and breadth (B)/length (L) ratio (BI=100 x B/L). Additionally, the breadth/height ratio (BHI=100 x B/H) and the vertebral length index (VLI=100 x L/(0.5 x (H + B))) (Brown Reference Brown1981) were also recorded. The B and H were measured on the posterior articular face. Finally, the B:L index (Welles Reference Welles1952), which records the ratio between the distal breadth and total length of propodials, was calculated. The body length of MLP 14-I-20-8 was estimated through comparison with SGO PV 260 (Otero et al. Reference Otero, Soto-Acuña and Rubilar-Rogers2012, fig. 2; specimen from the Área Paleontología, Museo Nacional de Historia Natural, Santiago, Chile), a juvenile aristonectine from the Quiriquina Formation, Chile, identified as Aristonectes quiriquinensis (Otero et al. Reference Otero, Soto-Acuña, O’Keefe, O’Gorman, Stinnesbeck, Suárez, Rubilar-Rogers, Salazar and Quinzio-Sinn2014). The comparison is based on the assumption that both MLP 14-I-20-8 and SGO PV 260 have similar body proportions.
Palaeohistological analysis
Palaeohistological samples were taken from the mid-diaphyseal of the right humerus of MLP 14-I-20-8. Three thin sections were prepared using standard palaeohistological techniques and examined with light microscopy (Chinsamy & Raath Reference Chinsamy and Raath1992). Nomenclature and definitions of structures used in this study are derived from Francillon-Vieillot et al. (Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg and de Ricqlés1990) and Chinsamy-Turan (Reference Chinsamy-Turan2005).
Systematic palaeontology
Sauropterygia Owen Reference Owen1860
Plesiosauria de Blainville Reference De Blainville1835
Plesiosauroidea Welles Reference Welles1943
Elasmosauridae Cope Reference Cope1869
Aristonectinae O’Keefe & Street Reference O’Keefe and Street2009 (sensu Otero et al. Reference Otero, Soto-Acuña and Rubilar-Rogers2012)
Aristonectinae

Fig. 2 Aristonectinae indet. MLP 14-I-20-8. Anterior cervical centrum in a. anterior, b. left lateral, c. dorsal and d. ventral views. Posterior cervical centrum in e. anterior, f. left lateral, g. dorsal and h. ventral views. Pectoral centrum in i. anterior, j. left lateral, k. dorsal and l. ventral views. Dorsal centrum in m. anterior, n. left lateral, o. dorsal and p. ventral views. q. Dorsal neural arch detail. r. Dorsal ribs. s. Gastralia. Scale bar=20 mm. pa=parapophyses, pf=pedicellar facet, vf=ventral foramina.

Fig. 3 Aristonectinae indet. MLP 14-I-20-8. Right scapula in a. dorsal, b. right lateral, c. anterior and d. medial views. Left coracoid in e. dorsal and f. symphyseal views. g. Left coracoid in ventral view. Scale bar=20 mm. dlp=dorsolateral process, mvp=mid-ventral process, rid=ridge, vr=ventral ramus.

Fig. 4 Aristonectinae indet. MLP 14-I-20-8. a. Ischia in dorsal? views. Pubis in b. dorsal and c. ventral views. Left humerus in d. dorsal, e. proximal, f. ventral, g. posterior and h. anterior. Left femur in i. ventral, j. proximal, k. dorsal, l. posterior and m. anterior. Scale bar=20 mm. cap=capitulum, tro=trochanter, tub=tuberosity.
Material
MLP 14-I-20-8 is a postcranial skeleton comprising two cervical, one pectoral and seven dorsal vertebrae, both coracoids, scapulae, pubes, ischia, humeri, femora dorsal ribs and gastralia. The palaeohistological samples were taken from the middle shaft of the right humerus (Fig. 5a).

Fig. 5 Histology and microstructure of MLP 14-I-20-8. a. Right humerus, black line indicates the approximate position of the thin section. Scale bar=2 cm. b. General view of the thin section. Scale bar=2 cm. c. General view of the medullar region showing abundant calcified cartilage with some erosion cavities, and deposition of endosteal bone. Scale bar=0.5 cm. d. The medullar and cortical tissues are separated by a Kastschenko’s line. Scale bar=1.75 cm. e. General view of the cortical bone showing abundant vascular canals. Scale bar=0.75 cm. Normal light. CC=calcified cartilage, EB=endosteal bone, FB=fibrolamellar bone, KL=Kastschenko’s line, PB=primary bone, VC=vascular canals.
Locality and horizon
Seymour Island (64°16'53.7''S, 56°45'21.00''W) James Ross Archipelago, Antarctic Peninsula. López de Bertodano Formation, limit between Klb 8 and 9, Upper Maastrichtian (Fig. 1d) (Macellari Reference Macellari1988).
Results
Morphological description
The MLP 14-I-20-8 was collected within an area of 1.2 m2. Only the left femur was displaced, probably by recent weathering.
Axial skeleton
Only two cervical centra are preserved and, as in the other vertebrae, they do not have the neural arches associated with the centra. Both centra are broader than high and higher than long (Table I). The pedicellar facets are rectangular shaped (Fig. 2c & g). The floor of the neural channel of the larger centra bears two foramina (Fig. 2g). The articular facets are elliptical but the smaller and anterior centra show almost flat dorsal and ventral limits (Fig. 2a). Both preserved cervical centra show a ventrolateral parapophysis (Fig. 2a & f). The ventral surface is flat in the smaller centrum and slightly transversely convex in the larger cervical centrum. Both centra show two ventral foramina separated by a rounded transversely broad ‘ventral keel’ (Fig. 2d & h) that is 29% anterior and 41% posterior of the centra transverse width.
Table I Aristonectinae indet. (MLP 14-I-20-8) measurements of vertebral centra (length (L), height (H), breadth (B) in mm) and height index (HI, 100 × H/L), breadth index (BI, 100 × B/L), breadth-height index (BHI, 100 × B/H) and vertebral length index (VLI, L/(0.5 × (H+B))). c=cervical, p=pectoral and d=dorsal.

One putative pectoral vertebra is preserved. It differs from the cervical centra by the inclined pedicellar facets and the absence of independent parapophysis (Fig. 2i & j). In general proportions, it is similar to the posterior-most cervical preserved. The ventral surface is pierced by two widely spaced foramina (Fig. 2l).
Seven dorsal centra are preserved, none of which have the neural arch and centra articulated. All dorsal centra are broader than high and higher than long (Table I). The pedicellar facets are mostly square in outline and only slightly laterally inclined (Fig. 2m). The articular faces are almost flat and elliptical in shape but with their dorsal areas divided into three parts: the two laterals correspond to the pedicellar facets and the medial third to the base of the neural canal (Fig. 2m). The lateral sides of the centra are slightly concave anteroposteriorly (Fig. 2n & o). Ventrally, two to four circular foramina are observed (Fig. 2p).
The dorsal neural arches are stocky elements with rounded pedicels and almost cylindrical diapophysis (Fig. 2q). The neural spines are short with a posterior rounded keel.
The dorsal ribs have a circular or elliptical cross-section, with a slightly concave proximal end (Fig. 2r), whereas the gastralia are shorter and strongly bent with a pointy ended apex (Fig. 2s).
Appendicular skeleton
Both scapulae and coracoids are preserved. The glenoid ramus of the scapula is stocky and it shows a slightly thicker acetabular facet and a smaller, triangular shaped coracoid facet proximally (Fig. 3a–d). However, the limit between both facets is not clear, indicating an extremely immature morphological stage. The typical domes and valleys of the surface observed in adult aristonectines (O’Gorman Reference O’Gorman2013, fig. 6.1.8.D) are absent. The ventral ramus is large and slightly ventromedially directed. The dorsolateral process is anteroposteriorly wide (55 mm) and proximodistally short (32 mm). The dorsal tip of the dorsolateral ramus is located far anteriorly to the distal end of the glenoid ramus (18 mm of 94 mm total length). The anterior margin of the ventral ramus and dorsolateral process is strongly convex (Fig. 3a & d). Ventrally, there is a faint ridge between the ventral ramus and the dorsolateral process (Fig. 3b).
The coracoid has the typical shape observed in elasmosaurids. The glenoid ramus bears well-defined scapular and glenoid facets (Fig. 3g). The anterior margin, between the glenoid ramus and the anterior process, is slightly concave (Fig. 3g). The symphyseal surface (Fig. 3f) exhibits a mid-ventral process located in the medial margin of the ventral surface (Fig. 3f). There is a rounded posterior ramus distally expanded that in the distal end shows the typical expansion (Fig. 3g). The posterior ramus limits the cordiform fenestra (Fig. 3e & g). The medial anterior and posterior processes are only slightly developed (Fig. 3e & g).
Both pubes are preserved. They are rounded, stocky and broader than long (Fig. 4b & c). The anterior margin is strongly convex without an anterolateral projection (Fig. 4b & c). A developed pelvic bar is absent. Only the right ischium is complete. The ischia have the typical ‘inverted L-shape’ and although the posterior ramus is not elongated it is larger than the anterior ramus (Fig. 4a). The thickest zone corresponds to the acetabulum. The symphyseal margin shows a thicker zone near the anterior margin.
Both humeri and femora are preserved. The humeri are rounded, stocky and dorsoventrally compressed (Fig. 4d–h, Table II). The proximal end forms the capitulum, but the trochanter is not well differentiated (Fig. 4e). The distal end is anteroposteriorly expanded (Fig. 4d & f). The femora are stocky and dorsoventrally compressed, proportionally similar to the humerus but slightly shorter and more gracile (Fig. 41–m, Table II). The proximal end forms the capitulum, but as in the humerus, a trochanter is not differentiated (Fig. 4j). The distal end of the femur is anteroposteriorly expanded, the posterior expansion being larger than the anterior one (Fig. 4i & k).
Table II Aristonectinae indet. (MLP 14-I-20-8) appendicular skeleton measurements.

Histology and microstructure
The whole section has a compact inner organization and no open medullary cavity (Fig. 5b). The medullary region is filled with a calcified cartilaginous core, showing remodelling processes with some erosion cavities and deposition of endosteal bone (Fig. 5c). These tissues are separated from the rest of the cortex by a distinct change in organization and a Kastschenko’s line (Fig. 5d). The cortex is entirely formed by fibrolamellar bone tissue consisting of a woven fibred matrix, isotropic under crossed polarized light (Fig. 5e). The cortical woven fibred bone is highly vascularized by simple radial and longitudinal vascular canals. The inner cortex shows osteocyte lacunae, irregularly shaped and randomly oriented with a few canalicular channels close to the medullary region that become more abundant near the outer cortex (Fig. 5e). The outer cortex, separated from the inner cortex by a distinct change in bone tissue, probably due to diagenetic alteration, contains a few osteocyte lacunae with a few canalicular channels. Bone tissues of all sections are characterized by the lack of primary or secondary osteons. Growth marks (annuli or lines of arrested growth) are also absent in the cortical bone.
Calculation
Body length
As many elements as possible were considered for the body length comparison between MLP 14-I-20-8 and SGO PV 260, including posterior cervical vertebrae, coracoid and ischia (Tables I, II & III). The SGO PV 260 body length is estimated to be between 5.5–6 m (Otero, personal communication 2015). Table III presents a ratio for the axial length for each element: M/S=MLP specimen measurement/SGO specimen measurement. A maximum and minimum estimation for MLP 14-I-20-8 body length was calculated by (M/S) × 6 m and (M/S) × 5.5 m, respectively. In summary, by comparing the measurements of the three elements, a maximum and minimum estimation of body length (2.16 and 1.41 m, respectively) is obtained.
Table III Aristonectinae indet. (MLP 14-I-20-8) body length estimation based on comparison of elements of MLP 14-I-10-8 and SGO PV 260 (5.5–6 m in length). Ratio M/S=ratio of length of element in MLP 14-I-10-8 over SGO PV 260.

The estimated body length of the adult of A. quiriquinensis is 8 m (Otero et al. Reference Otero, Soto-Acuña, O’Keefe, O’Gorman, Stinnesbeck, Suárez, Rubilar-Rogers, Salazar and Quinzio-Sinn2014), and therefore, the juvenile is 3.7–5.7 m, smaller than the adult form of the SGO PV 260 (A. quiriquinensis) (see Fig. 6)
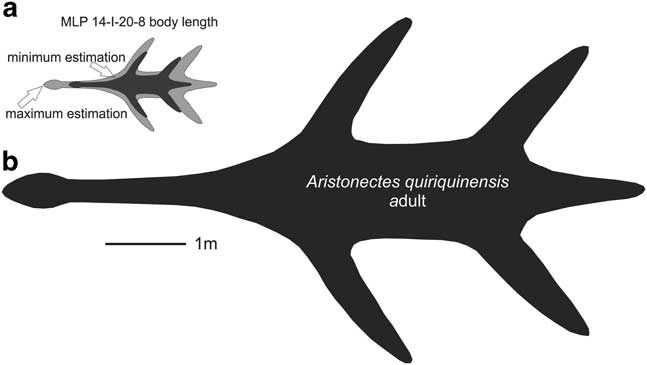
Fig. 6 Body length diagrams. a. Maximum and minimum estimated body length of MLP 14-I-20-8. a. Adult body length of Aristonectes quiriquinensis (SGO PV 957) taken from Otero et al. (Reference Otero, Soto-Acuña, O’Keefe, O’Gorman, Stinnesbeck, Suárez, Rubilar-Rogers, Salazar and Quinzio-Sinn2014).
Discussion
Maturity
According to the size-independent criteria used in plesiosaurs for osteological maturity determination (Brown Reference Brown1981, Otero et al. Reference Otero, Soto-Acuña and Rubilar-Rogers2012, O’Gorman et al. Reference O’Gorman, Gasparini and Salgado2014a), MLP 14-I-20-8 corresponds to a juvenile. Neural arches are not fused with centra, the sutures between the ribs and vertebral centra are not closed, bone edges are mainly rounded and the articular faces of the appendicular bones are poorly defined. Bone microstructure and histological data are consistent with those of the macromorphology and also indicate that MLP 14-I-20-8 was a perinatal individual at death. As this specimen was not found associated with a female, and distinction between unborn and new born is very difficult to assess, we use the term ‘perinatal’ in the sense of Horner et al. (Reference Horner, Padian and de Ricqlès2001). The evidence supporting a perinatal stage of the MLP 14-I-20-8 includes the incomplete endochondral ossification (i.e. retention of a calcified cartilaginous core in the medullary region), the predominance of primary bone tissue without secondary remodelling, the lack of primary or secondary osteons and growth marks in the cortical bone, and the open vascular spaces not surrounded by a thin coat of lamellar bone tissue (this last feature suggests the lack of an early formation of primary osteons). Another significant characteristic is the presence of the Kastschenko’s line (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg and de Ricqlés1990, Ricqlés et al. Reference Ricqlés, Se, Antunes and Taquet2001). In growing long bones this peculiar structure is a thin remaining coat of cartilage matrix that persists in the periphery of the young diaphysis after the condroblasts destroy most of the hypertrophic cartilage to produce the marrow cavity (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg and de Ricqlés1990).
When endosteal and periosteal bones are deposited centripetally and centrifugally, respectively, Kastschenko’s line separates them providing a clear evidence that the first periosteal bone deposit has not yet been reabsorbed by perimedullar osteoclasts (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg and de Ricqlés1990). The bone tissues of MLP 14-I-20-8 are exclusively formed by highly vascularized woven fibred bone, in which the channels within the bone are large and unfilled with deposits of lamellar bone (simple vascular canals) of a woven matrix. This bone pattern is characteristic of embryos and of very fast growing young individuals, especially among higher vertebrates (Francillon-Vieillot et al. Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg and de Ricqlés1990), and it is considered as an unambiguous indication of a high absolute rate (μm day-1) of periosteal accretion (De Buffrénil & Mazin Reference Buffrénil and Mazin1990, Horner et al. Reference Horner, Padian and de Ricqlès2001, Klein et al. Reference Klein, Houssaye, Neenan and Scheyer2015)
Systematic affinities
Although the MLP 14-I-20-8 specimen is incompletely preserved some conclusions about its systematic affinities can be made. MLP 14-I-20-8 has clear elasmosaurid affinities due to the presence of a coracoid with a posterior cordiform fenestra (Carpenter Reference Carpenter1999, Ketchum & Benson Reference Ketchum and Benson2011).
Systematic determination of juvenile elasmosaurids is a complex issue because of the great ontogenetic variation of the osteological features (Brown Reference Brown1981, Carpenter Reference Carpenter1999). Nonetheless, some information about the affinities of MLP 14-I-20-8 can be obtained using bivariate graphs based on cervical central proportions (O’Gorman et al. Reference O’Gorman, Gasparini and Salgado2013, Reference O’Gorman, Gasparini and Salgado2014a, Otero & O’Gorman Reference Otero and O’Gorman2013). The bivariate diagram in Fig. 7 shows a plot of the cervical vertebrae indexes for MLP 14-I-20-8. The proportions of MLP 14-I-20-8 are more similar to those recorded in aristonectines than in juvenile non-aristonectines elasmosaurids. Pending the discovery of additional diagnostic material, we refer MLP 14-I-20-8 to an indeterminate genus and species of Aristonectinae.

Fig. 7 Bivariate diagram (HI–BI) of cervical centrum values of juvenile aristonectine and non-aristonectine elasmosaurids and posterior cervical centrum values of adult Aristonectes sp. and the non-elasmosaurid Vegasaurus molyi (data taken from Welles Reference Welles1943, O’Gorman Reference O’Gorman2013, O’Gorman et al. Reference O’Gorman, Gasparini and Salgado2013, Reference O’Gorman, Gasparini and Salgado2014a, Reference O’Gorman, Olivero, Santillana, Everhart and Reguero2014b, Reference O’Gorman, Salgado, Olivero and Marenssi2015). Black triangles: Aristonectinae indet. specimens CM Zfr 104, MLP 89-III-3-2, MML PV 192, MUC PV 131 and Morturneria seymourensis specimen TTUP 9219, black pentagons: non-aristonectine elasmosaurid specimens AMNH 5261, CIT 2832, MLP 93-XII-20-1, MLP 99-XII-1-8, AM F9630-9928, MLP-86-X-28-(2-6), oval: non-aristonectine elasmosaurid specimen MLP 93-I-5-1 Vegasaurus molyi, green circles: Aristonectes sp. specimen MLP 89-III-3-1. Arrows indicate ontogenetic change in vertebral centrum proportions. Institution abbreviations: AM=Australian Museum, Sydney, Australia, AMNH=American Museum Natural History, New York, USA, CIT=California Institute of Technology, Pasadena, CA, USA, MLP=Museo de La Plata, Buenos Aires Province, Argentina, MML=Museo Municipal de Lamarque, Río Negro Province, Argentina, MUC=Museo de la Universidad del Comahue, Neuquén Province, Argentina, NZGS=Institute of Geological and Nuclear Sciences, Lower Hutt, New Zealand, TTUP=Museum of Texas Tech University, Lubbock, TX, USA.
Change of proportion in elements among aristonectine
Aristonectines show, as do other elasmosaurids, a marked modification of the morphology through the ontogeny (Carpenter Reference Carpenter1999), generating, as was previously mentioned, difficulties in systematic determination of juvenile specimens. However, there have been no attempts to describe this variation systematically.
The ontogenetic changes of the proportions of the cervical vertebrae have been recorded among elasmosaurids, mostly related to vertebral elongation (O’Keefe & Hiller Reference O’Keefe and Hiller2006). Figure 7 shows the HI and BI values of the cervical vertebrae and the proportion of change in the posterior-most cervical vertebrae. This modification involves strong decreases of the HI and less marked decreases of the BI, indicating that the increases in L are related to H and B. However, this trend does not continue, as the posterior cervical vertebrae of adult aristonectines show higher BI values relative to those recorded for non-aristonectine elasmosaurids (Fig. 7, O’Gorman Reference O’Gorman2013).
The scapula of MLP 14-I-20-8 was compared with the scapulae of the MLP 89-III-3-1 specimen identified as Aristonectes sp. (O’Gorman et al. Reference O’Gorman, Olivero, Santillana, Everhart and Reguero2014b). Figure 8 shows that the glenoid ramus is relatively stockier and shorter, differing in the convexity of the dorsolateral ramus from convex sides (juvenile) and parallel sides (adult) in the dorsolateral process. Additionally, it clearly shows the anteroposterior length of the dorsolateral process to be greater in the juvenile than in the adult. The ventrolateral ramus cannot be compared because it is not complete in MLP 89-III-3-1.
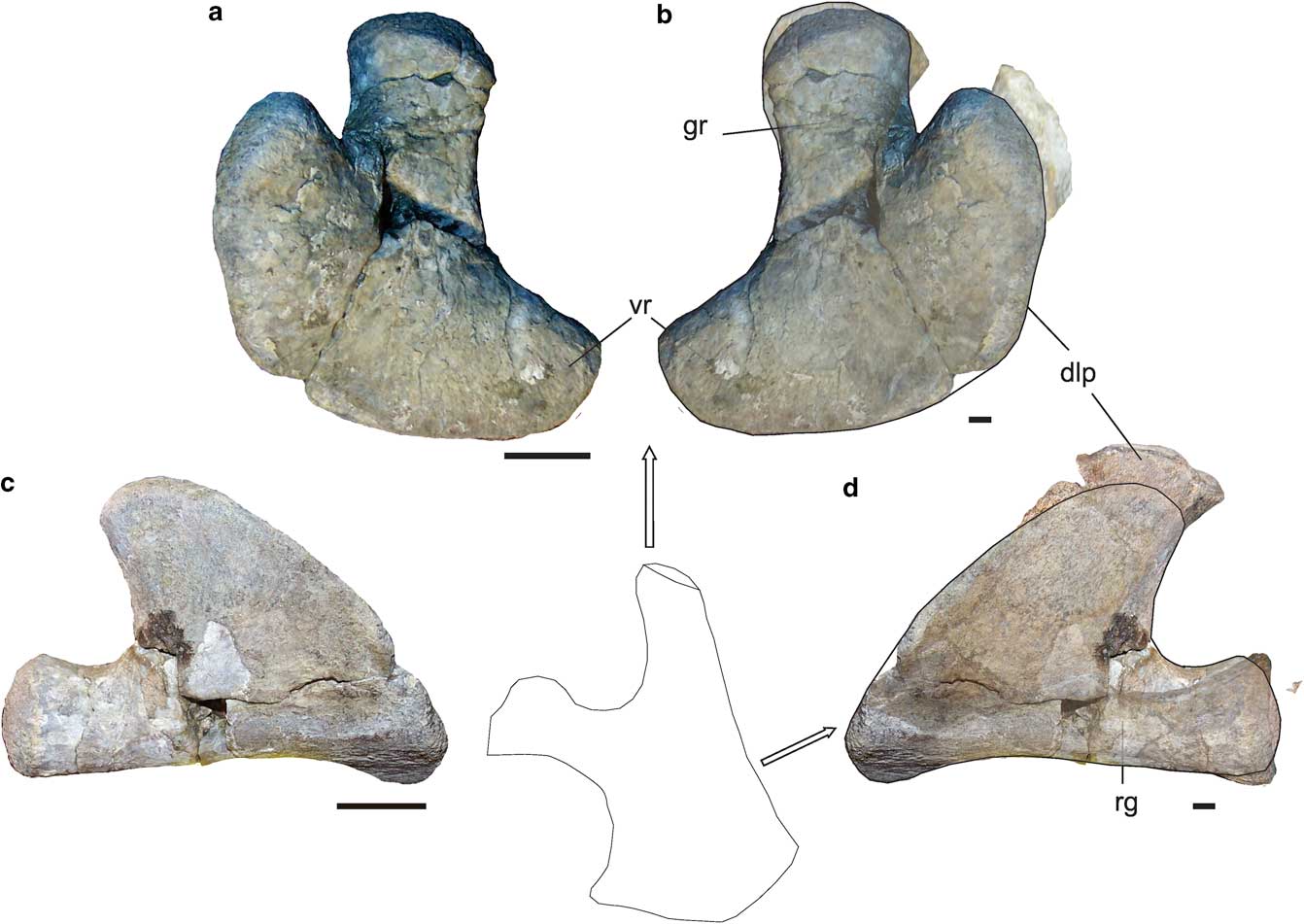
Fig. 8 Comparison between juvenile Aristonectinae indet. (MLP 14-I-20-8) and adult Aristonectes sp. (MLP 89-III-3-1). Right scapula in a. dorsal and c. lateral views. MLP 14-I-20-8 (reversed) and MLP 89-III-3-1 superposed in b. dorsal and d. lateral views. Scale bar=20 mm. dlp=dorsolateral process, gr=glanoid ramus, vr=ventral ramus. Scapula diagram modified from Hiller et al. 2005.
Figure 9 shows the B:L index and the total length of Aristonectinae propodials. The pattern seems to indicate that the ontogenetic change goes through two stages: the first is characterized by decreases in B:L, and the second shows an increase of length and B:L, indicating progressive development of the distal expansion.

Fig. 9 Bivariate diagram B:L and length of several aristonectine propodials. Hollow letters indicate juveniles. Black letters indicate adult specimens (data taken from O’Gorman Reference O’Gorman2013, Otero et al. Reference Otero, Soto-Acuña, O’Keefe, O’Gorman, Stinnesbeck, Suárez, Rubilar-Rogers, Salazar and Quinzio-Sinn2014, Araújo et al. Reference Araújo, Polcyn, Lindgren, Jacobs, Schulp, Mateus, Gonçalves and Morais2015). Italics indicate approximate values.
Baseline model of Aristonectinae ontogeny
The Aristonectinae shows some features that have intrigued palaeontologists since the XIX century, even before the description of Aristonectes. The descriptions of A. parvidens (Cabrera Reference Cabrera1941), Kaiwhekea katiki (Cruickshank & Fordyce Reference Cruickshank and Fordyce2002) and A. quiriquinensis (Otero et al. Reference Otero, Soto-Acuña, O’Keefe, O’Gorman, Stinnesbeck, Suárez, Rubilar-Rogers, Salazar and Quinzio-Sinn2014) provide information about the general morphology of aristonectines. The consequent recognition of the elasmosaurian affinities of aristonectines (Gasparini et al. Reference Gasparini, Bardet, Martin and Fernández2003, Ketchum & Benson Reference Ketchum and Benson2011, Otero et al. Reference Otero, Soto-Acuña and Rubilar-Rogers2012, O’Gorman et al. Reference O’Gorman, Gasparini and Salgado2013) allows us to compare the derived aristonectine morphology with the non-aristonectine morphology of the classic elasmosaurids. Several reasons, such as cervical morphology characterized by relatively short and wide centra (Cruickshank & Fordyce Reference Cruickshank and Fordyce2002, Gasparini et al. Reference Gasparini, Bardet, Martin and Fernández2003) and a relatively large body size of osteologically immature (juvenile) specimens indicate possible participation of pedomorphic processes during the evolution of the aristonectine morphology (O’Gorman Reference O’Gorman2013, O’Gorman et al. Reference O’Gorman, Gasparini and Salgado2014a). Additionally, palaeohistological evidence recorded by Araújo et al. (Reference Araújo, Polcyn, Lindgren, Jacobs, Schulp, Mateus, Gonçalves and Morais2015) indicates adult or sub-adult features in osteologically immature specimens. The MLP 14-I-20-8 specimen described here represents an earlier stage before remodelling, consistent with the ‘perinatal’ stage, and therefore, younger than that recorded by Araújo et al. (Reference Araújo, Polcyn, Lindgren, Jacobs, Schulp, Mateus, Gonçalves and Morais2015), enabling future comparative work with juvenile non-aristonectine elasmosaurids.
Finally, the specimen and the perinatal condition are consistent with the hypothesis that the Antarctic Peninsula was a breeding area for marine reptiles (Martin et al. Reference Martin, Sawyer, Reguero and Case2007) during the last part of the Cretaceous.
Acknowledgements
The authors thank the IAA (Instituto Antártico Argentino) and Fuerza Aérea Argentina for support in the field, Juan José Moly for help with the extraction of the specimen, Marcelo Reguero for allowing the study of the material, and P. Arregui for improvement of the English in this article. The authors would also like to thank E. Fordyce. This research was supported by PIP 0433, UNLP N 607, and PICT 2012-0748, PICT 2013-0663 and PIUNRN 40-A-404. The authors are also grateful to the reviewers for their comments on the manuscript.
Author contribution
José Patricio O’Gorman analysed the morphological data and wrote the paper. Marianella Talevi analysed the palaeohistological data and wrote the paper. Marta Fernández wrote the paper.















