Introduction
Deception Island (62°43′S, 60°57′W) is the most-westerly subaerial Quaternary active volcano of a series of subaerial and submarine volcanoes situated in Bransfield Basin between the Antarctic Peninsula and the South Shetland Islands (Fig. 1a & b). The recent tectonics of the Bransfield Basin is still controversial. It is currently explained by two different models: 1) the opening of the basin may be related to passive subduction of the former Phoenix Plate and rollback of the South Shetland Trench, or 2) the sinistral movement between the Antarctic and Scotia plates may be causing oblique extension thus generating the Bransfield Basin along the continental margin of Antarctic Peninsula (Maestro et al. Reference Maestro, Somoza, Rey, Martinez-Frias and Lopez-Martinez2007, and references therein).

Fig. 1. a. A map showing the location of Antarctica. The boxed area is enlarged in b. It shows Bransfield Strait which lies between the South Shetland Islands and Antarctic Peninsula (modified from Rey et al. Reference Rey, Somoza and Martínez-Frías1995). Deception Island is located at the western end of Bransfield Strait. The South Shetland Trench was created by subduction of the former Phoenix plate. c. Localities of samples used in this study (map simplified from Smellie & Lopez-Martinez Reference Smellie and López-Martínez2000). Fumarolic and bubbling gases were collected from Fumarole Bay and Telefon Bay, respectively, as shown by solid circles. Hot spring waters were collected from Fumarole Bay and Whalers Bay as shown by open squares. Surface waters (crater lake water and glacier meltwater) were collected from the points shown by crosses.
There are no systematic changes in lava composition along the central Bransfield Basin. All of the subaerial and submarine lavas show geochemical features indicating a contribution of the subduction components from the former Phoenix Plate (Keller et al. Reference Keller, Fisk, Smellie, Strelin, Lawver and White2002). The most MORB-like basalts with the least subduction components occur at both ends of the basin (Fretzdorff et al. Reference Fretzdorff, Worthington, Haase, Hekinian, Franz, Keller and Stoffers2004). It is probable that the mantle was contaminated during subduction of the former Phoenix Plate. The Bransfield Basin is thus thought to be an immature back-arc basin which appears to be at the transition from rifting to spreading. The volcanism there is believed to have occurred at the incipient spreading axis of the basin under extensional tectonics (Keller et al. Reference Keller, Fisk, Smellie, Strelin, Lawver and White2002, Lee et al. Reference Lee, Lee, Choe and Park2008). Submarine hydrothermal activity has also been reported in some of the ridge crests of Bransfield Basin (e.g. Klinkhammer et al. Reference Klinkhammer, Chin, Keller, Dahlmann, Sahling, Sarthou, Petersen, Smith and Wilson2001).
The rocks of the island are dominated by basalts and basaltic andesites but there is a minor proportion of evolved rocks (Smellie Reference Smellie2001). Deception Island is a horseshoe-shaped flooded caldera which has experienced frequent volcanic eruptions during the 19th century, the last one was in 1970 (Smellie & Lopez-Martinez 2000). There are several subaerial fumaroles and underwater degassing sites in Deception Island (Smellie & Lopez-Martinez 2000, Somoza et al. Reference Somoza, Martinez-Frias, Smellie, Rey and Maestro2004). Hydrothermal activity on the island has been documented by Rey et al. (Reference Rey, Somoza and Martínez-Frías1995) and Somoza et al. (Reference Somoza, Martinez-Frias, Smellie, Rey and Maestro2004). Based on a detailed acoustic survey, they revealed three different hydrothermal structures on the Port Foster seafloor; low-mounds, high-mounds and a spire. These structures were produced by sediment volcanism and seeps driven by high-temperature pore fluids related to the recent volcanic eruption of the island (Somoza et al. Reference Somoza, Martinez-Frias, Smellie, Rey and Maestro2004). From these studies, the Deception Island caldera can be said to be a site of high hydrothermal activity and strong magmatic degassing. In the present study, we report new noble gas and stable isotopic and chemical data obtained following sampling of thermal fluids from Deception Island.
Samples and analytical methods
Sampling was carried out in December 2006. The fumaroles from Fumarole Bay are currently the most active since the gas temperatures are as high as 97.8°C. The fumarolic gas was collected using a “Giggenbach bottle”, an evacuated glass bottle half-filled with concentrated KOH solution. Gases in the headspace of the bottle were analysed with gas chromatography. Chemical species soluble in KOH solution, such as CO2 and H2S, were determined using the wet chemical methods. The fumarolic gas was also collected using a lead-glass bottle with stopcocks at both ends for noble gas analysis. The use of lead-glass bottles prevents diffusive loss of helium during sample storage. We observed very strong gas bubbling at several places around and inside Port Foster, although gas temperature seemed low (atmospheric). We collected such low temperature, bubbling gas from a pond in Telefon Bay using a lead-glass bottle. Air in the bottle was completely replaced by the gas, and was tightly sealed for later laboratory analysis. Hot spring waters were sampled for chemical, stable isotopic and noble gas analyses from steaming ground on the beach of Fumarole Bay and Whalers Bay (Fig. 1b). The samples were collected in a hole of 10–20 cm deep on the beach. A lead-glass bottle was again used for the analysis of dissolved noble gases. Temperatures there were found to be as high as 60°C. We also collected crater lake waters and glacier meltwaters near Fumarole Bay and Telefon Bay to characterize hydrogen and oxygen isotopic features of the surface waters of the island.
The cation composition of hot spring waters was determined with atomic absorption spectrometry for Na+ and K+, and with inductively coupled plasma (ICP) spectrometry for Mg2+, Ca2+, Fe2+ Mn2+ and SiO2. The anion composition of hot spring waters was determined with ion chromatography. Carbon isotopic ratios of CO2 from fumarolic and bubbling gases were determined using a stable isotope mass spectrometer. The hydrogen isotopic ratios of waters were analysed with a Zn reduction method, while oxygen ratios were analysed using an automated H2O–CO2 exchange method. The sulphur isotopic ratio of SO42- dissolved in the hot spring waters was also analysed using SO2 as an analytical gas. Stable isotopic ratios are expressed in a conventional δ-notation using VSMOW, VPDB and Canyon Diablo troilite as the standard materials for hydrogen, oxygen, carbon and sulphur isotopic ratios respectively. Noble gas elemental and isotopic compositions were determined for fumarolic gases, bubbling gases and hot spring water. Noble gases were measured with a modified VG5400 (MS-III) mass spectrometer at the University of Tokyo.
Results
Table I shows the chemical analysis of fumarolic gas from Fumarole Bay and bubbling gas from Telefon Bay. The rate of fumarolic gas emission was highest at high tides. Hence the gas concentrations varied due to addition evaporating seawater at the time of sample collection. If H2O is excluded, then the most abundant gaseous components were CO2 (8~14 vol.%) followed by N2 (~0.8 vol.%) and H2S (0.05~0.11 vol.%). SO2 was found at only very low concentrations, i.e. 0.0010~0.0025 vol.%. The content of O2 was generally low (< 0.025 vol.%), indicating that contamination by air during sampling was small. Our gas analysis is consistent with high concentrations of CO2 (75~90 vol.%) and H2S (0.3~0.9 vol.%) reported by Valentin et al. (Reference Valentin, Martini and Diez-Gil1989) (cited in Somoza et al. Reference Somoza, Martinez-Frias, Smellie, Rey and Maestro2004), but their values cannot be directly compared with ours because they did not report H2O concentration. Of the minor components, H2 and He concentrations were 0.22 vol.% and from 12 to 16 ppmv, respectively. Ortiz et al. (Reference Ortiz, Valentin and Grimalt1987) (cited in Rey et al. Reference Rey, Somoza and Martínez-Frías1995) reported wider concentration ranges of these gases, i.e. 100~660 ppmv for H2 and 10~190 ppmv for He. The bubbling gas from Telefon Bay consisted mostly of CO2 with minor concentrations of the other gases, although we did not analyse components other than N2, Ar and He. Concentrations of these gases were very low, indicating air contamination during sampling was kept to a minimum.
Table I. Chemical and isotopic compositions of fumarolic and bubbling gases from Fumarole Bay and Telefon Bay, Deception Island.

n.m. = not measured, n.a. = not analysed
The following results are the first detailed noble gas analyses for hydrothermal fluids obtained from Deception Island. Elemental and isotopic analyses of noble gases are given in Table II as a) elemental composition of He, Ne, Ar, Kr and Xe, b) isotopic ratios of He, Ne and Ar, and c) isotopic composition of Xe. Analytical accuracies of elemental concentrations are about 10%, and analytical errors of isotopic ratios are given in 1σ. Helium isotopic ratios are close to but slightly lower those of the MORB-type He, suggesting predominant contribution of He of mantle origin. Neon isotopic ratios of gas and water samples from Fumarole Bay (DCF-5, DCF-6 and DCF-22 (see Table II for sample names.)) are atmospheric. Gas samples DCF-17 and DCF-18 from Telefon Bay, however, show 20Ne/22Ne and 21Ne/22Ne ratios slightly lower than the atmospheric values by 3.2 ± 0.8% (2σ) and 3.2 ± 1.6% (2σ) for DCF-17, and 2.1 ± 0.8% (2σ) and 1.5 ± 0.8% (2σ) for DCF-18. The low values may have been caused by mass fractionation that favours enrichment in heavier isotopes during transport of Ne from atmosphere to the bubbling gases. 38Ar/36Ar and Xe isotopic ratios agree with the atmospheric values. Small enrichment in 40Ar is observed in all the gas samples, which indicates contribution of Ar with high 40Ar/36Ar from mantle or crustal rocks beneath the island.
Table II. Elemental and isotopic compositions of noble gases of thermal fluids from Deception Island.

§ FB = Fumarole Bay, TB = Telefon Bay.
# Concentrations in water are given in cc/g water.
* Ozima & Podosek 2002.
** Kipfer et al. 2002.
Note: Number in parenthesis denotes the analytical error (1σ) corresponding to the last digit(s) of each analysis.
Chemical analyses of hot spring waters from Fumarole Bay and Whalers Bay are shown in Table III. The seawater contribution to these hot spring waters is high as exemplified by high concentrations of major cations and anions, i.e. Na+, K+, Ca2+, Mg2+, Cl- and SO42-, and by hydrogen, oxygen and sulphur isotopic ratios. However, Mn was highly enriched compared with seawater. Significant enrichment of SiO2 was also observed. Our observations suggest that these components were extracted from underlying volcanic rocks during water-rock interaction.
Table III. Chemical and isotopic compositions of hot spring waters from Fumarole Bay and Whalers Bay, Deception Island.

* CRC Handbook of Chemistry and Physics, 74th Edition (Lide 1993–1994).
** Estimated from Hydrographic Atlas of the World Ocean Circulation Experiment (WOCE) (Orsi & Whitworth Reference Orsi and Whitworth2005).
Discussion
Source of volatiles
Relative abundance of N2, He and Ar
The relative abundance of N2, He and Ar of volcanic and geothermal gases has been used to classify their sources (Giggenbach Reference Giggenbach and Barnes1997). These abundances have been calculated for Deception Island gases from the data in Table I and plotted in Fig. 2. Fumarolic gases from Fumarole Bay (DCF-5 and DCF-6) plot above a triangular area for mantle derived basaltic gases mixed with air or air dissolved in water (ADW). Bubbling gases from Telefon Bay (DCF-17 and DCF-18) plot close to air and ADW, indicating a large contribution of atmospheric components, although the atmospheric contamination of He to the observed He is less than 1% for these samples. Figure 2 suggests that the Fumarole Bay fumarolic gases are mixtures of mantle derived basaltic gases and gases from arc-type magma which are believed to be relatively enriched in N2. Enrichment of N2 in andesitic gases may be due to the contribution of nitrogen from sediments carried by a subducting slab beneath the arc systems (Matsuo et al. Reference Matsuo, Suzuki and Mizutani1978, Kita et al. Reference Kita, Nitta, Nagao, Taguchi and Koga1993). Thus the mantle beneath the Bransfield basin is suggested to contain some subducted components from the former Phoenix Plate.

Fig. 2. Relative N2-He-Ar composition of gases from Fumarole Bay (DCF-5 and DCF-6) and Telefon Bay (DCF-17 and DCF-18). Refer to Giggenbach (Reference Giggenbach and Barnes1997) for the areas for the mantle derived basaltic gases and andesitic gases.
Elemental abundance of noble gases
The concentrations of noble gases in Fumarole Bay gas samples (DCF-5 and DCF-6) and in Telefon Bay gas samples (DCF-17 and DCF-18) in Table II are plotted in Fig. 3a. The concentrations of Ar, Kr and Xe of the four gas samples are almost identical, whereas He and Ne concentrations of gases from Fumarolic Bay are significantly higher than those of the Telefon Bay gases. This suggests that magmatic He appears to be more effectively transported to the surface in the Fumarole Bay area compared to the Telefon Bay area. The concentrations of noble gases in the Fumarole Bay hot spring water (DCF-22) are plotted in Fig. 3b along with those for air dissolved in pure water (ADW) at 0° and 50°C for comparison. Concentrations of all noble gases except for He in the water sample are clearly lower than those saturated in water. The 20Ne, 36Ar, 84Kr and 132Xe concentrations in the DCF-22 water sample are lower than those in ADW at 50°C by factors 0.07, 0.31, 0.37, 0.52, respectively. The low concentrations may have resulted from partial removal of heavy noble gases from water phase when it vaporized, because noble gases tend to be preferentially partitioned into vapour phase (e.g. Ballentine et al. Reference Ballentine, Burgess, Marty, Porcelli, Ballentine and Wieler2002 and references therein).

Fig. 3. a. Abundance pattern of noble gases in the Fumarole Bay fumarolic gases (DCF-5 and DCF-6) and Telefon Bay bubbling gases (DCF-17 and DCF-18). b. Abundance pattern of noble gases dissolved in the Fumarole Bay hot spring water (DCF-22), and in water saturated by air (ADW) at 0°and 50°C (Kipfer et al. Reference Kipfer, Aeschbah-Hertig, Peeters, Stute, Porcelli, Ballentine and Wieler2002).
The higher concentration of He compared to the ADW may be due to a high supply of mantle He in the source region of the Fumarole Bay hydrothermal system. Relative elemental abundance patterns of the noble gases, normalized to air composition, are shown in Fig. 4 as a function of F(m). F(m) is defined as (mX/36Ar)sample/(mX/36Ar)air, where mX stands for 4He, 20Ne, 36Ar, 84Kr or 132Xe (Ozima & Alexander Reference Ozima and Alexander1976). The abundance patterns of ADW at 0°C and 50°C (Kipfer et al. Reference Kipfer, Aeschbah-Hertig, Peeters, Stute, Porcelli, Ballentine and Wieler2002) are also shown for comparison. The general features of Fig. 4 are: 1) thermal fluids from Deception Island are all enriched in He compared to ADW. Fumarolic gas is most enriched in He compared to ADW by approximately three orders of magnitude, bubbling gas by 20~30 times more than ADW, and gas dissolved in hot spring water by ~10 times more than ADW, 2) the thermal fluids from Deception Island are depleted in Ne relative to ADW, and 3) a significant enrichment of Xe compared to ADW is seen. Enrichment of He suggests the presence of He derived from magma, as supported by high 3He/4He ratios as will be discussed later. Other noble gases were probably derived from the atmosphere through groundwater and/or seawater, although a small contribution of radiogenic 40Ar from magma is recognizable as mentioned above. Depletion of Ne and enrichment of Kr and Xe relative to ADW may have resulted from elemental fractionation. The progressive enrichment in heavier noble gases may indicate the contribution from recycled noble gases trapped in subducted sediments (e.g. Fukumoto et al. Reference Fukumoto, Nagao and Matsuda1986, Ozima & Podosek Reference Ozima and Podosek2002). This is consistent with the N2 enrichment shown in Fig. 2.

Fig. 4. Noble gas abundance patterns of the fumarolic and bubbling gases from Deception Island relative to air composition. The relative abundance is expressed in terms of F(m) defined as F(m) = (mX/36Ar)sample/(mX/36Ar)air, where mX stands for 4He, 20Ne, 36Ar, 84Kr or 132Xe. FB and TB refer to Fumarole Bay and Telefon Bay, respectively.
3He/4He ratio
The 3He/4He values in deep seawater of the Bransfield Strait have been found to exceed the atmospheric value by up to ~7% (Schlosser et al. Reference Schlosser, Suess, Bayer and Rhein1988). Schlosser et al. interpreted this excess to result from addition of 3He into the deep waters of the Bransfield Strait. The estimated 3He/4He ratio of the added helium of 2.4-5.0 × 10-6 is lower than the ratio of mantle helium. Schlosser et al. suggested that the added helium contains radiogenic component from continental crust underlying the Bransfield Strait. In Deception Island, recent measurement of 3He/4He ratios in the Port Foster seawater clearly indicates an injection of deep magmatic helium (Park et al. Reference Park, Dziak, Matsumoto, Bohnenstiehl, Haxel and Lee2007). The Port Foster deep water at ~135 m has 3He/4He values as high as 3.5 × 10-6, 2.5 times higher than atmospheric. Park et al. (Reference Park, Dziak, Matsumoto, Bohnenstiehl, Haxel and Lee2007) discussed that the high 3He/4He ratio could be attributed to the venting of hot (> 200°C) hydrothermal fluids from the sea floor. Since hydrothermal activity is ubiquitous in and around Port Foster, we expect to find magmatic He uncontaminated by He dissolved in seawater in fumarolic gases. This may represent the end member He of the hydrothermal fluids discharging from the sea floor.
The 3He/4He ratios from thermal fluids from Deception Island listed in Table IIb show consistently high values of 9.37 × 10-6 to 9.78 × 10-6, whereas the 20Ne/22Ne, 21Ne/22Ne and 38Ar/36Ar ratios are very close to atmospheric values. The 40Ar/36Ar ratios are only slightly higher than atmospheric, implying addition of a minute amount of magmatic Ar. The high 3He/4He ratios suggest that the thermal fluids from Deception Island contain He of magmatic origin. The 4He/20Ne ratios are considered a good indicator of atmospheric contamination (Torgersen & Jenkins Reference Torgersen and Jenkins1982). The highest observed 4He/20Ne ratio of fumarolic gas from Fumarole Bay (DCF-5) was 1320, and about 4000 times higher than the atmospheric 4He/20Ne ratio of 0.32 (Table IIb). The 4He/20Ne ratios of bubbling gas from Telefon Bay (DCF-17) and from Fumarole Bay hot spring water (DCF-22) were 102 and 33, respectively. These values are 320 to 100 times higher than the atmospheric 4He/20Ne ratio. These observations suggest that these gases derive from magma, since magmatic gases are characterized by high 4He/20Ne ratios. In Fig. 5, the 3He/4He ratios are plotted against 4He/20Ne ratios for the samples under consideration. The mixing curves in Fig. 5 were drawn assuming that the atmospheric component of 3He/4He is 1.4 × 10-6 and 4He/20Ne is 0.32, the MORB source mantle component is 11.5 × 10-6 and 4He/20Ne is 40 000 (Aka et al. Reference Aka, Kusakabe, Nagao and Tanyileke2001), and the crustal radiogenic component of 3He/4He is 1.0 × 10-8 and 4He/20Ne is 100 000 (Ballentine & Burnard Reference Ballentine, Burnard, Porcelli, Ballentine and Wieler2002). In the 3He/4He-4He/20Ne space (Fig. 5), the Deception Island fluids plot slightly below the MORB-atmosphere mixing line and lie nicely on a mixing curve between a magmatic component end-member characterized by a high 3He/4He of ~9.8 × 10-6 and 4He/20Ne ratio greater than 1000, and the atmospheric end-member. The 3He/4He ratios of ~9.8 × 10-6 for Deception Island are slightly lower than those of MORB glasses from various areas (e.g. Graham Reference Graham, Porcelli, Ballentine and Wieler2002). Our preliminary 3He/4He analysis of gas extracted from rocks and minerals from the island is consistent with the above gas values in that the rock data never exceed 10 × 10-6 (K. Nagao, personal communication 2008)

Fig. 5. 3He/4He vs. 4He/20Ne plot of the Fumarole Bay fumarolic gases (DCF-5 and DCF-6), Telefon Bay bubbling gases (DCF-17 and DCF-18) and gases dissolved in the Fumarole Bay hot spring water (DCF-22). Mixing curves between air and gases directly derived from MORB-source mantle (3He/4He = 11.5 × 10-6) and between air and crustal rocks (3He/4He = 0.01 × 10-6) are shown. See text for the end-member compositions. The Deception Island gases plot slightly below the MORB-Air mixing curve with the end-member 3He/4He ratio of 9.8 × 10-6.
The MORB values are known to be homogeneous; 11.3 × 10-6 for Atlantic MORB, 11.4 × 10-6 for Pacific MORB, and 11.5 × 10-6 for Indian MORB (median values, Graham Reference Graham, Porcelli, Ballentine and Wieler2002). However, the 3He/4He ratios of back-arc basin volcanic rocks are highly variable. Although the mean data is close to the MORB values, the individual values are highly variable even in a given back-arc basin (Hilton et al. Reference Hilton, Fischer, Marty, Porcelli, Ballentine and Wieler2002). For example, the highest value of 30.9 × 10-6 and the lowest value of 1.2 × 10-6 were documented at Lau Basin (Poreda & Craig Reference Poreda and Craig1992). A large variation was also found at Manus Basin (Macpherson et al. Reference Macpherson, Hilton, Sinton, Poreda and & Craig1998). The high values have been interpreted to be related to the nearby hotspots. The 3He/4He ratios of back-arc basin volcanics that are lower than those of MORB may result from the situations where 1) the mantle wedge is depleted in helium, so radiogenic helium is accentuated, 2) the mantle wedge is enriched in U and Th derived from the subducted slab, 3) the magmas acquired crustal radiogenic helium during storage and/or ascent (Hilton et al. Reference Hilton, Fischer, Marty, Porcelli, Ballentine and Wieler2002), or 4) the mantle wedge contains enriched components related to a mantle plume. It is difficult to constrain the geochemical features of the mantle beneath the Bransfield back-arc basin on the basis of He isotopic variations alone. The geochemical characteristics of the mantle beneath Bransfield basin may be estimated from the analysis of trace element geochemistry of volcanic rocks collected from the area. High field strength elements such as Zr, Nb and Yb have been considered good geochemical indicators to characterize the slab influence on the wedge mantle, because they have low mobility in fluids and low concentration in sediments. We followed the approach of Pearce et al. (Reference Pearce, Baker, Harvey and Luff1995) which has been applied to estimate the subduction input to the wedge mantle beneath the South Shetland Islands by Lee et al. (Reference Lee, Lee, Choe and Park2008). In Fig. 6 we plot Xi/Yb against Nb/Yb where Xi denotes an incompatible element under consideration. Since neither Nb nor Yb are mobile during fluid formation from the subducting slab, any increment of the Xi/Yb ratio relative to mantle-derived oceanic basalts or “mantle array” could be interpreted to indicate the addition of Xi from the slab to the wedge mantle. The trace element data of volcanic rocks collected from the Bransfield back-arc basin have been obtained by Keller et al. (Reference Keller, Fisk, Smellie, Strelin, Lawver and White2002) and used for the construction of Fig. 6. The data for the mantle array which represents the trace element composition of MORB samples from the fossilized spreading centre of the Antarctic-Phoenix Ridge are from Choe et al. (Reference Choe, Lee, Lee, Hur and Jin2007). As seen in Fig. 6a, the Zr/Yb ratios plot in the mantle array as expected, suggesting that Zr and Yb are immobile and the abundance of Zr and Yb in the slab is low. On the other hand, approximately half of Sr/Yb, U/Yb and Pb/Yb ratios plot above the mantle array (Fig. 6b–d). Although it is not included in Fig. 6, the Th/Yb ratios plot is similar to the Sr/Yb, U/Yb and Pb/Yb ratios. Since Sr and Pb will be strongly partitioned into fluids relative to crystalline phases, it is conceivable that the above-mentioned elements (Sr, Pb, U and Th) contaminated the wedge mantle beneath the Bransfield basin in the form of fluid derived from the slab that carried altered oceanic crust or sediments. This interpretation is consistent with the geochemistry of Bransfield back-arc volcanics (Keller et al. Reference Keller, Fisk, Smellie, Strelin, Lawver and White2002). Keller et al. suggested that rocks from the south-west end of the Bransfield rift are almost MORB-like but enriched in Cs and Pb. The Pb enrichment was interpreted to have been derived from metalliferous sediments and fluids from a subducting slab underneath the Bransfield basin. A similar interpretation has been given to the trace element geochemistry of arc volcanics from South Shetland Islands (Kraus Reference Kraus2005, Lee et al. Reference Lee, Lee, Choe and Park2008). These observations are consistent with N2/He ratios of Fumarole Bay fumarolic gas that are higher than those of typical mantle-derived basaltic gases (Fig. 2). From the above discussion, we believe that the end member helium with the 3He/4He value of ~9.8 × 10-6 from the Deception Island hydrothermal fluids has been derived from magma generated in a mantle wedge that was influenced by subducted components from the former Phoenix Plate.

Fig. 6. Incompatible element ratios for volcanic rocks of the Bransfield Strait back-arc basin. a. Zr/Yb-Nb/Yb, b. Sr/Yb-Nb/Yb, c. U/Yb-Nb/Yb, and d. Pb/Yb-Nb/Yb. Data were taken from Keller et al. (2002). The shaded area indicates the range of incompatible element ratios in MORB from the fossilized spreading centre of the Antarctic-Phoenix Ridge (Choe et al. Reference Choe, Lee, Lee, Hur and Jin2007).
Carbon dioxide in the fumarolic and bubbling gases from Deception Island has a limited range of δ13C values of -6.3 to -5.0 ‰ (Table I). These values are typical for mantle carbon (e.g. Taylor Reference Taylor1986) and indicate that CO2 is also of mantle origin and that the CO2 contribution from sedimentary and limestone sources is small. CO2/3He ratios coupled with δ13C values have been used as an indicator to constrain the source of CO2 and He in volcanic fluids (e.g. Marty & Jambon Reference Marty and Jambon1987). Based on the CO2/3He versus δ13C relationship Sano & Marty (Reference Sano and Marty1995) evaluated the relative proportion of carbon sources in volcanic and hydrothermal fluids from subduction zones under the assumptions that the carbon sources were MORB-source mantle, marine carbonate and sedimentary organic materials in subducting slab. The end-member values used for the estimation are CO2/3He = 1.5 × 109 and δ13C = -6.5 ‰ for the MORB-source mantle, CO2/3He = 1 × 1013 and δ13C = 0 ‰ for the marine carbonate, and CO2/3He = 1 × 1013 and δ13C = -30 ‰ for the sedimentary carbon. In the case of Japanese volcanic gases the major carbon contributor was subducted marine carbonate and the mantle source was up to 20% (Sano & Marty Reference Sano and Marty1995). A similar conclusion has been obtained for the Lesser Antilles island arc geothermal fluids (van Soest et al. Reference van Soest, Hilton and Kreulen1998). Basalts and hydrothermal fluids from back-arc basins such as Lau Basin (Hilton et al. Reference Hilton, Hammerschmidt, Loock and Friedrichsen1993), Mid-Okinawa Trough (Ishibashi et al. Reference Ishibashi, Sano, Wakita, Gamo, Tsutsumi and Sakai1995), North Fiji Basin (Nishio et al. Reference Nishio, Sasaki, Gamo, Hiyagon and Sano1998) and Manus Basin (Shaw et al. Reference Shaw, Hilton, Macpherson and Sinton2004) were also studied using the same geochemical parameters. These studies showed a wide range of 3He/4He and C/3He ratios and indicate that mantle beneath the basins are affected by degassing and contamination of crustal carbon. The CO2/3He ratios of the Fumarole Bay fumarolic gases calculated from the data in Tables I & II are 0.7~0.9 × 109. These are slightly lower than the mean depleted mantle values of ~2 × 109. The reason why the Deception Island gases have CO2/3He ratios lower than those of MORB may be because part of CO2 has been fixed as carbonate through the water-rock interaction, e.g.
in the deep hydrothermal system (Giggenbach Reference Giggenbach and D'Amore1991). Since the carbon isotopic fractionation factor between calcite and CO2 is small at hydrothermal temperatures (-3‰ at 300°C and +1‰ at 150°C, Chako et al. Reference Chako, Cole, Horita, Valley and Cole2001), δ13C values at source would not be significantly influenced by the mineral control. Although the number of 3He/4He and CO2/3He ratios and δ13C values of the Deception Island gases is limited, they indicate that the majority of He and CO2 is likely to have derived from the MORB-like source mantle that was influenced by fluids from the slab as discussed above.
Subsurface hydrothermal system
Subsurface temperature
Hydrothermal manifestations such as fumaroles, steaming grounds, and hot springs along the beaches of Deception Island indicate the existence of a hydrothermal system beneath the island. Seismic and geochemical studies by Rey et al. Reference Rey, Somoza and Martínez-Frías1995 and Somoza et al. Reference Somoza, Martinez-Frias, Smellie, Rey and Maestro2004 also indicate hydrothermal activity on the island. Valentin et al. (Reference Valentin, Martini and Diez-Gil1989) (cited in Somoza et al. Reference Somoza, Martinez-Frias, Smellie, Rey and Maestro2004) suggested on the basis of gas chemistry that the temperature of the hydrothermal system is about 219°C. We estimated the temperature of the hydrothermal system using a hydrogen geothermometer. This was originally proposed by Taran (Reference Taran1986) and later revised by Giggenbach (Reference Giggenbach and D'Amore1991). He introduced a H2/Ar ratio into the geothermometer and assumed that H2 and Ar behave together. The H2/Ar ratio is used to minimize the effect on the geothermometer of preferential loss or addition of hydrogen after its separation from liquid phase. The fugacity ratio of H2/H2O in hydrothermal systems is governed by redox equilibria of Fe(II) and Fe(III) in rocks hosting a hydrothermal system, and is practically constant over a wide range of temperature (Giggenbach Reference Giggenbach1987). The hydrogen geothermometer essentially utilizes the temperature dependence of the hydrogen distribution coefficient between gas phase and liquid phase. The assumptions are that i) the hydrothermal reservoir consists of a liquid phase only, ii) the redox equilibrium between Fe(II) and Fe(III) in host rocks has been established, iii) a tiny fraction of vapour phase leaks out of the hydrothermal system to the surface without disturbing H2/H2O ratio of the system, and iv) Ar derives from air dissolved in groundwater. Accepting these assumptions, the observed vapour-phase H2/Ar ratio was used for calculations. Equation of the H2/Ar geothermometer is;
where XH2 and XAr denote mole fraction of hydrogen and argon in the gas analysed and tH2/Ar is in °C. The H2 and Ar data for Fumarole Bay fumarolic gases shown in Table I gave temperatures around 300°C for the underlying hydrothermal system. Similar temperatures were calculated for bubbling gases collected at Telefon Bay using a CO2/Ar geothermometer (Giggenbach Reference Giggenbach and D'Amore1991). The equation of the CO2/Ar geothermometer is:
where XCO2 and XAr denote mole fraction of CO2 and Ar in the gas and tCO2/Ar is in °C. Note that we did not analyse CO2 in the Telefon Bay bubbling gas but assumed that the gas consisted mainly of CO2 (Table I). Thus, we postulate that the hydrothermal system beneath Deception Island has temperatures of ~300°C.
The temperature of the hydrothermal system has also been estimated from the chemistry of hot spring waters of the island. Chemical analyses shown in Table III indicate that the hot spring waters are mainly seawater. This is exemplified by the following enrichment factor relative to seawater, (X/Cl)i/(X/Cl)SW where X denotes Na+, K+, Ca2+, Mg2+, SO42- etc., and suffixes i and SW are for sample and seawater, respectively. The enrichment factors for Na+, K+, Mg2+, and SO42- range 0.7 to 1.0, suggesting a large contribution of seawater to the Deception Island hot spring waters. The δ34S values of ~20‰ for SO42- measured for the hot spring waters (Table III) indicate that SO42- is mostly of seawater origin. Under such circumstances, the cation geothermometry (e.g. Na/K, K2/Mg, and Na/K/Ca (Fournier Reference Fournier and D'Amore1991)) that has been widely used to estimate temperatures of water-rock interaction in hydrothermal systems could be misleading. However, Mn and SiO2 in the Deception Island hot spring waters are enriched relative to seawater. Enrichment factors range from 6000 to 15 000 for Mn and from 29 to 37 for SiO2. Very high Mn enrichment of hot spring waters is consistent with high Mn-Zn-Fe contents in the Port Foster sediments related to the submarine volcanic activity (Rey et al. Reference Rey, Somoza and Martínez-Frías1995). The high Mn and SiO2 concentrations in the hot spring waters suggest that these components are derived from water-rock interaction in the hydrothermal system. SiO2 concentration in hydrothermal solution is governed by dissolution equilibrium with silica minerals. Temperature dependence of solubility of quartz, chalcedony, cristobalite and amorphous silica in water has been used as a method to estimate temperature of the water-rock interaction at depth. When applying such SiO2 geothermometers, however, it is always ambiguous which silica mineral controlled the SiO2 concentration of hot spring waters. For the Deception Island hot spring waters, we assumed that the waters were in equilibrium with quartz. The quartz saturation temperatures were calculated using an empirical equation proposed by Fournier & Potter (Reference Fournier and Potter1982), i.e.
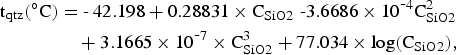
where CSiO2 is SiO2 concentration in ppm. The temperatures calculated were based on SiO2 concentrations shown in Table III. They range from 146° to 160°C for hot spring waters from Fumarole Bay and Whalers Bay. If chalcedony saturation was assumed, then temperatures range from 117° to 132°C. These temperatures depend on the assumption that the sampled hot spring waters represent a liquid phase of the hydrothermal system, which has not been diluted or contaminated by groundwater or seawater during upflow to the surface, and have attained chemical equilibrium. The SiO2 temperatures presented here (146°–160°C) are significantly lower than subsurface temperature of 300°C estimated using the H2/Ar geothermometer for fumarolic gases. The difference suggests that the deep part of the hydrothermal system of Deception Island is overlain by cooler fluid.
The island's seismicity has been found to be most active along the major fracture zone that runs in a NE–SW direction (Fumarole Bay to Pendulum Cove) in accordance with the occurrence of areas of high geothermal activity (Ibáñez et al. Reference Ibáñez, Del Pezzo, Almendros, La Rocca, Alguacil, Ortiz and Garcia2000). Gaseous components of magmatic origin such as H2, He and CO2 migrated upward through the fractures and faults to the surface. The volcanic earthquake focal depths are shallow and are estimated to occur at 2–5 km depths. This seismicity is probably related to vaporization of the fluid by magmatic gases discharging from the magma during the latest volcanic eruptions (Correig et al. Reference Correig, Urquizu, Vila and Marti1997). These shallow earthquakes occur during hydro-fracturing of crustal rocks when there is a sudden increase of volume associated with vaporization (Correig et al. Reference Correig, Urquizu, Vila and Marti1997, Fournier Reference Fournier1999, Kusakabe et al. Reference Kusakabe, Ohwada, Satake, Nagao and Kawasaki2003).
Hydrogen and oxygen isotopic features of waters
The hydrogen and oxygen isotopic ratios of fumarolic condensate, hot spring, crater lake and glacier meltwaters from Deception Island are listed in Table IV and graphically shown in Fig. 7. The δD and δ18O values of crater lakes and glacier meltwaters range from -82 to -64 ‰ in δD and -10.9 to -7.8 ‰ in δ18O. They are close to meteoric waters found in mid-latitudes and reflect a close affinity of the source atmospheric water vapour to nearby oceans. Some of the surface waters appear to have been subjected to isotopic enrichment due to evaporation (DCF-15, DCW-1, DCW-5, and DCW-7 (Table IV)). Excluding these samples, the mean δD and δ18O values of local meteoric waters are -78.8 ‰ in δD and -10.50 ‰ in δ18O. The hot spring waters plot on a mixing line between seawater and the local meteoric water. The mixing line is slightly below the global meteoric water line (MWL; δD = 8δ18O + 10). The hot spring waters were collected near the seashore, so that they were greatly influenced by seawater as shown in Fig. 7. The seawater contribution to the hot spring waters is 0.73 for the Fumarole Bay samples (DCF-7 and DCF-20) and 0.93 for the Whalers Bay sample (DCF-16). Water vapour in the Fumarole Bay fumarolic gas (DCF-1) may represent steam evaporated from the deep hydrothermal fluid of the island. The δD and δ18O values of the liquid may be calculated assuming a single step equilibrium evaporation at 300°C, which is the temperature estimated from the H2/Ar geothermometer. The calculation was done using an equation for isotopic fractionation between liquid and vapour phases, i.e. α = (1 + δ18Oliq/1000)/ (1 + δ18Ovap/1000) in the case of oxygen isotopes, where α is an oxygen isotopic fractionation factor at a given temperature. Hydrogen and oxygen isotopic fractionation factors between vapour and liquid of water including the salinity effect were taken from Horita et al. (Reference Horita, Cole and Wesolowski1993). The calculated δD and δ18O values are -40‰ and -6.0‰, respectively, and are plotted as a solid square in Fig. 7. The square lies close to the mixing line between seawater and local meteoric water and indicates a seawater fraction of approximately 0.43. A magmatic contribution cannot be detected from the δD and δ18O signatures (Fig. 7). However, helium in fumarolic, bubbling and hot spring gases was carried to the surface by magmatic CO2. The helium was not contaminated significantly by atmospheric helium dissolved in surface water and seawater, because it is enriched by a factor of up to 1000 relative to air (Fig. 4).

Fig. 7. δD and δ18O relationship of thermal waters and surface waters (crater lakes, ponds and glacier meltwater) from Deception Island, Antarctica. The hot spring waters plot on a mixing line between the surface water and seawater, slightly below MWL which is a global meteoric water line defined by δD = 8δ18O +10. Fumarolic water vapour from Fumarole Bay plot slightly above the MWL. Calculated composition of the liquid phase from which the fumarolic vapour evaporated at 300°C comes close to the hot spring water compositions. Stippled boxes indicate δD and δ18O values expected for magmatic fluids from andesitic volcanoes (top) and mantle (bottom).
Table IV. Hydrogen and oxygen isotopic ratios of waters from Deception Island.

Conclusions
The 3He/4He ratios of 9.4~9.8 × 10-6 and the δ13C values of -5 to around -6‰ for fumarolic gases, bubbling gases, and hot spring waters from Deception Island, Antarctica, suggest that He and CO2 are of mantle origin. The magma emplaced beneath the island was derived from the wedge mantle that was geochemically similar to MORB source mantle. But this mantle was contaminated by U, Th, Sr and Pb derived from a slab that had previously subducted beneath the Bransfield Basin back-arc basin. This interpretation is supported by the observed 3He/4He ratios that are slightly lower than the MORB values and the N2/He ratios of the gases that are higher than those of MORB-derived basaltic gases. Figure 8 illustrates a schematic view of the hydrothermal system. The subsurface temperature of deep part of the hydrothermal system is around 300ºC as indicated by the H2/Ar gas geothermometer. Gaseous components such as He and CO2 came from the underlying magma, moved through the fractures and faults without contamination by atmospheric components dissolved in groundwater and seawater, and reached the surface as a gas phase. Hot spring waters represent the shallow part of the hydrothermal system with temperatures around 150ºC as estimated by the SiO2 geothermometer. The major dissolved ionic components and δD-δ18O-δ34S values indicated that the hot spring waters were mixed with a large proportion of seawater.

Fig. 8. Schematic representation of the hydrothermal system beneath Deception Island along the NNW–SSE cross section (modified from Somoza et al. Reference Somoza, Martinez-Frias, Smellie, Rey and Maestro2004). Magmatic He and CO2 carrying the isotopic signatures of the source mantle beneath the Bransfield basin come from the solidifying magma and reach the surface through the faults and fractures. The hydrothermal system develops widely beneath the island. The temperature of deep part of the system is around 300°C and that of overlying shallow part is c. 150°C as shown by dashed curves. Fumarole Bay where the fumarolic activity is currently highest is off the cross section and is located at the western end of Port Foster (Fig. 1).
Acknowledgements
This study was supported by the KOPRI projects PE08020 and PP08030. We thank Mr Rafael Ayora Hirsh, commander of the Spanish Antarctic Base at Deception Island, and his team for their kind logistic support. The manuscript was prepared while MK was working for Univ. Toyama, Japan. We thank L. Somoza and D.R. Hilton for their critical review on the earlier version of the manuscript. English was improved by G.L. Scott.














