Introduction
Implementing the Convention on Biological Diversity amidst rapid change
Nations and regions could, and patchily did, undertake research to survey and monitor marine biodiversity and thus develop strategies to best protect and ‘sustainably’ utilize wildlife. The lack of progress, co-ordination and standardization led to pressure to formulate and apply the international Convention on Biological Diversity (CBD). 2010 was the United Nations declared International Year of Biodiversity and the year by which nations had committed to ‘a significant reduction of the current rate of biodiversity loss…’ from global to regional scales. Current analysis of international efforts to achieve even slight reductions shows near uniform failure (Butchart et al. Reference Butchart, Walpole, Collen, van Strien, Scharleman, Almond, Baillie, Bomhard, Brown, Bruno, Carpenter, Carr, Chanson, Chenery, Csirke, Davidson, Dentener, Foster, Galli, Galloway, Genovesi, Gregory, Hockings, Kapos, Lamarque, Leverington, Loh, McGeoch, McRae, Minasyan, Hernández Morcillo, Oldfield, Pauly, Quader, Revenga, Sauer, Skolnik, Spear, Stanwell-Smith, Stuart, Symes, Tierney, Tyrrell, Vié and Watson2010). To achieve meaningful reductions in loss requires three major actions. The first action is to gain estimates of the extent and distribution of biodiversity, its importance and vulnerability, and the accuracy of these estimates. This is the essence of articles 7a and 7b of the CBD (see http://www.cbd.int/). In this study we describe how a new project based at South Georgia will attempt to gauge these in a way that allows continuous monitoring and addition (of species distribution records). The second action required is to identify the key current and near-future impacts (CBD article 7c). Finally, appropriate action is then needed to provide some degree of protection to the communities and species identified as most vulnerable, (acting especially to support articles 6 and 8 of the CBD). Clearly the timing, location and nature of such conservative action can only be appropriate and adequate if the first two points have been undertaken with considerable care, unless they concern an individual problem to a community or species. An example of the latter in a Southern Ocean context would be Patagonian toothfish (Dissostichus eleginoides Smitt) (Collins et al. Reference Collins, Brickle, Brown and Belchier2010).
Except for those with research stations, the marine biodiversity around most remote islands is poorly known. Apart from megafauna, and ecosystems such as coral reefs, there are few biodiversity and vulnerability estimates, or meaningful protection measures in place for key areas. A notable exception is the recent decision to make a considerable marine protected area around the Chagos Archipelago in the Indian Ocean (Owen 2010 http://www.independent.co.uk/environment/nature/britain-sets-up-the-worlds-largest-marine-reserve-2121367.html). Given the poor state of progress in better known and more accessible areas, are the costs associated with implementing the CBD at remote or polar locations the best use of resources? We argue that it is for several reasons. Loss of species at remote islands such as South Georgia is loss of global as well as local biodiversity, because so many of the species occur nowhere else (Barnes et al. Reference Barnes, Griffiths and Kaiser2009a). The best possibility to monitor biological response to climate change is probably where many species are highly thermally sensitive and at range edges, in an area of extreme warming (Whitehouse et al. Reference Whitehouse, Meredith, Rothery, Atkinson, Ward and Korb2008). There is a long record of historical information for South Georgia (for example on physical conditions, phytoplankton, krill and higher predator population sizes and reproductive success) and lack of complicating factors (no known established non-indigenous species, no [terrestrial] land use changes, distance from urban centres and negligible pollution). These should be helpful in understanding responses. There is also considerable merit in monitoring a near ‘natural’ continental shelf fauna. Although some populations of target species are still recovering from past fishing activity at South Georgia the intensity and damage is probably much lower than on most of the world's continental shelves and furthermore it is not skewed ecologically by species invasions. In contrast to most of Antarctica's continental shelf there are clear areas unreached by the last glacial maximum (shelf beyond moraines in Fig. 1 insert). This means we can compare the response of communities tens of thousands of years old compared with those hundreds of thousands of years old. Little detail of the influence of glaciations of marine life around South Georgia is known but most, possibly all, of the inner shelf would have been covered by grounded ice (Graham et al. Reference Graham, Fretwell, Larter, Hodgson, Wilson, Tate and Morris2008). In addition fast ice would have extended further north as would the Polar Front. These would mean that benthos would be absent from the shallows, in more turbid conditions (sediment resuspension caused by ice), and be further away (geographically and bathymetrically) from primary production food. Finally, South Georgia has a highly unusual oceanographic context making it very productive but highly variable.
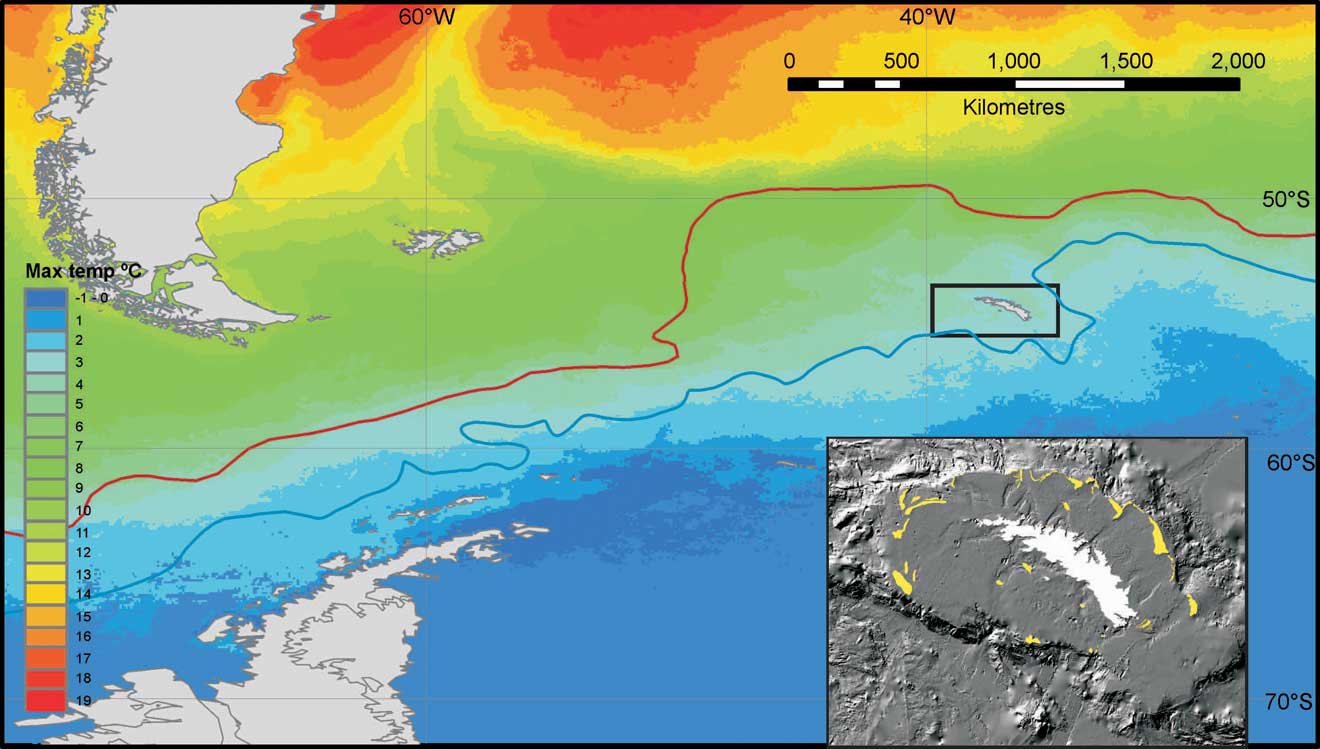
Fig. 1 Schematic to show the oceanographic, bathymetric and historic context of South Georgia. The position of nearby continents, ocean front systems and sea surface temperature (main figure) are shown relative to South Georgia (enclosed in box). The Polar Front (PF) is shown as a red line and the South Antarctic Circumpolar Current Front (SACCF) is shown as a blue line. The figure insert (bottom right - modified after Graham et al. Reference Graham, Fretwell, Larter, Hodgson, Wilson, Tate and Morris2008) shows detail of the continental shelf, shelf break and moraines (shown in yellow) indicating the recent maximum extent of grounded ice around South Georgia.
South Georgia bisects the powerful Antarctic Circumpolar Current (ACC), with the major boundary of the Polar Front (PF) to the north and the Southern ACC Front (SACCF) to the south (Fig. 1). However, the Polar Front does significantly vary (by several hundred kilometres) in its position to the north of South Georgia (e.g. see Trathan et al. Reference Trathan, Brandon and Murphy1997). This shelf has both the warmest (∼4°C) and biggest seasonal range (∼5°C) of sea surface temperatures within the Southern Ocean (Barnes et al. Reference Barnes, Fuentes, Clarke, Schloss and Wallace2006). This oceanographic position results in South Georgia being a key region for phytoplankton, krill productivity and the associated fisheries making the region significant economically. South Georgia's position within the ACC also makes the shelf there subject to high levels of physical and biological variability (linked to the Antarctic Circumpolar Wave and temperature fluctuations in the Pacific due to El Niño events, Trathan et al. Reference Trathan, Brierley, Brandon, Bone, Goss, Grant, Murphy and Watkins2003). Time series analysis indicates that there has been periodicity in temperature anomalies which lag around four years after El Niño events. Warm sea-surface temperature anomalies at South Georgia are associated with reduced krill numbers. Low krill abundances influence higher predators’ populations of birds and seals, such that periods of reduced predator breeding performance are strongly correlated with the warmer waters (Trathan et al. Reference Trathan, Murphy, Forcada, Croxall, Reid and Thorpe2006). Trenberth & Hoar (Reference Trenberth and Hoar1996) related the increasing frequency of El Niño events to decadal climate change throughout the Pacific, with potentially serious implications for already vulnerable locations such as South Georgia.
Response of marine ecosystems to climate change
The rise in atmospheric carbon dioxide (CO2) over the last century has driven massive uptake of CO2 in the oceans (e.g. Thomas et al. Reference Thomas, Prowe, Lima, Doney, Wanninkhof, Greatbatch, Schuster and Corbière2008), resulting in increased levels of dissolved oceanic CO2 and thus decreasing levels of pH (‘ocean acidification’) (see Turner et al. Reference Turner, Bindschadler, Convey, Di Prisco, Fahrbach, Gutt, Hodgson, Mayewski and Summerhayes2009 for a review of climate change in the region). In addition it has driven temperature increases, which in turn drive changes in sea level, precipitation, ice mass balance, salinity and storm frequency. Current data and model projections show, however, that the severity of impacts is spatially uneven (Parry et al. Reference Parry, Canziani, Palutikof, Van Der Linden and Hanson2007, Turner et al. Reference Turner, Bindschadler, Convey, Di Prisco, Fahrbach, Gutt, Hodgson, Mayewski and Summerhayes2009). Parts of the Southern Ocean have amongst the highest rates of sea temperature and salinity change (Meredith & King Reference Meredith and King2005, Whitehouse et al. Reference Whitehouse, Meredith, Rothery, Atkinson, Ward and Korb2008), glacier retreat and ice shelf loss (Cook et al. Reference Cook, Fox, Vaughan and Ferrigno2005) and sea ice loss (Stammerjohn et al. Reference Stammerjohn, Martinson, Smith and Iannuzzi2008). This region is also projected to be the most severely influenced by ocean acidification (Orr et al. Reference Orr, Fabry, Aumont, Bopp, Doney, Feely, Gnanadesikan, Gruber, Ishida, Joos, Key, Lindsay, Maier-Reimer, Matear, Monfray, Mouchet, Najjar, Plattner, Rodgers, Sabine, Sarmiento, Schlitzer, Slater, Totterdell, Weirig, Yamanaka and Yool2005). In the Scotia and Bellingshausen seas phytoplankton blooms are now more prolonged, with new blooms more likely to become established (e.g. Peck et al. Reference Peck, Barnes, Cook, Fleming and Clarke2010). There have also been changes in zooplankton and higher predator populations (Forcada & Trathan Reference Forcada and Trathan2009), and a range shift of native species with the prospect of invasions by non-indigenous species probable (Barnes et al. Reference Barnes, Griffiths and Kaiser2009a).
Biological response to environmental change is linked to the magnitude, frequency, predictability or variety of physical changes. However, it also depends on the particular characteristics of species and populations. Vulnerability of fauna in the South Georgia context is likely to be associated with population size, high endemism, proximity to range edges, slow growth rates, long life-spans, high age at first reproduction, dispersal abilities (there are few places to disperse to from South Georgia) and physiological sensitivity (e.g. to temperature). Old, large, isolated regions (and habitats) tend to have highest endemism levels whereas those on the edge of geographic biomes should have many species at range edges (Longhurst Reference Longhurst1998). The fauna of polar and deep sea environments tend to have slow growth, extended ages and late onset of reproductive activity (Arntz et al. Reference Arntz, Brey and Gallardo1994). Experimental work suggests that ectotherms in the tropics and poles seem to be most sensitive to thermal or acidity changes (e.g. Compton et al. Reference Compton, Rijkenberg, Crent and Piersma2007). Thus, in summary, the fauna around old, remote polar islands close to the oceanic fronts should be amongst the most vulnerable localities in biological terms. Combining these with the geography of physical change suggests that the archipelago of South Georgia should be a key locality to investigate. Recent technical advances in Geographic Information Systems (GIS), mapping and multinational input into biodiversity databases such as SCARMarBIN have revealed that South Georgia is anomalously high in both endemism and numbers of species at range edges (Barnes et al. Reference Barnes, Griffiths and Kaiser2009a). The problem is that, as with most polar areas, marine biodiversity there is poorly characterized, georeferenced or understood. This represents a major barrier to assessment, monitoring and achieving reductions in the loss of biodiversity.
Over recent decades, but also dating back several centuries, anthropogenic impacts of overfishing, pollution, habitat destruction and non-indigenous species invasions were thought to be the dominant pressures on marine biodiversity (Jackson et al. Reference Jackson, Kirby, Berger, Bjorndal, Botsford, Bourque, Bradbury, Cooke, Erlandson, Estes, Hughes, Kidwell, Lange, Lenihan, Pandolfi, Peterson, Steneck, Tegner and Warner2001). Aspects of climate change, such as warming, are likely to exacerbate most of these, for example by increasing establishment and spreading success in non-indigenous species (Walther et al. Reference Walther, Post, Convey, Menzel, Parmesan, Beebee, Fromentin, Hoegh-Guldberg and Bairlein2002). There are few sites where we have enough knowledge of southern polar marine biodiversity to assess impacts (Clarke & Johnston Reference Clarke and Johnston2003). Currently the only discrete marine area, for which a biodiversity estimate (mega- and macrofauna only) has been undertaken in the Southern Ocean, is the South Orkney Islands (Barnes et al. Reference Barnes, Kaiser, Griffiths and Linse2009b). However, little of the South Orkney Islands’ biodiversity is quantified or georeferenced and there are few endemics or species at range edges. In this paper we report the start of the first polar Darwin Initiative project and the first attempt to generate a baseline for a polar marine locality at which the CBD might be meaningfully applied. Finally, we consider why it is particularly important to implement the CBD at South Georgia, what are the most likely current and near-future impacts, and what the corresponding biological responses will be to change.
Towards an interactive GIS model of the South Georgian marine environment
South Georgia is surrounded by a continental shelf which is about 200 m deep and 50–150 km wide, punctuated by a series of deep canyons (Fig. 1 insert). With the major boundary of the PF sweeping a few hundred kilometres north its marine fauna is essentially Antarctic in character (Griffiths et al. Reference Griffiths, Barnes and Linse2009). Modelling suggests that the SACCF transports water masses, krill and maybe larvae of many species from the Antarctic Peninsula to South Georgia (e.g. Hofmann et al. Reference Hofmann, Klinck, Locarnini, Fach and Murphy1998). Acute thermal tolerance probably determines which species survive such a journey and thus also their range sizes (Barnes et al. Reference Barnes, Peck and Morley2010).
Models of fine scale oceanography are needed to understand this productive and rich region. Young et al. (Reference Young, Meredith, Murphy and Carvalho2009) recently adapted the Proudman Oceanographic Laboratory Coastal Ocean Modelling System (POLCOMS). Their model used existing high-resolution multibeam sonar data for the region and linked this to conductivity-temperature-depth (CTD) deployments including tidal and freshwater flux flow. Outputs of the Young et al. (Reference Young, Meredith, Murphy and Carvalho2009) model (at ∼3 km spatial scale) can be used to generate georeferenced temporal (e.g. monthly) means of sea temperature, salinity and current velocity and direction for a grid of both the South Georgia shelf and continental slope. These physical oceanographic data can then be overlain on topographical (bathymetry) data in the South Georgia GIS (http://www.sggis.gov.gs), a visualization tool for spatial data developed by British Antarctic Survey for the Government of South Georgia and the South Sandwich Islands (GSGSSI) (Fig. 2). When this data is layered into this 3D GIS model the geography and interactivity of regional scale physical variables can be powerfully and visually linked to the biodiversity information.

Fig. 2 Layers of information that will be available in the interactive GIS model of South Georgia including seabed current flow, temperature and salinity, primary production and bathymetry along with records of benthic species. The example biodiversity data are superimposed on bathymetry are all Crustacea (green dots) and an example crustacean species, the crab Paralomis spinosissima (red dots). Data from SCARMarBIN open access database and the Government of South Georgia and South Sandwich Islands.
The new Darwin Initiative project is collecting, digitizing and analysing biodiversity data from reports and papers dating back to the German Polar Expedition of 1882. Significant information has already been gained from early scientific research expeditions to South Georgia (see Table I). The rapid development of new international open access databases (e.g. SOMBASE (Southern Ocean Mollusc database) and SCARMarBIN (Scientific Committee for Antarctic Research Marine Biodiversity Information Network database – see http://www.scarmarbin.be/)), checked by expert taxonomists, has greatly increased the potential for application of the CBD to South Georgia (and other localities). This is because such databases have made viewing of hotspots of richness, endemics, rare species, sampling, or any other category more widely and easily viewable and analysable.
Table I Sources of biological data. For more detail on scientific expedition history, see http://www.sght.org/science.htm.

Estimating marine biological diversity at South Georgia
The CBD highlights the need for identifying existing biodiversity, assessing its vulnerability and threats to it, as well as devising management plans to safeguard this resource. Clearly this cannot be undertaken simultaneously for all areas so strong prioritization must be undertaken. There are several a priori reasons for hypothesizing that South Georgia's marine biodiversity should be rich and globally important. These include that it is large, old and isolated, close to a major ocean boundary and has virtually no human population. The nearby South Orkney Islands are old, have a similar sized shelf area and are highly speciose, with 1026 marine species recorded (Barnes et al. Reference Barnes, Kaiser, Griffiths and Linse2009b). Yet recent comparisons of species rarefaction curves and richness residual analyses showed that South Georgia was one of the most important species richness hotspots in the whole Southern Ocean for bryozoans (Barnes Reference Barnes2008), gastropod and bivalve molluscs, amphipod crustaceans, ophiuroid echinoderms and particularly hexacorals (Barnes et al. Reference Barnes, Griffiths and Kaiser2009a). Open access international biological databases show that more decapod crustacean and fish species are recorded from South Georgia than any other similarly sized region within the Southern Ocean (e.g. run searches in http://www.scarmarbin.be). Commencing the first polar Darwin Initiative project we found recent literature reporting 640 species, of which most were endemic (0–36%) or at northern (5–53%) or southern (2–49%) range limits (Table II). Species at range edges are close to their physiological limits (e.g. temperature) so some South Georgia species are sensitive to small physical changes (Morley et al. Reference Morley, Hirse, Pörtner and Peck2009). We have started collation, databasing and analysis of South Georgia marine biodiversity records over the last 120 years. It is already clear that South Georgia will prove biodiverse. The importance of this richness is here argued to be globally important in the sense of containing major populations of ‘figurehead’ top predators and many species at the edges of their ranges found nowhere else.
Table II Status of benthic biodiversity information for selected taxa prior to the South Georgia marine biodiversity GIS project. Variability in species numbers, endemism and species with range limits at South Georgia, in the Southern Ocean. Decapod data refer to malacostracan crustaceans (crabs and shrimps). Additional data from from GSGSSI (fisheries) and Estefania Rodriguez personal communication 2010 (anemones).

Biodiversity data held in pre-existing databases show that there are 3205 records of identified species from 1800 sites across the South Georgia Shelf. These georeferenced records represent around 340 species (just 53% of species from Table I) from six phyla, among which Mollusca and Crustacea have the highest representation with 120 and 109 species respectively. Twenty-four phyla have been recorded from the South Orkney Islands (Barnes et al. Reference Barnes, Griffiths and Kaiser2009a), which are similarly old, remote and in the same approximate region. Therefore, there are probably many records of biodiversity at South Georgia which are not yet represented in international open access databases and so our ability to assess biodiversity is extremely restricted. Some phyla are very poorly represented, such as Cnidaria and Nematoda, whilst others (e.g. Annelida and Porifera) remain unquantified. Crustacea dominate records of biodiversity there and outnumber all other phyla combined. Unsurprisingly krill (Euphausiacea), the key pelagic group which are food for huge populations of higher predators in the region, represent more (43%) records than any other species. The most abundant nine species of crustaceans (krill, planktonic amphipods and copepods) constitute 60% of all records. The distribution of reported samples is patchy, with most north of the island along the northern shelf break. By comparison knowledge of the shelf south of the island remains relatively impoverished, with two areas > 300 km2 devoid of biodiversity information. The geographic position and species composition of recorded samples suggests much of the existing available information is from targeted sampling of the epi-pelagic zone (e.g. by fisheries) and that the South Georgian benthos are poorly characterized.
Early results from the Darwin Initiative project are already significantly increasing our understanding of marine biodiversity around South Georgia. For example, there were just 51 records, comprising 10 species, of cnidarians catalogued. Updating database records with all ISI and grey literature to date reveals 150 species now known from 700 records. The update has also shown species data to be much less patchy - there is now data representing much of the major shelf areas around the archipelago. Large areas of the coastal south-east and parts of the west remain unstudied. Amongst these poorly sampled areas we have identified four key areas as potential biodiversity hotspots.
The CBD explicitly refers to conservation and management of genetic resources. Currently the Zoological Society of London are investigating the genetic structure of octocorals, which are abundant and rich components of fishery by-catch. Scleractinian corals are less frequently caught and one of the few CITES (Convention on International Trade in Endangered Species of wild fauna and flora) listed and most vulnerable taxa. To date little is known about the connectivity between species that are shared between South Georgia and the Patagonian Shelf to the north and the Antarctic Peninsula to the south. Some studies have demonstrated that the PF acts as a barrier to benthic brooders (Hunter & Halanych Reference Hunter and Halanych2008) and feely dispersing pelagic organisms (Thornhill et al. Reference Thornhill, Mahan, Norenburg and Halanych2008) while others indicate that this is not a general rule (e.g. Wilson et al. Reference Wilson, Hunter, Lockhart and Halanych2007). The commercially important Patagonian toothfish also appears to have population discontinuity across the PF (Shaw et al. Reference Shaw, Arkhipkin and Al-Khairulla2004). Recent studies are highlighting the dynamic nature of the shallow water (> 50 m) benthos. Nikula et al. (Reference Nikula, Fraser, Spencer and Waters2010) found that even organisms which were thought to be poor dispersers seem to have been capable of considerable migration since the last glacial maximum. If any generality can be drawn it is that ‘circumpolar’ shelf species tested so far tend to have quite marked population structures (e.g. Wilson et al. Reference Wilson, Hunter, Lockhart and Halanych2007). Work on octopus from the region has shown depth to be an isolating factor, even across very small spatial scales. For example, Allcock et al. (Reference Allcock, Brierley, Thorpe and Rodhouse1997) identified a panmictic population of Pareledone turqueti (Joubin) from around South Georgia (500 km range) which was genetically distinct from the individuals from nearby Shag Rocks (150 km away). The barrier in this case is a deep channel between the two shelf regions. The most spatially comprehensive molecular study to date that compares South Georgia individuals with those of surrounding shelves was by Linse et al. (Reference Linse, Cope, Loerz and Sands2007). They used two genes to study the population structure of the small bivalve Lissarca notorcadensis Melvill & Standon from around the Scotia Arc and the Antarctic continental shelf. Although sample sizes were small (n = 1–12), each discrete shelf region had its own mitochondrial haplotypes, and both gene regions demonstrated that individuals from the Scotia Arc were distinctly different from those from the Antarctic shelf. To really understand the connectivity of South Georgia marine biota with that of the Antarctic Peninsula more phylogeographic and population genetic studies are required.
Implications of the extension of the CBD to South Georgia's marine environment
The area within the South Georgia and South Sandwich Islands Maritime Zones (SGSSI MZ) are principally managed by GSGSSI, however the SGSSI MZ falls within the area of the Convention for the Conservation of Antarctic Marine Living Resources (CCAMLR), which regulates fishing activities. GSGSSI implements all applicable CCAMLR Conservation Measures, but in many cases has more stringent requirements than CCAMLR. Both the GSGSSI and CCAMLR are working towards the establishment of Marine Protected Areas (MPAs) in the Southern Ocean, although CCAMLR's remit in this context is limited to fisheries. The information required to meaningfully establish where, how big and how many MPAs is essentially the same as would be required for the CBD. The CBD has not as yet been extended to South Georgia, but if extended, would apply to both the marine and terrestrial environments of South Georgia and the South Sandwich Islands. Conforming to the CBD is unlikely to drastically alter the information collected, or how it is utilized, but it would put the onus on GSGSSI and the UK government to demonstrate that biodiversity is being quantified and not lost. Work towards assessing biodiversity, such as by the Darwin Initiative project, should be both complementary to, and informative for, CCAMLR fisheries management. Certainly, the history of marine life harvesting in the Southern Ocean, and particularly South Georgia, clearly illustrates the need for better ecosystem resource management.
Historical impacts to, and responses of, marine biodiversity at South Georgia
Exploitation of marine biodiversity at South Georgia began in the 1780s when sealers arrived at South Georgia and targeted fur seals (Arctocephalus gazella (Peters)) for their skins and elephant seals (Mirounga leonina (L.)) for their blubber. This heavily depleted the fur seal population by the 1820s so sealing moved elsewhere, but small numbers of fur seals were taken sporadically. In the early 20th century the British government introduced a strict licensing regime on all marine exploitation. Fur seal populations remained protected, but a small number of bull elephant seals (3000–6000) were still taken each year until the mid 1960s.
Grytviken whaling station opened in 1904 followed by five more whaling stations by 1912 thus making South Georgia the centre of Southern Ocean whaling. With the advent of whaling from factory ships in the late 1920s the ability of the British government to restrict catches was lost and the number of whales killed in the Southern Ocean more than doubled (to over 40 000 total by 1930). This rapid expansion of whaling led to an over-production of oil and the establishment of the International Committee for the Regulation of Whaling, the precursor of the International Whaling Commission. The success of pelagic whaling, and the availability of alternative products and increasing scarcity of whales caused the stations on South Georgia to close in 1965.
Large-scale commercial fishing began around South Georgia in the late 1960s, with ex-Soviet-bloc bottom trawlers initially targeting marbled rock-cod (Notothenia rossii Richardson) but later mackerel icefish (Champsocephalus gunnari Lönnberg) and yellow finned rock-cod (Patagonotothen guntheri Norman). There is considerable doubt about catch levels and composition, but 400 000 tonnes of marble rock-cod were the reported take for the 1969/70 season (Agnew Reference Agnew2004). Four decades later populations of marbled rock-cod are yet to recover and likewise mackerel icefish have not recovered to the levels reported from 1976/77 and 1981/84. Current fisheries target three species, Antarctic krill (Euphausia superba Dana), mackerel icefish and Patagonian toothfish (Dissostichus eleginoides) all of which are managed within the framework of CCAMLR. Krill fishing, which began around South Georgia in the early 1980s, peaked in 1987/88 (at 300 000 tonnes) and led to the establishment of CCAMLR. Since 1992 the average annual catch in South Georgia waters has been 36 000 tonnes. The bottom trawl fishery for mackerel icefish fishery was closed by CCAMLR in 1990, so they were trawled pelagically. Abundance of mackerel icefish is volatile and recently allowable catches have been between 2000 and 4500 tonnes. A targeted longline fishery for Patagonian toothfish began in the late 1980s, which rapidly expanded to see catches of over 6000 tonnes in 1990. CCAMLR and GSGSSI have subsequently enforced strict regulations on this fishery, with current catch levels at around 2500 tonnes.
Current threats to marine biodiversity and future protection outlook
At South Georgia and Shag Rocks, within 22 km of the coast is a no-take zone, within 352 km (the Management Zone) and < 550 m depth bottom fishing is banned (including longlining and potting). Longline fishing is still likely to represent a key impact to benthic organisms, particularly corals, on the continental slope (> 550 m). Other significant threats to marine biodiversity have been identified including the potential introduction of invasive species on the hulls or in ballast water of visiting fishing and tourist vessels (Lewis et al. Reference Lewis, Riddle and Smith2005). With the exception of Cumberland Bay, fishing vessels are generally restricted to 22 km (12 nm) offshore, but tourist vessels (∼70 per year) visit many bays on the north coast of the island. The threat of invasive species establishment and spread is exacerbated by warming.
Current protection of South Georgia waters is mainly achieved through licensing conditions. No licences are issued for bottom trawling anywhere in the South Georgia Maritime Zone (SGMZ), for fishing within 21 km of coast or for bottom fishing < 550 m. The presence of observers on every vessel and the requirement of vessels to carry Vessel Monitoring Systems aids compliance. New Wildlife & Protected Areas legislation, which is currently being drafted, will allow GSGSSI to declare Marine Protected Areas within the 200 nm Maritime Zone and enshrine the current protection in law. Further work is required to identify important areas both inshore and in deep water that may also require protection. An overview of protection outlook for areas south of 60°S and other polar areas beyond national jurisdiction is discussed in Rayfuse (Reference Rayfuse2008).
Conclusions
Physical data show South Georgia shallow waters are in a region of drastic current and projected temperature change. Biological data show that fish and whale populations have not recovered from past fishing pressure, and that most of its biodiversity occurs on the continental shelf. However, this biodiversity has been poorly and unevenly sampled and most of this data is not georeferenced. None of the established marine species recorded to date are non-indigenous and most of the existing species meet various vulnerability criteria (slow growth, high endemism and many at range limits). We argue that remote islands are particularly significant case for the application of the Convention on Biological Diversity, and that even amongst these, South Georgia is particularly important. There are very few places on our planet at which we can overlay such strong geographical, physical, oceanographic, historical human pressure and biodiversity data (Fig. 1 for schematic) into, what will be, an open access GIS model. Our project, initiated by the GSGSSI and South Georgia Heritage Trust, has now become the first polar Darwin Initiative project and has begun to show where some biodiversity hotspots may lie. Benthic biodiversity may already appear considerable (Table II) but we found that existing databases encompassed < 7% of the species records of cnidarians (which include CITES listed corals) and < 8% of sample records for the same taxon. It is clear that from a century of exploration there is much information available if it can be collected, assessed by experts and linked to a wealth of other types of data for the same area. Development of this model should, by 2012/13, enable us to meaningfully apply the CBD to South Georgia. The model should facilitate assessment of the status and vulnerability of South Georgia's shelf marine biodiversity. In turn this can be used to inform on the number, size, location and management strategy of marine protected areas there. Finally, we may then get powerful insights on the response of life to differing aspects of climate change, away from most direct anthropogenic pressures.
Acknowledgements
We thank Andrew Fleming for sea surface temperature data in Fig. 1, Emma Young for access to an ‘in press’ oceanographic model paper and Alistair Graham for the image in Fig. 1 inset. This work was funded by grants from the Government of South Georgia and South Sandwich Islands, the South Georgia Heritage Trust and the Darwin Initiative (DEFRA). Finally we thank the care and attention paid by two anonymous referees which improved our manuscript considerably.






