Introduction
Antarctic ecosystems have unique characteristics, resulting from a long evolutionary process under extreme environmental conditions and isolation, which provide outstanding opportunities for studying the natural cycles of the elements. Due to the high degree of endemism and eco-physiological adaptations of many species, the Antarctic food web is relatively simple (Hempel Reference Hempel, Siegfried, Condy and Laws1985) and thus studies focussing on Antarctic systems may be more able to characterize transfer processes between trophic levels (Nygard et al. Reference Nygard, Lie, Nils and Steinnes2001) than work in more complex systems. Furthermore, Antarctica can be considered largely unpolluted, facilitating the investigation of the natural processes without the confounding influence of anthropogenic factors. Trace elements are natural constituents of any ecosystem. In the Antarctic marine environment, they are introduced through natural processes, such as the activity of submarine volcanoes (Deheyn et al. Reference Deheyn, Gendreau, Baldwin and Latz2005), wet deposition of windborne soil particles (Mahowald et al. Reference Mahowald, Baker, Bergametti, Brooks, Duce, Jickells, Kubilay, Prospero and Tegen2005) and direct release from the sea ice (Grotti et al. Reference Grotti, Soggia, Ianni and Frache2005). Their concentration in Antarctic abiotic matrices (sediment, seawater, sea ice and suspended particulate matter) are generally within or lower than values reported for remote polar regions of the Northern Hemisphere and can be considered as background levels (Sanchez-Hernandez Reference Sanchez-Hernandez2000, Bargagli Reference Bargagli2000). Conversely, concentrations in Antarctic biota are comparable or even higher than those from polar and temperate areas of the Northern Hemisphere; for example, cadmium in sponges (10–80 µg g-1; Bargagli et al. Reference Bargagli, Nelli, Ancora and Focardi1996), the sea star Odontaster validus (Koehler) (80–180 µg g-1; Dalla Riva et al. Reference Dalla Riva, Abelmoschi, Magi and Soggia2004) and in the digestive gland of the bivalve mollusc Adamussium colbecki (Smith) (100–200 µg g-1; Mauri et al. Reference Mauri, Orlando, Nigro and Regoli1990, Bargagli et al. Reference Bargagli, Nelli, Ancora and Focardi1996, Dalla Riva et al. Reference Dalla Riva, Abelmoschi, Magi and Soggia2004).
In order to explain the naturally occurring elevated concentrations of trace elements in Antarctic organisms, several environmental and biological factors favouring metal accumulation have been considered. The elevated cadmium accumulation found in the coastal environment of Terra Nova Bay (Ross Sea) has been ascribed to the increased bioavailability due to the upwelling of Cd-rich deep waters, rapid regeneration in surface sediments and algal blooms (Bargagli et al. Reference Bargagli, Nelli, Ancora and Focardi1996). Biomagnification of mercury in the same area has been associated with the trophic connections between organisms in well developed benthic communities, phytoplankton and fish, as well as with enhanced persistence of mercury species due to the upwelling of cold waters and reduced photodegradation processes (Bargagli et al. Reference Bargagli, Monaci, Sanchez-Hernandez and Cateni1998). Local volcanism has been reported to increase bioavailability of trace elements in the marine ecosystem of Deception Island, thereby enhancing their accumulation in organism tissues (Deheyn et al. Reference Deheyn, Gendreau, Baldwin and Latz2005). Finally, decrease in detoxification mechanisms due to the low temperature of Antarctic water, peculiar feeding strategies (Nigro et al. Reference Nigro, Regoli, Rocchi, Orlando, Battaglia, Valencia and Walton1997) and longevity (de Moreno et al. Reference De Moreno, Gerpe, Moreno and Vodopivez1997) may further increase bioaccumulation of trace elements in Antarctic organisms.
Hence, it is becoming increasingly clear that metal concentration values in Antarctic biota cannot be used as global background levels; on the other hand, investigating temporal variations in metal concentration by using suitable biomonitors could be of great value to assess local or global changes. Accordingly, several authors proposed Antarctic molluscs (e.g. Adamussium colbecki and Laternula elliptica (King and Broderip)) and fish (e.g. Trematomus bernacchii Boulenger) as appropriate biomonitor organisms (Ahn et al. Reference Ahn, Lee, Kim, Shim and Kim1996, Jimenets et al. Reference Jimenets, Fossi, Nigro and Focardi1999, Nigro et al. Reference Nigro, Regoli, Rocchi, Orlando, Battaglia, Valencia and Walton1997, Dalla Riva et al. Reference Dalla Riva, Abelmoschi, Chiantore, Grotti, Magi and Soggia2003, Reference Dalla Riva, Abelmoschi, Grotti, Soggia, Bottaro and Vacchi2006). However, to make this approach really effective, it is crucial to establish the natural variability in the metal concentration within the same population and between different sites.
In this work, the concentrations of trace elements in representative marine organisms from two pristine coastal environments (Terra Nova Bay and Cape Evans, Ross Sea, northern Victoria Land) were measured and compared with literature data for other uncontaminated coastal ecosystems. The main goal of the research was to improve the estimation of the natural variability of trace elements in Antarctic biota, necessary to obtain a more confident interpretation of the results from biomonitoring programmes.
Materials and methods
Sampling sites
Terra Nova Bay is an 80 × 30 km inlet in the south-western part of the Ross Sea, delimited to the north by the narrow peninsula of Cape Washington and to the south by the Drygalski Ice Tongue (Fig. 1). The bay is a continental shelf, with an average depth of about 450 m and greater depths up to 1100 m in the Drygalski Basin. The bay is covered by sea ice for at least nine months of the year, usually from the end of March to the beginning of January. Ice dynamics are strongly influenced by the katabatic winds, which form and preserve polynyas persisting during winter. The dynamics of ice melting influences water column stratification and phytoplankton temporal distribution. The sampling site (74°41′–74°43′S, 164°02′–165°05′E) lies between the coast and the edge of a polynya zone. Within this area, at the sampling depth of 10–30 m, the seafloor is primarily granitic rock, with softer substrates of coarse sands or gravels.

Fig. 1. Sampling sites.
Cape Evans (77°38′05′′S, 166°24′51′E) is a small ice free area in the south-west of Ross Island, 10 km to the south of Cape Royds and 22 km north of Hut Point Peninsula on Ross Island (Fig. 1). The ice free area is composed of till-covered basalt bedrock. The site is within 200 m of Scott's Terra Nova Hut, but sufficiently far from the impacts of McMurdo Station to be considered as a pristine reference site against which the contamination of the US Station can be assessed (Negri et al. Reference Negri, Burns, Boyle, Brinkman and Webster2006).
Sample collection and storage
Benthic organisms were collected at 10–30 m by SCUBA diving. Representative organisms collected both at Terra Nova Bay and Cape Evans included the red alga Phyllophora antarctica (Gepp), the bivalve mollusc Laternula elliptica, the sea urchin Sterechinus neumayeri (Meissner), the sea star Odontaster validus and the fish Trematomus bernacchii (Table I). Specimens of the red alga Iridaea cordata (Turner), of the bivalve mollusc Adamussium colbecki, of the nemertine worm Parbolasia corrugatus (McIntosh) and of the sea cucumber Holothuria were also sampled at Terra Nova Bay. All the samples were stored at -80°C until analysis. Surface (1–2 cm deep) marine sediments were also collected in acid clean polycarbonate containers and stored at -30°C until analysis. All the samples were stored in the Antarctic Environmental Specimen Bank (Soggia et al. Reference Soggia, Abelmoschi, Dalla Riva, De Pellegrini and Frache2000, Reference Soggia, Ianni, Magi, Frache, Caroli, Cescon and Walton2001).
Table I. Collected organisms and analysed tissues.

Notes: a number of pooled organisms, b mean±standard deviation (minimum ÷ maximum).
Sample preparation and analysis
Organisms were thawed and their body length and weight measured (Table I). Using acid clean stainless steel dissecting tools, the following tissues were collected: arms (whole tissue) and soft tissues from the disc (including gonad and caeca pyloric) from Odontaster validus, digestive gland and all the remaining soft tissues from Odontaster validus; digestive gland and all the remaining soft tissues from Laternula elliptica; muscle, liver (or digestive gland), spleen and gonads from Trematomus bernacchii. For the other specimen, all the soft tissues of each organism have been collected.
100 mg of freeze-dried and homogenized (agate mortar) sub-sample (250 mg for the CRMs (Certified Reference Materials)) was weighed with a precision of ± 0.1 mg into the 12 cm3 quartz tubes of the microwave heated autoclave UltraCLAVE III (EMLS, Leutkirch, Germany) and 2 ml (5 ml for the CRMs) of doubly distilled 65% nitric acid (Merck, further purified with an MLS Duopur, sub-boiling unit) were added. The tubes were closed with Teflon® caps and placed into the quartz rack of the autoclave. Before the heating programme started the autoclave was loaded with Argon to a pressure of 4*106 Pa. The temperature was then ramped in 40 min to 250°C and kept at this temperature for further 45 min. After cooling to < 80°C the pressure was released and the autoclave opened. The mineralized samples were transferred into polyethylene tubes and diluted to 20 ml (50 ml for the CRMs) with Milli-Q water (Millipore, Bedford, MS, USA). The solutions were determined with a 7500 ce ICPMS from Agilent Technologies (Agilent, Waldbronn, Germany). Germanium, indium and rhenium were added online as internal standards.
In order to improve the confidence in the analytical data, a number of samples were also analysed according to another analytical procedure, carried out in a different laboratory. 100–200 mg quantities of freeze-dried and homogenized sub-sample were weighed to ± 0.1 mg and solubilized with 3 ml of 37% hydrochloric acid, 1 ml of 65% nitric acid and 1 ml of 40% hydrofluoric acid, using the microwave digestion system MDS-2000 (CEM, Matthews, NC, USA). Digestion was conducted for 50 min at 175°C, with a maximum (control) pressure of 150 psi, using 640 W power. After cooling, 5 ml of saturated boric acid solution were added and the heating programme performed again for 20 min. Finally, the samples were transferred into graduated flasks and diluted to 20 ml with Milli-Q water (Millipore, El Paso, TX, USA). For each run, two blanks and one certified reference sample were also prepared to check contamination and analytical accuracy. Determination of trace elements in the digests was performed using the ICP-AES Vista PRO from Varian (Springvale, Australia). Online internal standardisation using the Lu 291.139 nm reference line was applied.
Sediment samples were separated into two different granulometric fractions (particle size < 63 µm and < 2000 µm) using a stainless steel sieve, dried in an oven at 40°C and homogenized. Sub-samples (c. 200 mg) were weighed to ± 0.1 mg and solubilized with 3 ml of 37% hydrochloric acid, 1 ml of 65% nitric acid and 2 ml of 40% hydrofluoric acid, using the microwave digestion system MDS-2000. The operating conditions were the same as for the digestion of the biological samples. 10 ml of saturated boric acid were used in this case. Determination of trace elements in the digests was performed using the ICP-AES Vista PRO from Varian under the same operating conditions as for the organisms. All acids were of suprapure grade quality from Merck.
For the textural analysis of the sediments, a known amount of each sample was separated into four granulometric fractions: 2000–500 µm (coarse sand), 500–250 µm (medium sand), 250–63 µm (fine sand) and < 63 µm (silt and clay), using stainless steel sieves. Each fraction was dried in an oven at 40°C and weighed.
Validation of analytical accuracy
The accuracy of the analytical procedures was tested by analysing several certified reference materials: CRM-414 (plankton) from the Institute for Reference Materials and Measurements; DORM-2 (dogfish muscle), TORT-2 (lobster hepatopancreas), DOLT-3 (dogfish liver) and MESS-2 (marine sediment) from the National Research Council Canada; MURST-ISS-A1 (Antarctic sediment) and MURST-ISS-A2 (Antarctic krill) from PNRA - Istituto Superiore di Sanità, Rome; IAEA-407 (fish tissue) from the International Atomic Energy Agency. Results are reported in Table II. By comparing the analysis values with the certified ones (t-test, 95% confidence interval), it was concluded that all the analytical procedures used were accurate and suitable for the task.
Table II. Validation of analytical accuracy (concentrations in µg g-1 dry mass).

Notes: a determined by acid dissolution and ICP-AES, b determined by acid dissolution and ICPMS, c indicative value.
In order to further improve the confidence in the analytical data, a number of samples were analysed in two different laboratories and using two independent analytical procedures, as previously described. The agreement between the results was good, thereby confirming the accuracy of the analytical data. For example, the scatter plots of data obtained by the two methods for arsenic, cadmium and zinc were characterized by a slope of 0.94, 1.06 and 0.99 respectively, with correlation coefficients higher than 0.99 (n = 9).
Statistics
Data processing and statistical analysis were performed using the software tool XLSTAT (Microsoft Co., USA). The two-tailed Student t-test at the 95% confidence level was used to compare groups of data, with variances not assumed to be equal. Both the classical parametric coefficients and the Spearman's non-parametric rank correlation coefficients have been used to calculate the correlation. Principal Component Analysis has been performed after autoscaling of data.
Results and discussion
Marine sediments
Textural analysis of the sediments collected at Terra Nova Bay and Cape Evans showed that both sediments are mainly formed by sands, with a very small pelitic (clay and silt) fraction (1.8% and 0.3%, respectively). The Cape Evans sediments are dominated (81%) by coarse sand (0.5–2 mm), while the sediments collected at Terra Nova Bay consist mainly (78%) of finer sand (63–250 µm).
For element determination, two different granulometric fractions were considered, namely the < 2000 µm and < 63 µm fractions. The first fraction was chosen because it constituted the bulk of the samples; the second, although present in these sediments in a very low percentage, was considered because its capability to bind trace elements by complex mechanisms of absorption/adsorption which occur at the water/sediment interface, and which have significant implications for filter-feeding organisms (Tessier et al. Reference Tessier, Campbell, Auclair and Bisson1984).
Trace element concentrations in the sediments from Terra Nova Bay and Cape Evans are reported in Table III. These data are in good agreement with the ranges reported by Giordano et al. (Reference Giordano, Lombardi, Ciaralli, Beccaloni, Sepe, Ciprotti and Costantini1999) for Terra Nova Bay and by Negri et al. (Reference Negri, Burns, Boyle, Brinkman and Webster2006) for Cape Evans. In general, the concentration levels had the same order of magnitude as, or were lower than, those indicated as natural for deep sea sediments (Turekian & Wedepohl Reference Turekian and Wedepohl1961). As expected, the concentrations in the < 63 µm fraction were generally higher than those found in the coarser one.
Table III. Trace element concentrations (µg g-1 dry mass) in marine sediments from Terra Nova Bay and Cape Evans (mean values and relative standard deviations).

Analytical data for marine organisms
Trace element concentrations in pooled organisms collected in Terra Nova Bay and Cape Evans are summarized in Table IV. Pooling the individuals was assumed to better reflect the mean concentration within each species, as it reduced the weight of individual variability. Hence, the reported standard deviations are simply an estimation of the analytical precision and they do not reflect the concentration variability among the individuals. Since a preliminary analysis showed that the analytical precision do not depend on the matrix, but only on the concentration level (F-test, 95% probability level), the pooled standard deviations have been computed after grouping the samples according to the order of magnitude of their analytical concentration.
Table IV. Trace element concentrations (µg g-1 dry mass) in marine organisms from Terra Nova Bay and Cape Evans (mean values and pooled standard deviation of duplicate analysis).

Arsenic concentration ranged from 3.34 to 78 µg g-1 dry mass. The highest values were found in the muscle of Trematomus bernacchii (74 µg g-1) and in the digestive gland of Laternula elliptica (78 µg g-1), both collected at Cape Evans. High arsenic concentrations were also observed in the same species from Terra Nova Bay (47 and 62 µg g-1 in the muscle and liver of T. bernacchii, respectively, and 49 µg g-1 in the digestive gland of L. elliptica). Arsenic concentration in the other investigated species was in the 3–30 µg g-1 range, with higher values for the organisms collected at Cape Evans. A comparison of arsenic data obtained in this work with literature values was possible only for L. elliptica and Adamussium colbecki species. The arsenic content found in the soft tissues of these organisms (Table IV) is in good agreement with the concentration ranges reported for whole organism analyses of L. elliptica specimens sampled at Winter Quarters Bay, close to McMurdo Station, and at three reference sites (14–35 and 19–25 µg g-1, respectively; Sanchez-Hernandez Reference Sanchez-Hernandez2000). Comparable values (10–66 µg g-1) have also been obtained by Negri et al. (Reference Negri, Burns, Boyle, Brinkman and Webster2006) for specimens of L. elliptica collected at eight impacted and pristine sites in McMurdo Sound. The highest values (25–66 µg g-1) were found in the organisms sampled in the pristine sites of Turtle Rock and Cape Evans. Finally, the high concentration found in the digestive gland of the L. elliptica is in good agreement with the results by Nigro et al. (Reference Nigro, Regoli, Rocchi, Orlando, Battaglia, Valencia and Walton1997), who reported concentration up to 400 µg g-1 in the organs of Antarctic molluscs A. colbecki, L. elliptica and Yoldia eightsi collected at Terra Nova Bay.
Cadmium concentration ranged from 0.07–267 µg g-1 dry mass, thereby extending over 4 order of magnitude. There were relatively low cadmium concentrations (< 5 µg g-1) in the seaweed Phyllophora anatarctica and in the tissues of the benthic primary consumers Sterechinus neumayeri and T. bernacchii. On the other hand, cadmium accumulated in the liver of T. bernacchii (27 µg g-1) and in the tissues of the filter feeders, detritivorous and omnivorous benthic invertebrates (20–267 µg g-1). The highest concentrations were observed in the digestive gland of L. elliptica (59–80 µg g-1) and in the tissues of the sea star O. validus (76–267 µg g-1). Cadmium accumulation in Antarctic biota is a well-documented fact indicating its high bioavailability in the Antarctic marine environment, probably related to the upwelling of Cd-enriched deep waters and algal blooms (Bargagli 1996). Petri & Zauke (Reference Petri and Zauke1993) reported cadmium concentration in decapods as high as 13 µg g-1, a concentration among the highest observed in marine crustaceans. Elevated cadmium concentrations have also been reported for sponges (Bargagli et al. Reference Bargagli, Nelli, Ancora and Focardi1996, Negri et al. Reference Negri, Burns, Boyle, Brinkman and Webster2006), the amphipod Themisto gaudichaudii Guerin (Rainbow Reference Rainbow1989), the sea star O. validus (de Moreno et al. Reference De Moreno, Gerpe, Moreno and Vodopivez1997, Dalla Riva et al. Reference Dalla Riva, Abelmoschi, Magi and Soggia2004), the bivalve molluscs L. elliptica (Ahn et al. Reference Ahn, Lee, Kim, Shim and Kim1996, Nigro et al. Reference Nigro, Regoli, Rocchi, Orlando, Battaglia, Valencia and Walton1997, Negri et al. 2006, Lohan et al. Reference Lohan, Statham and Peck2001) and A. colbecki (Mauri et al. Reference Mauri, Orlando, Nigro and Regoli1990, Nigro et al. 1992, Minganti et al. Reference Minganti, Capelli and De Pellegrini1998). Our results are consistent with the state of knowledge on cadmium accumulation in Antarctic biota. In particular, cadmium accumulation in the liver of the T. bernacchii agrees with the results by Leninhan et al. (Reference Lenihan, Oliver, Oakden and Stephenson1990) and Bargagli et al. (Reference Bargagli, Nelli, Ancora and Focardi1996). Finally, remarkably high concentrations of cadmium for the sea star O. validus have also been reported by de Moreno et al. (Reference De Moreno, Gerpe, Moreno and Vodopivez1997) and Dalla Riva et al. (Reference Dalla Riva, Abelmoschi, Magi and Soggia2004).
Cobalt concentration was generally at the sub-µg g-1 level, except for the digestive gland of L. elliptica, where a concentration of 1.8–1.9 µg g-1 was detected for specimens collected in both sites. A slight cobalt accumulation may be observed also in the organs of T. bernacchii (0.09–0.16 µg g-1, while its concentration in the muscle is below the limit of detection). Literature data on cobalt levels in Antarctic biota are scarce. Kahle & Zauke (Reference Kahle and Zauke2003) determined a number of trace metals in Antarctic copepods from the Weddell Sea, reporting cobalt concentrations lower than 0.1 µg g-1 in all species investigated. Sures & Reimann (Reference Sures and Reinmann2003) analysed different tissues of the fish Notothenia coriiceps, caught at King George Island, South Shetland Islands. They found cobalt concentration below the limit of detection in the muscle and detectable concentrations in the liver and intestine (< 0.1 µg g-1), in good agreement with our results for T. bernacchii.
Chromium concentration values were at the sub-µg g-1 level in the benthic primary consumers Sterechinus neumayeri (0.7–0.8 µg g-1) and T. bernacchii (0.05–0.15 µg g-1) and in the arms of O. validus (0.7 µg g-1), without any significant difference between the sampling sites. Higher chromium concentration were found in the L. elliptica, mainly in its digestive gland (2–2.5 µg g-1), and in the soft tissues of O. validus (2.9–3.8 µg g-1). Higher concentrations of chromium (> 2 µg g-1) were also observed in the algae Phyllophora antarctica from both the sites. Chromium concentration in the soft tissues of A. colbeckii (1.0 µg g-1) collected in Terra Nova Bay is in good agreement with the values reported by Mauri et al. (Reference Mauri, Orlando, Nigro and Regoli1990) for the same organism and sampling site (0.3–1.5 µg g-1, depending on the tissue). Chromium accumulation in the L. elliptica (0.3–6.7 µg g-1, depending on the tissue) was also reported by Lohan et al. (Reference Lohan, Statham and Peck2001), although these authors indicated the kidney as the main target organ. Finally, the level of chromium found in the tissues and organs of T. bernacchii is in accordance with that reported by Sures & Reimann (2003) for the Antarctic fish Notothenia coriiceps (< 0.5 µg g-1).
Copper concentration values ranged from 1–41 µg g-1 dry mass and the distribution pattern was rather similar to that found for Cd, Co and Cr. The lowest value (about 1 µg g-1) was recorded in the muscle of T. bernacchii. However, metal accumulation was evident in its organs, mainly in the liver (16 µg g-1). High concentration values were also found in digestive gland of L. elliptica (23 µg g-1) and in the soft tissues of O. validus (30–41 µg g-1). Finally, considerably elevated copper concentrations (10–18 µg g-1) were found in the algae. The concentration value in the soft tissues of A. colbeckii (4.3 µg g-1) is consistent with the results by Mauri et al. (Reference Mauri, Orlando, Nigro and Regoli1990), Berkman & Nigro (Reference Berkman and Nigro1992) and Minganti et al. (Reference Minganti, Capelli and De Pellegrini1998) for specimens collected in the same site. The higher copper accumulation in the tissues of L. elliptica compared to A. colbeckii agrees with the finding by Ahn et al. (Reference Ahn, Lee, Kim, Shim and Kim1996). These authors reported a mean concentration value of 38 µg g-1 in the soft tissues of L. elliptica from Maxwell Bay, King George Island, which is significantly higher than that found in other marine bivalve molluscs in temperate and Antarctic waters. Finally, Negri et al. (Reference Negri, Burns, Boyle, Brinkman and Webster2006) reported copper concentration ranging from 4.2–23.8 µg g-1 (whole tissue) for specimens of L. elliptica sampled in the pristine sites Turtle Rock and Cape Evans, in good agreement with our data.
Manganese concentration in the muscle of T. bernacchii (0.7–1.3 µg g-1) was lower than in its organs (1.9–9.6 µg g-1), as already found for cadmium, cobalt and copper. Significant concentrations of manganese were also found in the algae Phyllophora antarctica (13–27 µg g-1) and the L. elliptica (3–36 µg g-1). For all the other organisms, levels of manganese were in the 1–8 µg g-1 range. Few data on manganese in Antarctic marine biota have been published. Ahn et al. (Reference Ahn, Lee, Kim, Shim and Kim1996) reported very high manganese concentrations in the organs of L. elliptica, ranging from 18.6 µg g-1 in the digestive gland up to 190 µg g-1 in the kidney. Conversely, significantly lower (1.4–27.5 µg g-1) values have been reported for the same organism by Lohan et al. (Reference Lohan, Statham and Peck2001). Manganese concentration in the tissues of A. colbeckii from Terra Nova Bay is in agreement with the measurements by Mauri et al. Reference Mauri, Orlando, Nigro and Regoli1990 and by Berkman & Nigro (Reference Berkman and Nigro1992), who obtained values in the 1.1–15.3 µg g-1 range. Finally, Sures & Reimann (2003) showed manganese accumulation in the organs of the fish Notothenia coriiceps, in agreement with our results for T. bernacchii.
Nickel concentration ranged from 0.14 to about 30 µg g-1 dry mass and the distribution pattern was similar to that found for manganese. Nichel concentration was at sub-µg g-1 level in the muscle of T. bernacchii (0.1–0.5 µg g-1), while slight higher values were detected in its organs (up to 1.1 µg g-1). Intermediate values (1–4 µg g-1) were found in the soft tissues of Sterechinus neumayeri, O. validus, L. elliptica and A. colbecki. Finally, significantly higher concentrations were observed in the arms of O. validus (8–10 µg g-1), in the digestive gland of L. elliptica (9–15 µg g-1) and in the seaweed Phyllophora (20–30 µg g-1). Nickel accumulation in the bivalve L. elliptica has been also indicated by Ahn et al. (Reference Ahn, Lee, Kim, Shim and Kim1996), who reported nickel concentration ranging from 2.74–21 µg g-1. Kidney appeared to be the preferential target organ, although a high concentration was also found in the digestive gland, gonad and gills. Significant nickel accumulation in the organs of L. elliptica (mainly kidney) has also been reported by Lohan et al. (Reference Lohan, Statham and Peck2001). Finally, the nickel concentration found in the tissues of A. colbecki is in good agreement with the values reported by Berkman & Nigro (Reference Berkman and Nigro1992) for specimens collected in different sites around Antarctica.
Lead concentration in the tissues of the investigated organisms was generally lower than 1 µg g-1, ranging from values below 0.1 µg g-1 in the tissues of Sterechinus neumayeri and T. bernacchii to concentrations close to 1 µg g-1 detected in the tissues of L. elliptica and in the seaweed. The analytical concentrations recorded were generally lower than those in literature. Negri et al. (Reference Negri, Burns, Boyle, Brinkman and Webster2006) found lead concentrations ranging from 0.3–5.9 µg g-1 in the whole tissue of L. elliptica sampled at Cape Evans. For the same organism, Ahn et al. (Reference Ahn, Lee, Kim, Shim and Kim1996) reported a mean concentration of 4.0 µg g-1 and Lohan et al. (Reference Lohan, Statham and Peck2001) reported values in the range 0.12–4.53 µg g-1. Finally, Minganti et al. (Reference Minganti, Capelli and De Pellegrini1998) found 0.64 ± 0.77 µg g-1 by the analysis of 25 samples of A. colbecki collected at Terra Nova Bay.
Vanadium concentration values ranged from 0.05 to about 18 µg g-1 dry mass. The lowest concentration was found in the muscle of T. bernacchii (0.05 µg g-1), while one order of magnitude higher values were detected in its organs (0.3–0.5 µg g-1). Relatively low vanadium concentrations (< 1 µg g-1) were also recorded in the tissues of Parbolasia corrugatus and in the arms of O. validus. On the other hand, vanadium accumulation was evident in the tissues of the other benthic invertebrates investigated, mainly in the digestive gland of L. elliptica (7–18 µg g-1). Finally, quite high vanadium concentrations (5–10 µg g-1) were found in the seaweed, from both the sampling sites, as already found for Cr, Cu, Mn and Ni. Literature on the occurrence of vanadium in Antarctic biota is very scarce. Apparently the only available data is that by Minganti et al. (Reference Minganti, Capelli and De Pellegrini1998), who measured vanadium concentrations in 25 samples of A. colbecki collected in Terra Nova Bay. The mean concentration for vanadium was 1.0 ± 0.3 µg g-1, in good agreement with the result obtained in this work.
Zinc concentration values ranged from 32–284 µg g-1, on an average of about 130 µg g-1. Zinc accumulation was evident in the organs of T. bernacchii (105–137 µg g-1) and in the tissues of most of the investigated benthic invertebrates. These results are generally consistent with literature data. Zinc concentrations in A. colbecki fall within the ranges reported by Mauri et al. (Reference Mauri, Orlando, Nigro and Regoli1990), Berkman & Nigro (Reference Berkman and Nigro1992) and Minganti et al. (Reference Minganti, Capelli and De Pellegrini1998). Similarly, levels of zinc in the tissues of L. elliptica agree with the values reported by Lohan et al. (Reference Lohan, Statham and Peck2001) for the same organism collected around Adelaide Island in the Antarctic Peninsula (96–289 µg g-1, depending on the organ). On the other hand, Ahn et al. (Reference Ahn, Lee, Kim, Shim and Kim1996) found much higher zinc concentration in the organs of L. elliptica from Maxwell Bay, King George Island (up to 1687 µg g-1 in the kidney), while Negri et al. (Reference Negri, Burns, Boyle, Brinkman and Webster2006) reported slightly lower values (48–172 µg g-1, in the whole organism) for specimens of L. elliptica sampled in the pristine sites of Turtle Rock and Cape Evans, in McMurdo Sound. Finally, concentration values similar to those obtained in this work were reported by dos Santos et al. (Reference Dos Santos, Silva-Filho, Schaefer, Sella, Silva, Gomes, Passos and Ngan2006) for the fish Trematomus newnesi (99 µg g-1), caught at King George Island.
Correlations and accumulation patterns
In order to analyse the correlation between the elements and to highlight statistically significant differences among the investigated organisms, all the data were treated by principal component analysis (PCA). Each object has been labelled as “XYZ”, where X indicates the sampling site (1 = Terra Nova Bay, 2 = Cape Evans), Y the species (T = Trematomus bernacchii, L = Laternula elliptica, and so on) and Z the analysed tissue (D = digestive gland, S = soft tissue, and so on). After autoscaling, three significant components were identified, explaining the 40%, the 21% and the 16% of the total variance, respectively. Hence, these components account for approximately 77% of the total variance. The loadings of the variables on these components are plotted in Fig. 2.
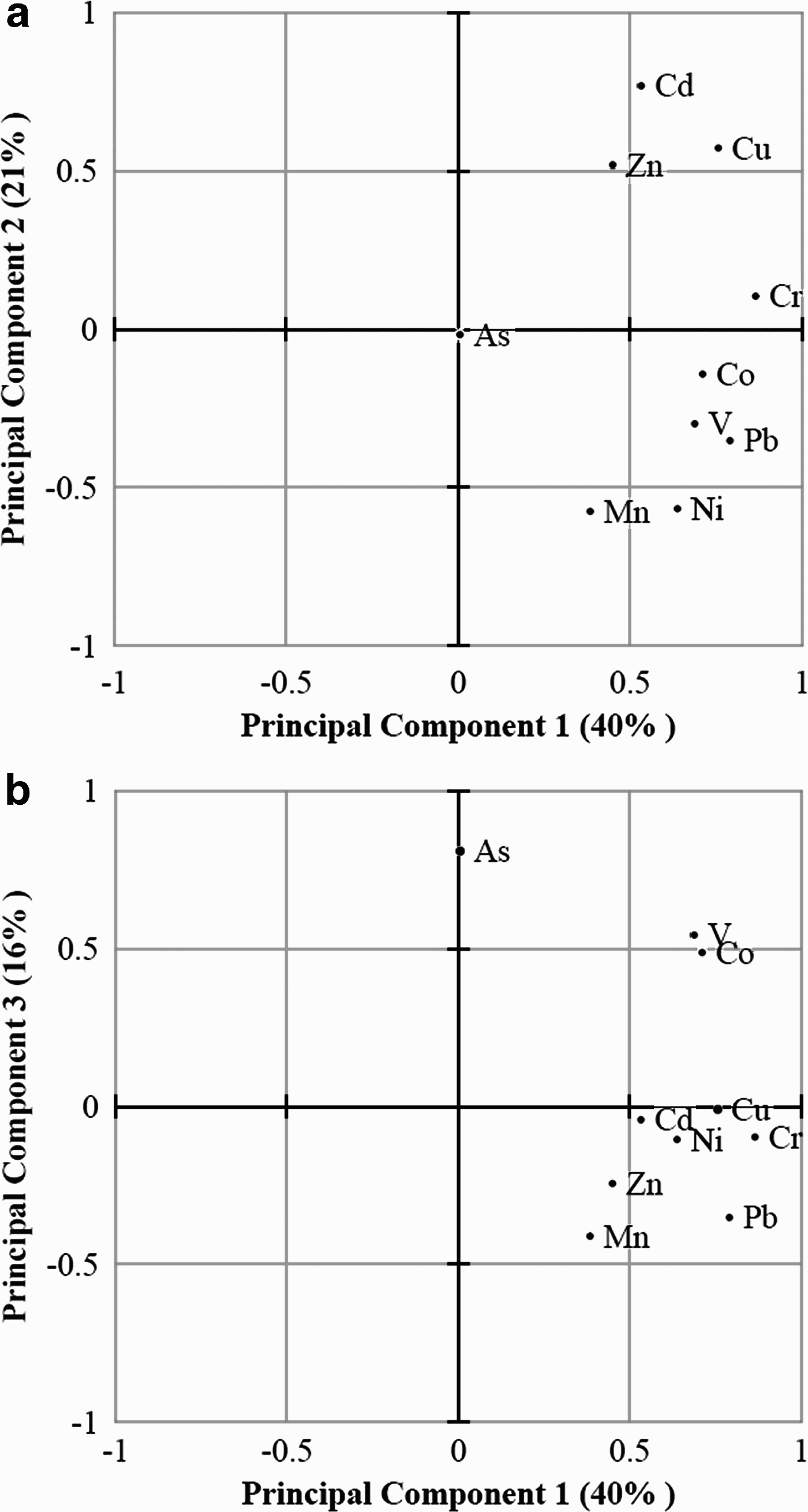
Fig. 2. Loading plots. a. Principal components 1 and 2, b. Principal components 1 and 3.
A good correlation between zinc, cadmium and copper may be observed, as well as among Pb, Cr, Co, V, Ni and Mn. Correlation is statistically significant at 95% probability level (except for Mn–Co and Mn–Cr). Arsenic behaves unlike the other elements and a third component was needed to explain data variability with respect to arsenic concentration. All the elements, except arsenic, directly load on the first component, meaning that an increase in that component corresponds to an increase in the metal concentration. On the other hand, an increase in the second principal component corresponds to an increase in the levels of cadmium, zinc and copper, but a decrease in the concentration of the other elements, mainly manganese and nickel. Finally, arsenic directly loads on the third component. The scores of the objects on the first three components are showed in Fig. 3. It can be clearly seen that O. validus, L. elliptica and Phyllophora antarctica differ significantly from the other species which form a quite homogeneous group. By considering the loading plots (Fig. 2), it is evident that these samples are separated from the others by significantly higher metal concentrations. In particular, the soft tissues of O. validus are primarily separated by the high concentrations of cadmium, zinc and copper (Fig. 2a), the P. antarctica by the high concentrations of manganese and nickel (Fig. 2a), and the digestive gland of L. elliptica by high concentrations of all the elements. Hence, metal accumulation was not the same for all the analytes, which depended on the organism and its characteristics. The strong accumulation of cadmium, zinc and copper found in the sea star O. validus can be ascribed to several factors: i) the wide range of utilized food items and types of feeding behaviour (Arnaud Reference Arnaud and Llano1977), ii) low pressure of predation, which determines high longevity (maybe more than 100 years), iii) detoxification processes due to the presence of metallothionein-like proteins (den Besten et al. Reference Den Besten, Herwig, Voogt, Zandee and Vernet1989).

Fig. 3. Score plots. a. Principal components 1 and 2, b. Principal components 1 and 3.
The seaweed Phyllophora antarctica exhibited rather high trace element concentrations, mainly nickel, manganese and, at minor extent, vanadium and lead. The concentrations were significantly higher than those observed in the other investigated species of algae (Iridaea cordata). This difference might be explained by the dissimilar sampling period. In fact, P. antarctica was collected in the early summer, before the beginning of significant biological activity, while I. cordata was sampled at the end of December after the phytoplankton bloom. In the latter situation, the metal concentration could be decreased by a dilution effect produced by an increase in the biomass, as already showed for the Antarctic scallop A. colbecki (Nigro et al. Reference Nigro, Regoli, Rocchi, Orlando, Battaglia, Valencia and Walton1997). Furthermore, the difference in the metal content between the species can be due to the peculiar physiological characteristics of each species, such as specific binding sites on the cell wall or specific decontamination processes, including extra-cellular release of certain compounds. For the same reasons, preferential accumulation toward specific elements (nickel and manganese in our case) can be observed.
The bivalve L. elliptica strongly accumulated all the elements, especially in its digestive gland (Fig. 3). This finding substantially confirms the results obtained by other authors, as reported above. Conversely, a discrepancy may be noted in the literature concerning the target organ. Some authors (Ahn et al. Reference Ahn, Lee, Kim, Shim and Kim1996, Lohan et al. Reference Lohan, Statham and Peck2001) observed preferential metal accumulation in the kidney, while others (Bargagli et al. Reference Bargagli, Nelli, Ancora and Focardi1996, Nigro et al. Reference Nigro, Regoli, Rocchi, Orlando, Battaglia, Valencia and Walton1997), indicated the digestive gland as the target organ for bivalve molluscs.
Comparison between the sampling sites can also be obtained by analysing the PCA plots. In fact, for each sample, the differences between the areas are highlighted by the relative position in the score plot of the objects 1YZ and 2YZ. As before, this information has to be analysed together with the relative loading plot. In this way, it can be clearly showed that the concentrations of cadmium and copper in the soft tissues of O. validus are significantly higher for specimens from Cape Evans than from Terra Nova Bay (2OS-1OS, Fig. 3a). Similarly, concentrations of manganese and nickel in the seaweeds P. antarctica (2PL-1PL, Fig. 3a), as well as the metal concentrations found in the digestive gland of L. elliptica (2LD-1LD, Fig. 3a & b) were higher for the specimens from Cape Evans than those from Terra Nova Bay. From raw data, it can be seen that this difference is particularly marked for arsenic, cadmium, manganese and vanadium. Since no relevant difference was observed between the sampling sites with regards to the element concentrations in the marine sediments, except for arsenic and lead (Table III), these differences cannot be ascribed to a different metal availability in the environment, but must be due to natural variability. This includes: i) different size and age of the pooled organisms, ii) different feeding strategies depending on the local food web, iii) temporal variation of organism biomass (Nigro et al. Reference Nigro, Regoli, Rocchi, Orlando, Battaglia, Valencia and Walton1997). Concerning the body size, specimens of O. validus collected at Cape Evans were smaller than those collected at Terra Nova Bay (Table I) and this could explain the differences in the content of cadmium, copper and zinc. In fact, negative correlation of these elements with body size has been observed in other benthic organisms and attributed to a higher growth rate and faster metabolism in the younger and smaller organisms than in the older ones, as well as higher surface absorption in the former animals (Boyden Reference Boyden1974, Hornung et al. Reference Hornung, Kress and Rameloc1991).
Natural variability of trace elements in Antarctic organisms
In an attempt to improve the understanding of the natural variability of trace elements in Antarctic organisms coming from different coastal sites around Antarctica, literature data for the bivalve L. elliptica have been considered together with data obtained in this work. The organism was selected because it has been seen as a suitable biomonitor for long-term monitoring of heavy metal contamination in the Antarctic coastal waters (Berkmann & Nigro 1992). Both the concentration in the whole tissue and in the digestive gland has been considered. If not directly reported, the concentration values in the whole tissue have been calculated by multiplying mean metal concentration in each organ by the percentage contribution of the organ to whole body burden and then combining all the multiplications. The ranges of concentration obtained are reported in Fig. 4. Since the mean values have been considered, these ranges indicate the variability in the mean metal content among the sites. These are Terra Nova Bay, Cape Evans and Turtle Rock in the Ross Sea, King George Island and Adelaide Island in the Antarctic Peninsula. All these sites have been considered as pristine environments (e.g. without significant anthropogenic impact), on the basis of the results from surface sediment analysis or environmental monitoring and assessment studies (Giuliani et al. Reference Giuliani, Kuneshka, Testa, Caroli, Cescon and Walton2001, Andrade et al. Reference Andrade, Poblet, Scagliola, Vodopivez, Curtosi, Pucci and Marcovecchio2001, Lohan et al. Reference Lohan, Statham and Peck2001, Negri et al. Reference Negri, Burns, Boyle, Brinkman and Webster2006). Even the values reported for the coastal environment of King George Island, which hosts ten research stations, have been evaluated to be natural, since the human contamination affects only the immediate surroundings of the stations (Ahn et al. Reference Ahn, Lee, Kim, Shim and Kim1996, Andrade et al. Reference Andrade, Poblet, Scagliola, Vodopivez, Curtosi, Pucci and Marcovecchio2001).

Fig. 4. Concentration ranges reported for the bivalve Laternula elliptica from different Antarctic coastal environments (Terra Nova Bay, Cape Evans, Turtle Rock, King George Island, Adelaide Island): a. mean values in the whole tissue, b. mean values in the digestive gland, c. all values reported. Sources: this work, Ahn et al. Reference Ahn, Lee, Kim, Shim and Kim1996, Nigro et al. Reference Nigro, Regoli, Rocchi, Orlando, Battaglia, Valencia and Walton1997, Lohan et al. Reference Lohan, Statham and Peck2001, Dalla Riva et al. Reference Dalla Riva, Abelmoschi, Chiantore, Grotti, Magi and Soggia2003, 2004, Negri et al. Reference Negri, Burns, Boyle, Brinkman and Webster2006.
Therefore, the reported variation ranges reflect the natural variability, which must be taken into account when referring to baseline levels. The natural variation in the metal content is even greater when taking into account also the variance among individuals and the different tissues, as showed in Fig. 4c, where the comprehensive ranges of all the reported concentration values for L. elliptica are visualized. The high variability can be ascribed both to the individual variability and to the different accumulation patterns of trace metals by aquatic invertebrates (Rainbow Reference Rainbow2002, Luoma & Rainbow Reference Luoma and Rainbow2005).
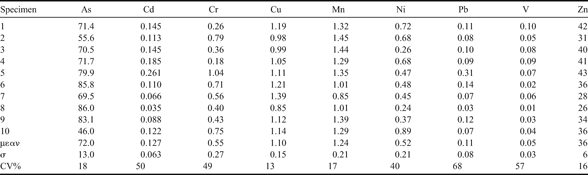
Variations of element concentration in individuals have also been evaluated for the muscle of the fish T. bernacchii, another suitable bioindicator organism (Jimenez et al. 1999, Dalla Riva et al. Reference Dalla Riva, Abelmoschi, Chiantore, Grotti, Magi and Soggia2003). Results are reported in Table V. Percentage variations (n = 10) were about 15–20% for As, Cu, Mn and Zn and 50–70% for Cd, Cr, Ni, V and Pb. Finally, no correlation between the element concentration and the body size was found. The finding is in agreement with the lack of correlation between heavy metals and fish size reported for different fish species from the Mediterranean Sea (Canli et al. Reference Canli and Atli2003) and South Indian Ocean (Bustamante et al. Reference Bustamante, Bocher, Cherel, Miramand and Caurant2003).
Table V. Element concentrations (µg g-1 dry mass) in muscle from ten specimens of Trematomus bernacchii from Cape Evans.
Conclusions
The assessment of the current status and the future degree of environmental pollution in Antarctica requires a confident definition of the baseline levels of potential contaminants. The main goal of this research was to improve the estimation of the natural variability and distribution of trace elements in Antarctic biota from uncontaminated coastal ecosystems. Multivariate analysis was used to highlight the correlation between the elements and to show general accumulation patterns.
Cadmium, copper and zinc were significantly correlated, thereby indicating similar accumulation pathways. These elements accumulated in the tissues of various benthic organisms, mainly in the soft tissues of Odontaster validus. The other elements analysed were also correlated, except for arsenic which showed a specific distribution. The general distribution trend among the benthic invertebrates was: primary consumers (Trematomus bernacchii and Sterechinus neumayeri) < filter feeders (Adamussium colbecki and Laternula elliptica) and detritivorous (Parbolasia corrugatus and Holoturia) < opportunistic predator (O. validus). The red alga Phyllophora antarctica also accumulated trace elements, mainly manganese and nickel. The metal accumulation was higher than that found for the other investigated algae species (Iridaea cordata), probably due to the different sampling period and peculiar characteristics of the species.
Finally, comparison of the data presented here with literature data for other uncontaminated coastal ecosystems highlighted a remarkably high natural variability in the metal content (up to 2 order of magnitude), which must be taken into account when interpreting results from biomonitoring programs.
Acknowledgements
This work was financially supported by the Italian National Antarctic Research Programme (PNRA), in the framework of project 9.2 involving the Antarctic Environmental Specimen Bank (BCAA).











