Introduction
Polar subglacial hydrologic systems have garnered much interest since the recognition of Lake Vostok in 1996. In Antarctica, these environments are hydrologically diverse, including isolated lakes of different sizes, river–lake flow-through systems, “swamps” and groundwater (Siegert Reference Siegert2016). The refreezing of subglacial meltwater is also an important process beneath a large portion of the East Antarctic Ice Sheet (Bell et al. Reference Bell, Ferraccioli, Creyts, Braaten, Corr, Das and Damaske2011). As subglacial water refreezes it exsolves salts, potentially leaving behind saline and hypersaline brines. Brines thought to derive from this cryoconcentration process have been observed in the northern polar permafrost regions and in the McMurdo Dry Valleys (MDVs) region of Antarctica. Additionally, sediments in the Victoria Land Basin have diagenetic signatures produced by brine movement dating from 3–11 m.y.a, suggesting hypersaline brines have existed in the McMurdo region since at least this time (Staudigel et al. Reference Staudigel, Murray, Dunham, Frank, Fielding and Swart2018).
Two other processes are known to produce subglacial hypersaline brines. Brines along the Antarctic coast are thought to come from the cryoconcentration of seawater (e.g. Frank et al. Reference Frank and Gui2010). An isolated complex of hypersaline subglacial lakes was discovered beneath the Devon Ice Cap in the Canadian Arctic, probably originating from a local salt-bearing geologic unit (Rutishauser et al. Reference Rutishauser, Blankenship, Sharp, Skidmore, Greenbaum and Grima2018).
Antarctic subglacial lakes can support viable microbial ecosystems (Christner et al. Reference Christner, Priscu, Achberger, Barbante, Carter and Christianson2014). The geochemistry of subglacial brines is inextricably linked to the structure and function of possible subglacial ecosystems where coupled biogeochemical cycles may support life. This short note demonstrates the variability of geochemical compositions of subglacial Antarctic inland brines potentially formed through cryoconcentration.
Methods
The chemical thermodynamic model FREZCHEM version 13.3 (Marion & Kargel Reference Marion and Kargel2008) and previously published subglacial fresh water data from Antarctica were used to model the chemical evolution of these waters as they are cryoconcentrated to −8°C. This temperature was used because it is the approximate temperature of the observed Taylor Glacier englacial brine (Lyons et al. Reference Lyons, Mikucki, German, Welch, Welch and Gardner2019). Data came from Lake Whillans (LW) (Christner et al. Reference Christner, Priscu, Achberger, Barbante, Carter and Christianson2014, Michaud et al. Reference Michaud, Skidmore, Mitchell, Vick-Majors, Barbante and Turetta2016), pore waters from the Kamb (KIS) and Bindshadler (BIS) ice stream sediments in West Antarctica (Skidmore et al. Reference Skidmore, Tranter, Tulaczyk and Lanoil2010), the predicted water chemistry of Lake Vostok (LV) in East Antarctica (Siegert et al. Reference Siegert, Tranter, Ellis-Evans, Priscu and Lyons2003) and subglacial outburst waters near Casey Station (CS) (Goodwin Reference Goodwin1988). Recently measured geochemistry of the englacial brine associated with Taylor Glacier (TG) in the MDVs is included for comparison, and is interpreted as having derived from the cryoconcentration of seawater modified by chemical weathering (Lyons et al. Reference Lyons, Mikucki, German, Welch, Welch and Gardner2019).
Results and discussion
The compositions of these brines are diverse (Table I). Although the LW, LV, TG, and CS brines are classified as Na–Cl waters, LW and CS have much higher Na:Cl ratios (1.26 and 1.36, respectively) than LV (0.74) and the observed TG brine (0.82). The LW and CS brines are similar, having much lower Ca and Mg concentrations, higher HCO3 concentrations than LV and TG, and similar elemental ratios. LW solutes are thought to come primarily from microbially mediated silicate mineral weathering with an additional seawater input (Michaud et al. Reference Michaud, Skidmore, Mitchell, Vick-Majors, Barbante and Turetta2016). The modelled KIS and BIS brines are Mg–SO4 > Cl waters. These may reflect different water−rock interactions, such as an influence of bedrock, reactivity, biogeochemical weathering or hydrological flow path. Provenance work on the tills in the Ross Embayment shows diverse geology (Lang Farmer et al. Reference Lang Farmer, Licht, Swope and Andrews2006). Skidmore et al. (Reference Skidmore, Tranter, Tulaczyk and Lanoil2010) argued that the solute concentrations in KIS and BIS pore waters are the result of both cryoconcentration and biogeochemical weathering through sulphide and organic matter oxidation. All the original subglacial waters have Na as the primary cation; initial KIS and BIS waters have Na:Cl ratios approximately an order of magnitude higher than the others. The higher sulphate concentrations in BIS and KIS lead to removal of Na and Ca though mirabolite (Na2SO4·10H2O) and gypsum (CaSO4·2H2O) precipitation, leaving Mg-SO4–rich brines.
Table I. Measured geochemistry and modelling results** of five subglacial waters cryoconcentrated to −8°C with modelled precipitated solid phases.
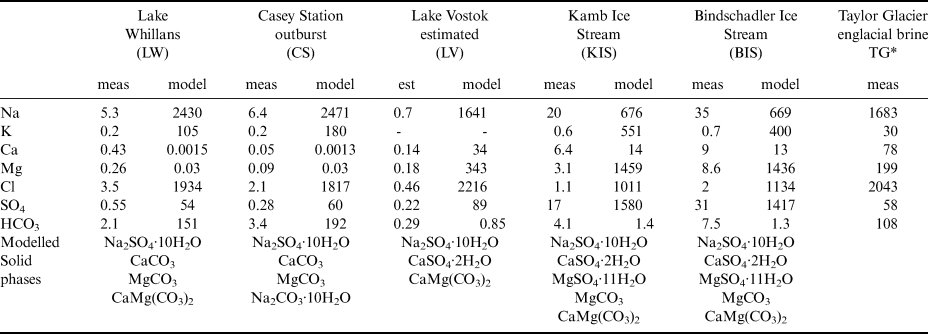
* Taylor Glacier englacial brine concentrations as measured at ~−8°C (Lyons et al. Reference Lyons, Mikucki, German, Welch, Welch and Gardner2019).
** Units are mmol l−1; meas = measured; est = estimated; model = modelled at −8°C.
There is evidence of microbial sulphide oxidation in both LW (Michaud et al. Reference Michaud, Skidmore, Mitchell, Vick-Majors, Barbante and Turetta2016) and the KIS and BIS pore waters (Skidmore et al. Reference Skidmore, Tranter, Tulaczyk and Lanoil2010). The modelling discussed here illustrates the potential for sulphate mineral precipitation (primarily mirabolite and gypsum) to control the chemistry of brines undergoing cryoconcentration, and the diversity of geochemical compositions that may occur in subglacial regions of Antarctica where meltwater freeze-on has occurred. Hypersaline waters as demonstrated in Table I could exist in various subglacial features, from lakes to much smaller cavity fillings, and even groundwater. Diverse brines have been documented in subaerial evaporative environments where subtle differences in the initial freshwater can occur. Evolution of brines and precipitation of salts are well understood theoretically. The modelling here suggests the possibility of a wide range of brine compositions in Antarctica's subglacial environments.
Acknowledgments
This work was supported in part by NSF grant ANT-1144176.
Author contribution
C.B. Gardner performed FREZCHEM modelling and both authors contributed to manuscript preparation.