Introduction
Even under the conditions of extreme dryness and low temperature that prevail in the McMurdo Dry Valleys, the soils contain organic carbon (Burkins et al. Reference Burkins, Virginia, Chamberlain and Wall2000, Barrett et al. Reference Barrett, Virginia, Parsons and Wall2005, Reference Barrett, Virginia, Hopkins, Aislabie, Bargagli, Bockheim, Campbell, Lyons, Moorhead, Nkem, Sletten, Steltzer, Wall and Wallenstein2006, Elberling et al. Reference Elberling, Gregorich, Hopkins, Sparrow, Novis and Greenfield2006, Hopkins et al. Reference Hopkins, Sparrow, Elberling, Gregorich, Novis, Greenfield and Tilston2006a, Reference Hopkins, Sparrow, Gregorich, Elberling, Novis, Fraser, Scrimgeour, Dennis, Meier-Augenstein and Greenfield2009, Sparrow et al. Reference Sparrow, Gregorich, Hopkins, Novis, Elberling and Greenfield2011), active organisms as indicated by CO2 production from respiration (Burkins et al. Reference Burkins, Virginia and Wall2002, Parsons et al. Reference Parsons, Barrett, Wall and Virginia2004, Barrett et al. Reference Barrett, Virginia, Hopkins, Aislabie, Bargagli, Bockheim, Campbell, Lyons, Moorhead, Nkem, Sletten, Steltzer, Wall and Wallenstein2006, Elberling et al. Reference Elberling, Gregorich, Hopkins, Sparrow, Novis and Greenfield2006, Hopkins et al. Reference Hopkins, Sparrow, Novis, Gregorich, Elberling and Greenfield2006a, Reference Hopkins, Sparrow, Elberling, Gregorich, Novis, Greenfield and Tilston2006b, Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a, Ball et al. Reference Ball, Virginia, Barrett, Parsons and Wall2009, Cary et al. Reference Cary, McDonald, Barrett and Cowan2010), enzymatic activities involved in the biogeochemical transformation of carbon, nitrogen, phosphorus and sulfur (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a, Zeglin et al. Reference Zeglin, Sinsabaugh, Barrett, Gooseff and Takacs-Vesbach2009), and communities of heterotrophic soil organisms with surprising diversity albeit with low total biomass (Friedmann Reference Friedmann1982, Treonis et al. Reference Treonis, Wall and Virginia1999, Stevens & Hogg Reference Stevens and Hogg2002, Barrett et al. Reference Barrett, Virginia, Wall and Adams2008, Smith et al. Reference Smith, Barrett, Tusnady, Rejtoe and Cary2010, Cary et al. Reference Cary, McDonald, Barrett and Cowan2010).
The relative harshness of the environment of the Dry Valleys makes them sensitive to change. Experimental manipulations of water content, nutrient addition and warming have all led to changes in the composition of the soil communities and the biological processes in the soils (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a, Simmons et al. Reference Simmons, Wall, Adams, Ayres, Barrett and Virginia2009, Sparrow et al. Reference Sparrow, Gregorich, Hopkins, Novis, Elberling and Greenfield2011). The respiration rates and enzyme activities respond to substrate and nutrient additions indicating that the community of soil organisms exhibits a physiological response to increased resource supply (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a, Sparrow et al. Reference Sparrow, Gregorich, Hopkins, Novis, Elberling and Greenfield2011).
We have used soil samples from a field experiment established in the Garwood Valley in the Dry Valleys region of southern Victoria Land to investigate the effects of addition of carbon and nitrogen on soil processes (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a, Sparrow et al. Reference Sparrow, Gregorich, Hopkins, Novis, Elberling and Greenfield2011). The specific objective of this experiment was to determine the effects of amending the soil with organic carbon and inorganic nitrogen, both singly and in combination, as glucose and ammonium chloride, respectively, on the soil biological responses. We have previously shown initial and small increases in soil microbial respiration in response to nitrogen addition were followed by larger and sustained respiratory responses to carbon addition, indicating that carbon supply was the main limitation to microbial activity (Sparrow et al. Reference Sparrow, Gregorich, Hopkins, Novis, Elberling and Greenfield2011). The soil enzyme activities indicative of the biogeochemical potential for carbon, phosphorus and sulfur transformations were altered to different extents by carbon and nitrogen additions. However, the increases in most enzymes assayed were smaller than those for respiration, indicating prioritization of the resources for energetic metabolism (catabolism) over biosynthesis (anabolism; Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a). This is presumably due to the severe limitations on energetic and nutrient resources in the Dry Valley soils.
Carbon and nitrogen additions to soils in the Maritime Antarctic have resulted in shifts in the soil microbial community composition (Dennis et al. Reference Dennis, Newsham, Rushton, Ord, O'Donnell and Hopkins2012) in a comparable carbon and nitrogen addition experiment to the Garwood Valley experiment. It is probable, therefore, that the physiological responses by soil organisms observed in the Garwood Valley experiment were also accompanied by any compositional changes in the soil community in responses to carbon and nitrogen addition. Therefore, in the present study, we have examined the ester-linked fatty acid (ELFA) concentrations and profiles in the soils to assess responses to carbon and nitrogen addition.
Materials and methods
The Garwood Valley experiment
The Garwood Valley is a small and relatively sheltered, coastal valley in the lee of the Royal Society Range in southern Victoria Land (Ross Dependency; 78°01′S, 163°53′E). The experiment was set up in the upper part of the valley which is a basin bounded by the Joyce and Garwood glaciers measuring c. 3 x 4 km containing Lake Colleen (0.6 km2 area, 350 m above sea level). Lake Colleen has an ice-free moat several metres wide most summers and occasionally has large ice-free expanses at the surface, and is relatively productive with conspicuous accumulations of lacustrine detritus, mainly of cyanobacterial origin, common at the lake edge (Gregorich et al. Reference Gregorich, Hopkins, Elberling, Novis, Greenfield, Sparrow and Rochette2006) which can be redistributed around the valley by the wind (Hopkins et al. Reference Hopkins, Sparrow, Gregorich, Novis, Elberling and Greenfield2008b). The experiment comprised 94 circular plots with 25 cm radius each marked in the centre with an aluminium stake laid out in an area of frost-heave polygons on soil that contained 1.1 mg organic carbon g-1 soil, 0.05 mg total nitrogen g-1 soil, 1.1 μg NO3--N g-1 soil and 1.0 μg NH4+-N g-1 soil, had pH 8.4, and was composed of mainly sand-sized particles and contained a negligible quantity of clay-sized particles. The plots were arranged in a randomized block design with four blocks each on a separate polygon. The experimental treatments were imposed over a period of three days during January 2003 (further details of the experiment are contained in Hopkins et al. (Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a) and Sparrow et al. (Reference Sparrow, Gregorich, Hopkins, Novis, Elberling and Greenfield2011)). For the current work we have analysed soil samples from 36 of the plots which had received glucose and ammonium chloride in factorial combinations, replicated four times (Table I). During January 2006, three years after the treatments were imposed, soil samples from the 0–5 cm depth of the plots were collected and sieved to pass a 2 mm sieve in the field to remove large stones, sealed in double polythene bags, stored at the field temperature (in the range -5 to +2°C) for up to seven days. They were transported to Scott Base on Ross Island, where they were stored below 0°C until transport to New Zealand and subsequent transport by refrigerated air freight to the UK where they were stored frozen.
Table I Summary of experimental treatments applied to field plots in the Garwood Valley in January 2002 and sampled in January 2006.

Laboratory incubation
While still frozen, each soil sample was divided into two portions one of which was kept frozen. The other portion was thawed and incubated for ten days at 10°C. This laboratory incubation was performed to simulate peak summer conditions in the Garwood Valley and allowed the further response to carbon and nitrogen addition to be monitored. Both the incubated and the unincubated (frozen) soils were subject to ELFA analysis as below.
Ester-linked fatty acid analysis
The analysis of ELFAs limited to known microbial fatty acids allows the rapid fingerprinting of lipids in the soil of microbial origin (Drijber et al. Reference Drijber, Doran, Parkhurst and Lyon2000, Steger et al. Reference Steger, Jarvis, Smårs and Sundh2003, Hinojosa et al. Reference Hinojosa, Carreira, Garcia-Ruız and Dick2005; Table II). Ester-linked fatty acids were extracted from 10 g of soil “spiked” with a 3 μg aliquot of the fatty acid 23:0 as an internal standard, and were then methylated using the method of Schutter & Dick (Reference Schutter and Dick2000) and analysed by gas chromatography (GC). The GC analyses were conducted on an Agilent Technologies 6890N Network GC fitted with a flame ionization detector and an Agilent HP-5 column (Agilent Technologies, UK) with helium as the carrier gas. The temperature programmed resolution of individual fatty acid methyl esters was carried out at 160°C for 2 min, 160–270°C at 4°C min-1, and then at 270°C for 10 min. Ester-linked fatty acids were identified by comparing retention times with commercial (bacterial acid methyl esters mixture; Supelco, USA) and in-house standards, and then quantified by relating the area of individual peaks to that of the internal standard. The concentrations of ELFAs with 14–19 carbon atoms expressed in nmol ELFA g-1 dry weight soil were used as proxies for soil microbial biomass. Ester-linked fatty acids with fewer than 14 or more than 19 carbon atoms were excluded from the analysis because they are usually from non-microbial sources (Zelles et al. Reference Zelles, Bai, Rackwitz, Chadwick and Beese1995, Leckie et al. Reference Leckie, Prescott, Grayston, Neufeld and Mohn2004). Changes in the ELFA profiles were assessed by comparing the Shannon-Weiner diversity index (Shannon Reference Shannon1948) for the ELFA profiles which emphasizes the richness of the ELFA profile (i.e. the number of ELFAs present), and the Simpson diversity index (Simpson Reference Simpson1949) which emphasizes the evenness or equitability of the ELFA profile.
Table II Summary of ester-linked fatty acids (ELFAs) used as markers.
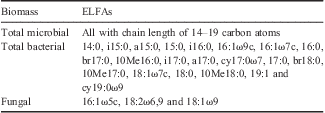
ELFAs are designated as the number of carbon atoms followed by a colon, then the number of double bonds and their position(s) from the aliphatic (ω) end. The prefixes a, i, Me, OH, cy, and br refer to anteiso, iso, methyl, hydroxyl, cyclopropane, and unknown branching respectively. A number followed by Me indicates the position of a methyl group.
Statistical analysis
The effects of the experimental treatments (carbon and nitrogen amendment, and the incubation in the laboratory) were tested by analysis of variance using the GenStat statistical system (GenStat 11th edition, Lawes Agricultural Trust, VSN International, Hemel Hempstead, UK). Post-hoc comparisons of individual means was done using Tukey's honestly significant difference statistic calculated at the P < 0.05 level. When necessary the data were logarithm-transformed, square root-transformed or transformed by addition of half the minimum value to remove zero values and then taking logarithms to meet assumptions of normality. For each parameter (total ELFA concentration, Shannon index and Simpson index) the effects of carbon and nitrogen additions and of incubation were analysed by three-factor analyses of variance, with the carbon and nitrogen treatments each having three levels (0, 1 and 10 mg carbon or nitrogen g-1 soil) and the incubation treatment having two levels (incubated and unincubated).
Results
Ester-linked fatty acid concentrations
The ELFA concentration of the unincubated soil was 56 nmol ELFA g-1 soil, but declined to 37 nmol ELFA g-1 soil after incubation for ten days at 10°C, a decline of 33% (Fig. 1). Declines of similar magnitude during incubation were observed for the soils that had received the large carbon amendments, but they were not statistically significant (Fig. 1).

Fig. 1 Total ester-linked fatty acid (ELFA) concentrations for Garwood Valley soils from substrate amendment experiment before and after laboratory incubation. Each bar is the mean of four replications and the error bars are ± standard error.
Ester-linked fatty acid responses to carbon and nitrogen addition
There was no significant effect of low nitrogen treatment and the high nitrogen treatment reduced the total ELFA concentration in both the unincubated and incubated soil (Fig. 1). There were positive responses in both the unincubated and incubated treatment to the carbon additions, with the high carbon addition leading to significantly greater ELFA concentrations than the low carbon additions (Fig. 1). It was also notable that the high carbon addition treatments led to increases in the variance in ELFA concentrations (Fig. 1). When carbon and nitrogen were added in combination, the positive effects of carbon were significantly repressed or removed completely by nitrogen addition in the low carbon addition (Fig. 1). The same trend was observed for the high carbon addition, although the effect of the low nitrogen addition was not strong enough to reduce the effect of carbon addition significantly (Fig. 1). In all cases the ELFA concentrations were less for the incubated compared with the unincubated treatment except for the two high carbon additions, for which the differences were not significant (Fig. 1).
Ester-linked fatty acid profile diversity
The only differences in the Shannon or the Simpson diversity indices were significant decreases for both the high carbon and the high carbon with low nitrogen treatments, indicating reductions in both richness and evenness, respectively (Figs 2 & 3).

Fig. 2 Shannon index values derived for ester-linked fatty acid (ELFA) diversity for Garwood Valley soils from substrate amendment experiment before and after laboratory incubation. Each bar is the mean of four replications and the error bars are ± standard error.

Fig. 3 Simpson index values derived for ester-linked fatty acid (ELFA) diversity for Garwood Valley soils from substrate amendment experiment before and after laboratory incubation. Each bar is the mean of four replications and the error bars are ± standard error.
Discussion
The microbial biomass of a range of UK agricultural soils measured using the same EFLA approach was in the range 74–94 nmol ELFA g-1 soil (Gregory et al. Reference Gregory, Watts, Whalley, Kuan, Griffiths, Hallett and Whitmore2007), compared with 37 or 56 nmol ELFA g-1 soil in the control soils from the Garwood Valley experiment (Fig. 1). These smaller estimates of microbial biomass for the Garwood Valley soils are consistent with earlier measurements of low respiration rates from dry valley soils (Parsons et al. Reference Parsons, Barrett, Wall and Virginia2004, Barrett et al. Reference Barrett, Virginia, Parsons and Wall2005, Elberling et al. Reference Elberling, Gregorich, Hopkins, Sparrow, Novis and Greenfield2006, Hopkins et al. Reference Hopkins, Sparrow, Elberling, Gregorich, Novis, Greenfield and Tilston2006a) and both the low enzyme activities and the respiration rate for soils from the Garwood Valley experiment (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a, Sparrow et al. Reference Sparrow, Gregorich, Hopkins, Novis, Elberling and Greenfield2011). The data also contrast with less environmentally-extreme soils in the Maritime Antarctic which tend to have larger and more active microbial communities (Pietr et al. Reference Pietr, Tatur and Myrcha1983, Bölter et al. Reference Bölter, Kandeler, Pietr and Seppelt2002, Tscherko et al. Reference Tscherko, Bölter, Beyer, Chen, Elster, Kandeler, Kuhn and Blume2003).
The control soils from the Garwood Valley experiment had ELFA concentrations in the range 37–56 nmol ELFA g-1 soil and contained only 0.11% organic carbon, whereas Gregory et al. (Reference Gregory, Watts, Whalley, Kuan, Griffiths, Hallett and Whitmore2007) reported temperate soils with 74–94 nmol ELFA g-1 soil for soils containing 4.0–8.7% organic carbon. So, although the Garwood Valley soil contained between 40 and 80 times less organic carbon than the temperate soils, they had only about half the EFLA concentration. Therefore, relative to the organic carbon content of the soils, the microbial biomass as indicated by ELFA concentration in the Garwood Valley soil was large. A large microbial biomass-to-organic carbon ratio is consistent with the fast turnover of organic matter in these soils (Burkins et al. Reference Burkins, Virginia, Chamberlain and Wall2000, Elberling et al. Reference Elberling, Gregorich, Hopkins, Sparrow, Novis and Greenfield2006, Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a). The larger experimental carbon addition increased the soil organic carbon concentration from 0.11% to a maximum of 1.11% before any respiration loss and was accompanied by increases in ELFA concentrations to 211 or 290 nmol ELFA g-1 soil three years later. It is not possible to estimate the amount of added C that remained in the soil after three years, but even though it will have been depleted to some extent, the ELFA concentration of the Garwood Valley soils substantially exceeded the ELFA (range 74–94 nmol ELFA g-1 soil) for the temperate soils containing substantially more organic carbon (4.0 and 8.7% carbon; Gregory et al. Reference Gregory, Watts, Whalley, Kuan, Griffiths, Hallett and Whitmore2007). This indicates efficient conversion of glucose carbon into microbial biomass in the Garwood Valley soil.
The decline of 33% in ELFA concentration from 56–37 nmol ELFA g-1 soil in the control (un-amended) soil during incubation in the laboratory is probably the result of substrate depletion in the control soil. The declines in ELFA concentrations in the carbon-amended soils during incubation were of similar magnitude, but not significant, and also suggestive of substrate depletion. Like the positive response of ELFAs to carbon addition, these observed declines in ELFA during incubation also suggest relatively rapid assimilation of carbon into microbial biomass, and are consistent with the high biomass-to-organic carbon ratio in these soils and the proposed small reserves of energetic substrate relative to microbial biomass. The ELFA concentration decline of 33% during ten days’ incubation is large by comparison with the data of Hopkins et al. (Reference Hopkins, Waite and O'Donnell2011) who reported biomass declines of between 10 and 80% during incubation of temperate grassland soil for 200 days at 20°C, especially since the Q10 values for respiration in the Garwood Valley soil are between 2.0 and 4.4 for the 9–20°C range (Hopkins et al. Reference Hopkins, Sparrow, Elberling, Gregorich, Novis, Greenfield and Tilston2006a). Overall, therefore, the proportions of ELFA-to-organic carbon in the Garwood Valley soils and the rapid depletion of both the indigenous and the added carbon indicate that the Garwood Valley soils are characterized by small and rapidly utilized organic (energetic) resources and microbial communities capable of rapidly utilizing the available carbon provided there are suitable environmental conditions, and is supported by the relatively rapid turnover times of organic carbon in dry valley soils (Burkins et al. Reference Burkins, Virginia and Wall2002, Elberling et al. Reference Elberling, Gregorich, Hopkins, Sparrow, Novis and Greenfield2006, Hopkins et al. Reference Hopkins, Sparrow, Gregorich, Elberling, Novis, Fraser, Scrimgeour, Dennis, Meier-Augenstein and Greenfield2009).
The positive responses of total ELFA concentration to the high carbon addition indicate carbon limitation leading to biosynthesis consistent with increased enzyme activities previously reported (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a), however, no such effect of nitrogen addition on total ELFA were detected (Fig. 1). In a comparable experiment at sites in the Maritime Antarctic (less environmentally extreme), increases in total ELFA concentrations were observed for both carbon and nitrogen addition (Dennis et al. Reference Dennis, Newsham, Rushton, Ord, O'Donnell and Hopkins2012). This suggests that the microbial response in the more extreme Garwood Valley was not constrained by nitrogen supply over the three year period of the field experiment, although we have shown that the respiratory response were initially (0–6 days) limited by nitrogen supply (Sparrow et al. Reference Sparrow, Gregorich, Hopkins, Novis, Elberling and Greenfield2011), but that other factors intervened between carbon and nitrogen to limit the microbial response. The lack of a sustained nitrogen limitation to microbial activity is consistent with a carbon-to-nitrogen ratio of this soil (22) which is well within the range for temperate soils, where N limitation rarely limits microbial activity (Killham Reference Killham1994, Brady & Weil Reference Brady and Weil1999) and the likely availability of inorganic forms of nitrogen (ammonium and nitrate) in the Dry Valleys (Barrett et al. Reference Barrett, Virginia and Wall2002, Elberling et al. Reference Elberling, Gregorich, Hopkins, Sparrow, Novis and Greenfield2006).
Considering the unincubated treatments, there were 5.6-fold and 4.1-fold increases in total ELFA concentrations following the high carbon and the high carbon plus low nitrogen additions, respectively (Fig. 1). These increases contrast with 2.0-fold and 5.5-fold increases in activity of acid phosphatase and aryl sulfatase activities, respectively, no significant change for alkaline phosphatase and β-glucosidase, and a reduction in dehydrogenase activity following the high carbon additions (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a). Thus, the relationships between biomass responses to glucose and enzyme activity responses were variable. There are at least two possible reasons for this variability. First, there may have been greater demands for phosphorus or sulfur relative to carbon which would have triggered induction of phosphatase and sulfatase activities. Second, the enzymes catalysing a step in carbon mineralization (β-glucosidase) or in cellular respiration (dehydrogenase) are constitutively expressed so no changes would be expected, whilst those involved in phosphorus and sulfur cycling are inducible.
Considering the incubated treatments which are the most directly comparable set of treatments to the respiration measurements in Hopkins et al. (Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a), the total ELFA concentration increased 5.9-fold due to the high carbon addition (Fig. 1), whilst the respiration from similarly incubated soil increased by 8.8-fold (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a). These are both high responses as would be expected from glucose addition, but it is notable that the respiratory response was greater than the ELFA response. By contrast, the high nitrogen addition, which had no significant effect on the ELFA concentration in the incubated treatments (Fig. 1), led to a small but significant increase of 1.8-fold in the respiration rate (Hopkins et al. Reference Hopkins, Sparrow, Shillam, English, Dennis, Novis, Elberling, Gregorich and Greenfield2008a).
The changes in ELFA concentrations associated with carbon additions are indicative of a community structure shift reflected by the diversity indices, with both the Shannon and Simpson indices being significantly less for the high carbon additions indicating reductions in both richness and equitability (Figs 2 & 3). Community structure shifts, with a select group of organisms increasing in abundance whilst others declined to levels below which their contribution to the ELFA profile were not detected, therefore occurred as a result of the carbon additions.
Conclusions
This work has provided evidence for the large microbial biomass relative to organic carbon in the McMurdo Dry Valleys soils, compared with other soils, and the rapid organic matter processing indicative of some of the micro-organisms having an efficient scavenging strategy suited to a resource-poor and environmentally challenging habitat. The positive response in the ELFA concentration to carbon addition, but not consistently to nitrogen additions, confirms that carbon supply was the main constraint to biosynthesis. It is well established that soil microbial activity, biomass and community diversity in the Dry Valleys are influenced by available water, temperature, soil pH and other physical and chemical factors including electrical conductivity, nitrogen and carbon supply. Of these factors, availability of water, which is largely controlled by temperature, is probably the single most influential, but there are likely to be cascade effects as increased temperature and water availability will increase carbon and nitrogen supply.
Acknowledgements
We are grateful to Antarctica New Zealand for fieldwork and logistic support in the 2004–05 and 2005–06 field seasons. DWH also wishes to acknowledge additional support from the Royal Society (of London), the TransAntarctic Association, the Carnegie Trust for the Universities of Scotland, and the UK Natural Environment Research Council (NERC). This paper is an output from NERC grant (NE/D00893X/1; Biodiversity in Antarctic Soils). PMN acknowledges support from the Foundation for Research, Science and Technology (New Zealand). We also wish to thank Sandra Caul and Charlie Scrimgeour at SCRI for technical support and advice, and David Wardle for helpful discussions during the design of the field experiment. SCRI is supported in part by the Scottish Government. The constructive comments of the reviewers are also gratefully acknowledged.







