Introduction
Baleen whales (mysticetes) are a key component of the Antarctic marine ecosystem. Of the 13 species of baleen whale currently recognized globally, six species can be defined as true Antarctic whales: humpback (Megaptera novaeangeliae (Borowski)), blue (Balaenoptera musculus intermedia Burmeister), minke (B. bonaerensis Burmeister), fin (B. physalus (L.)), sei (B. borealis Lesson) and southern right whales (Eubalaena australis (Desmoulins)) which are generally found south of the Polar Front, at c. 50–60°S (Fig. 1) (Leaper et al. Reference Leaper, Zerbini, Bannister, Branch, Clapham, Donovan, Matsuoka and Reilly2008). Antarctic whales are defined as those populations that rely on the Southern Ocean as a habitat i.e. as a critical part of their life history, either through the provision of habitat for breeding or through the provision of a major source of food (Boyd Reference Boyd2009). While the Southern Ocean may not be a critical habitat for breeding for baleen whales, it is a critical habitat for major food resources. Baleen whales feed almost exclusively on plankton and krill (Nicol et al. Reference Nicol, Worby and Leaper2008). As the most abundant secondary producer in the Antarctic marine ecosystem, krill are also a key prey item for a number of other vertebrate predators. Hence, in a food web context, the link between baleen whales and krill is an interaction likely to influence other dynamic interactions in the Antarctic as well as ecosystem structure and function.
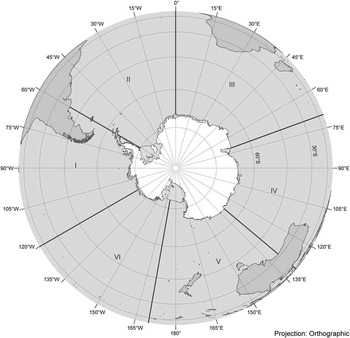
Fig. 1 International Whaling Commission Southern Hemisphere Management Areas for baleen whales (excluding Bryde's whales). Antarctica data is from the Antarctic Digital Database version 5 © Scientific Committee on Antarctic Research 1993-2006. Map provided by the Australian Antarctic Data Centre.
Antarctic baleen whales are generally large animals that have long reproductive lives and relatively low mortality rates. Given their life history characteristics, and the fact that they were once numerous predators in the Antarctic marine ecosystem, baleen whales are also ecologically significant as storers and movers of nutrients (especially carbon and nitrogen) and energy, within and between different components of the ecosystem and across its boundaries (Trites et al. Reference Trites, Bredesen and Coombs2004). Baleen whales transfer biological production directly from the bottom of the animal food chain to the top trophic level, and across ocean basins through their long-range annual migrations that link breeding and calving events in low latitude tropical waters to feeding events in high latitude polar waters (Nicol et al. Reference Nicol, Worby and Leaper2008).
At the beginning of the 20th century Antarctic baleen whales, like other targets of harvesting such as seals and flightless birds, were heavily depleted in a relatively short period. In 1904, the first commercial whaling operation in the Antarctic harvested 195 whales by way of a single Norwegian catcher boat (Nicol & Robertson Reference Nicol and Robertson2003). Between 1910 and 1930 there was a thirteen-fold increase in the numbers of whales taken, and at the height of the commercial period (1930/31) over 40 000 whales were taken in just one year. Consistently high catches were reported throughout the 1950s and mid 1960s, but by the late 1960s all but the smallest species of baleen whale in Antarctica had been severely depleted, some to near extinction (Tønnessen & Johnsen Reference Tønnessen and Johnsen1982, Gambell Reference Gambell1993, Baker & Clapham Reference Baker and Clapham2004). Concerns over both the conservation status of baleen whales and the state of the commercial whaling industry led to a global moratorium on commercial whaling that came into force in 1986. But by then, the rapid and systematic hunting of over 1.3 million whales (in only 70 years) had almost eliminated an entire trophic level of the marine ecosystem (Leaper et al. Reference Leaper, Zerbini, Bannister, Branch, Clapham, Donovan, Matsuoka and Reilly2008). It is not unreasonable to suppose that the loss of baleen whales had important and unique effects on the Antarctic marine ecosystem.
While the harvesting history of baleen whales is well documented, the response of the Antarctic marine ecosystem to this harvesting is far from understood. Not only is data with which to assess how the Southern Ocean changed before and during the depletion of baleen whales sparse, but data on whale numbers is often lacking for many populations, as is information on the impact of whales on other species and ecosystem processes (Kareiva et al. Reference Kareiva, Yuan-Farrell and O'Conner2006). This has meant that recent research efforts to understand the potential influence of whales and whaling on ocean ecosystems have had to rely on retrospection, analogy with other systems and broad ecological theory (see Estes et al. Reference Estes, Demaster, Doak, Williams and Brownell2006 and references therein). A clear understanding of the ecological effects of whales and whaling remains elusive. However, and alternatively, a great opportunity lies in asking the question “if whales recover, what will this mean for the Antarctic marine system?” Inarguably not killing whales allows for the potential for populations to recover, but current limitations on directed take do not expedite or promote the recovery process. As the diversity and intensity of human activities increase, rather than decrease in the Antarctic marine ecosystem (Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno, Pfeiffer, Scott and Snape2009) new threats will, quite conceivably, constrain baleen whale recovery.
In this review we discuss how the effective management of the contemporary Antarctic ecosystem necessitates a comprehensive consideration of baleen whales. By virtue of their large size, (once) large biomass, prey choice and potential to interact trophically with other species, baleen whales are an important ecological component of the Antarctic marine ecosystem. We synthesize our scientific understanding of baleen whale species recovery in light of threats not confined to direct take, and within the historical context of over-exploitation. We split the review into three parts. First, we discuss the history of exploitation of Antarctic baleen whales and their management by the International Whaling Commission (IWC) and other relevant International Organizations (IOs). Second, we review the current population status of baleen whales. Third, we qualitatively assess those threats believed to have some impact on Antarctic baleen whales with respect to species, time frames and Antarctic areas. We also discuss which threats are likely to have the greatest effect on Antarctic baleen whales. Finally, we discuss the possible ways to take forward this scientific understanding to underpin conservation efforts that include ecosystem-based management and the precautionary approach, concepts that form the modern basis for oceans management but are yet to be explicitly incorporated into baleen whale management in the Antarctic marine ecosystem.
Management of whales in the Antarctic
In order to clarify the historical development of whaling, Fig. 2 provides a chronology of some of the key events affecting the management of modern whaling in Antarctica (here south of 50–60°S). The culmination of the pre-war efforts to protect whale stocks and regulate the whaling industry came in 1948 with the entry into force of the International Convention for the Regulation of Whaling (ICRW) (signed in 1946) and the establishment of the International Whaling Commission (IWC). The original signatories of the ICRW adopted ‘a Convention to provide for the proper conservation of whale stocks and thus make possible the orderly development of the whaling industry’, implicitly recognizing the need to balance conservation and economics. The ICWR also formally assigned importance to the need for scientific advice, requiring that amendments to the regulations ‘shall be based on scientific findings’. However, despite this laudable aim, because the IWC could not restrict operations by numbers or nationality nor allocate quotas by operation, it struggled to manage the problems associated with increasing numbers of vessels chasing limited quotas. In particular, the use of the blue whale unit (BWU) (Donovan Reference Donovan1995) allowed catching of depleted species below levels at which catching that species alone would be economically unviable, and as blue whale catches declined, so fin whale catches increased until they too were overexploited and sei whale catching began (Fig. 3). In practice there was no agreed scientific procedure in place to calculate recommended catch limits, and the values chosen were largely the result of political negotiations (Donovan Reference Donovan1995). By the end of the 1960s over 657 000 whales had been taken commercially, of which 457 000 were fin whales (Fig. 3).

Fig. 2 Chronology of some key events in the management of whaling in Antarctica.

Fig. 3 Reported catch for five species of baleen whale in the Norwegian and Factory Ship Eras south of 40°S. Catch data as reported in Leaper et al. Reference Leaper, Zerbini, Bannister, Branch, Clapham, Donovan, Matsuoka and Reilly2008.
Efforts to manage baleen whales subject to commercial whaling operations were also hindered by illegal unreported and unregulated (IUU) whaling. Although not known at the time, 90 000 whales were taken in IUU operations in the Antarctic between 1947–72 (Brownell & Yablokov Reference Brownell and Yablokov2009). The former USSR alone was responsible for a total of 48 702 catches of humpback whales between 1947–73 (Clapham et al. Reference Clapham, Mikhalev, Franklin, Paton, Baker, Ivashchenko and Brownell2009). More than one-third of these (25 474 whales, of which 25 192 came from Areas V and VI) were taken in just two seasons, 1959–60 and 1960–61, and were taken at a time when humpbacks were already in serious decline from commercial hunts (Clapham et al. Reference Clapham, Mikhalev, Franklin, Paton, Baker, Ivashchenko and Brownell2009). IUU exploitation was not only restricted to humpbacks although they represented over half the catches and 3364 southern right whales (1950–71) and 23 165 sei whales (1947–72) were also taken illegally by Soviet whalers (Brownell & Yablokov Reference Brownell and Yablokov2009). Although there are known instances of illegal whaling by other nations, the catches made by the former USSR are by far the most significant (Brownell & Yablokov Reference Brownell and Yablokov2009).
By the 1970s the IWC adopted a more ‘science based’ approach to allocating catch quotas by using computer models to simulate the behaviour of the exploited populations. The work was conducted by a newly formed Scientific Committee (IWC-SC). These models required estimates of certain life history parameters such as natural mortality rates, pregnancy rates, recruitment rates, and in effect assumed that management stocks were the equivalent of biological stocks (Holt & Young Reference Holt and Young1990, Donovan Reference Donovan1995). This ‘New Management Procedure’ (NMP) was aimed at bringing all stocks of whales to an optimum level at which the largest number of whales could be taken consistently (the maximum sustainable yield or MSY) without depleting the stock. Complete protection was offered to those stocks thought to be below 54% of the original stock size, while catch limits were set below MSY (assumed to be 60%) for stocks greater than 54% of the original stock size, the degree to which depended on how far below the MSY level the stock was. In practice, although crude estimates of these parameters could be obtained, they could not be obtained to the level of precision required, even assuming the models really did reflect the dynamics of the populations. Ten years under the NMP resulted in an almost complete failure to protect whale populations from overexploitation and in 1982 the IWC agreed to introduce a ‘moratorium’, setting zero catch limits for all forms of commercial whaling for implementation in the 1985/86 summer. The designation of sanctuaries, or ‘no take’ areas in the Indian and Southern Oceans followed in the 1990s. Of particular importance for the Antarctic region was the Southern Ocean Sanctuary (SOS) which was established in 1994 with the explicit purpose of establishing an area in which commercial whaling was prohibited. The northern boundary of the SOS lies at 40°S except in the Indian Ocean sector where it provides the southern boundary at 55°S, and around South America and into the South Pacific where the boundary is at 60°S. Despite the ban on commercial whaling, scientific whaling has taken place in the SOS since 1987 under research programmes established by the Government of Japan. CRW Article VIII permits a Contracting Government to grant to any of its nationals a special permit authorizing that national to kill, take and treat whales ‘for purposes of scientific research subject to such restrictions as to number and subject to such other conditions as the Contracting Government thinks fit, and the killing, taking, and treating of whales in accordance with the provisions of this Article shall be exempt from the operation of this Convention.’
The amendment to the regulations had also included a clause that ‘the Commission will undertake a comprehensive assessment of the effects of this decision on whale stocks and consider modification of this provision and the establishment of other catch limits’. The IWC-SC defined such a comprehensive assessment as ‘an in-depth evaluation of the status of all whale stocks in the light of management objectives and procedures’ that ‘would include the examination of current stock size, recent population trends, carrying capacity and productivity’. Thus began the task of developing a management procedure that considered the limitations of both the data the IWC had and the data it was likely to obtain (Donovan Reference Donovan1995).
The Revised Management Scheme
Unlike the NMP which based its stock assessment on only the ‘best’ set of assumptions, the IWC developed and adopted ‘a management procedure approach’. Conceptually the new catch allocation approach (‘The Revised Management Procedure’, RMP) differed from the NMP in that management advice was to be based on a fully specified set of rules that would be tested in simulations of a wide variety of scenarios that specifically would take uncertainty into account (Punt & Donovan Reference Punt and Donovan2007). Central to the RMP was a generic method for calculating safe catch limits that could be applied to any baleen whale population on its feeding grounds given perfect knowledge of stock structure (referred to as the ‘Catch Limit Algorithm’, CLA). Again unlike the NMP, the RMP required only two types of data for the CLA - a series of historical catches by sex, and a series of absolute abundance estimates together with information on their uncertainty. In cases where there was uncertainty about stock boundaries in the region being managed so-called ‘multi-stock rules’ could also be used to modify the catch limits. Performance of the RMP rules were evaluated by computer simulation where a virtual whale industry was modelled over a 100 year period (Butterworth & Punt Reference Butterworth and Punt1999).
The RMP was developed over a six year period and formally accepted by the IWC-SC in 1994, but has never been formally adopted into the schedule. It has not yet been used to set catch limits given that the commercial whaling moratorium is still in place and no requests for advice on catch limits have been issued by the IWC. In addition the RMP is only the first step toward developing a programme to manage commercial whaling. Even though the RMP was adopted by the Commission, the Commission decided that before the RMP could be implemented for any set of whale stocks, several further aspects needed to be addressed as part of wider scheme to manage whaling under the ‘Revised Management Scheme’ (RMS) (Fig. 4). Since the 1990s discussions have centred on the question of what supervision and control measures should be included in the RMS. Negotiations on the RMS came to a halt in 2006, where unresolved issues included: 1) the level of international observer coverage required, 2) the type and level of tracking of whaling vessels required, 3) the frequency with which reporting information must be provided, 4) the maintenance and accessibility of a register of DNA profiles of all whales killed, 5) procedures to monitor the origins of whale products on the market, 6) oversight and review of the operation of the Scheme, and 7) the funding of the Scheme.

Fig. 4 IWC Management Procedure (adapted from Punt & Donovan 2007).
Contemporary management in the Antarctic marine ecosystem
Ocean governance has developed enormously since the negotiation of the ICRW, especially through the growth of a network of international law and institutions to govern human impacts on the seas (Gillespie Reference Gillespie2005). Those particularly important to the Antarctic marine ecosystem are detailed in Table I and include, the Antarctic Treaty System (ATS) and its associated instruments, the Convention on the Conservation of Antarctic Seals (CCAS), the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR) and the Protocol on Environmental Protection to the Antarctic Treaty (Madrid Protocol) (Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno, Pfeiffer, Scott and Snape2009). A variety of other instruments external to the ATS, and more global in nature, include the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), the Convention on the Conservation of Migratory Species of Wild Animals (CMS), the UN Convention on the Law of the Sea (UNCLOS) and the Convention on Biological Diversity (CBD) (Grant Reference Grant2005). A common feature of the IOs that have evolved since the ICRW is that they provide for action on emerging contemporary issues in the marine environment. These include the conservation and management of marine biodiversity (e.g. CBD), the application of precaution when dealing with uncertainty in the management of multiple-species and whole ecosystems (e.g. CCAMLR) and the potential effects of environmental change on marine organisms.
Table I International conservation instruments and their importance for baleen whales in Antarctica (adapted from Grant Reference Grant2005).

1Specifically for the Antarctic region.
Working relations between the IWC and other International Organizations (IOs)
Despite the fact that few international conservation instruments focus solely on whales, there is a clear cross-over between issues of broader oceans governance and the purposes of the ICRW (Table I). The IWC was built upon the intention that it would become the sole body to deal with whaling-related issues, and this has been reaffirmed by the international community several times since, even as whaling has ceased to be the main human interaction with whales (Gillespie Reference Gillespie2005). As overlapping IOs have developed, the IWC has been increasingly asked to contribute and take part in a number of international activities through cooperation between Conventions (IWC 1991). For many, especially within the direct UN family, the co-operation has been on an ad hoc basis and has been bolstered by strong working relations between the bodies, for example with the Food and Agriculture Organisation (FAO) and the United Nations Environment Programme (UNEP) when working on areas of mutual interest such as the FAO/UNEP Global Action Plan for Marine Mammals (adopted in 1984 but currently under review) (Gillespie Reference Gillespie2005). Conversely, where treaties have dealt directly with migratory species or the trade in endangered species and whaling issues surrounding Antarctica, they have been explicit in their deference to the IWC when dealing with whales. Even though the IWC has built strong relationships with CITES, CMS and CCAMLR, cooperation has been predicated upon the recognition of the Vienna Convention on the Law of Treaties (VCLT) principle, that when a treaty specifies that it is subject to an earlier or later treaty, the provisions of that treaty prevail (Gillespie Reference Gillespie2005).
CITES provides for the conservation of whales through, for example, the regulation of wildlife trade, and CMS the restoration of habitats and mitigation of obstacles to migration. Both conventions list species in ‘Appendices’ according to the degree of protection they need (Table II). Under CITES species threatened with extinction are listed in Appendix I of the Convention, and trade in specimens of these species is permitted only in exceptional circumstances. Species not necessarily threatened with extinction, but in which trade must be controlled in order to avoid utilization incompatible with their survival are listed in Appendix II of the Convention. Under CMS, migratory species threatened with extinction are listed in Appendix I, and species that need or would significantly benefit from international cooperation are listed in Appendix II. All six species of Antarctic baleen whales are listed in the CITES and CMS appendices.
Table II Conservation status of Antarctic baleen whales.

1With respect to Appendix I of the Convention on Trade in Endangered Species (CITES), Iceland, Norway and Japan hold reservations to specific listings that differ between the countries and populations. See http://www.cites.org/eng/app/reserve_index.shtml. However, even for these countries the reservations do not apply for the sei whale in areas from 0–70°E and from the equator to the Antarctic Continent, and for the fin whale in areas from 40°S to the Antarctic Continent and from 120–60°W. 2The subspecific taxonomy of blue whales is not yet fully elucidated.
CITES and CMS have based their decisions relating to Antarctic baleen whales on those of the IWC, consulting on both scientific data and coordination with any conservation measures. Support for the IWC has appeared in separate resolutions in both conventions and as such, the scientific basis by which CITES and CMS have opted for operating has in effect followed the lead of the IWC. A clear example of this is demonstrated through the process of listings of baleen whales under CITES. Prior to 1985 only blue, humpback and right whales were listed in Appendix I, with sei and fin whales listed in Appendix II. By the time of the Conference of the Parties at Buenos Aires in 1985, all six Antarctic baleen whale species were in Appendix I. The listing of minke whales on Appendix I was especially controversial, as for some member states it was a position that was thought to be more protective than the IWC. However, given that there was no scientific assessment to suggest that they were not endangered, and that more importantly, the IWC had listed them as protection stocks, in 1986 CITES agreed to include them in Appendix I (Gillespie Reference Gillespie2005). Japan, Iceland, Norway entered reservations to various species and some of these parties have made regular attempts to downgrade the listing of great whales to Appendix II since 1986. The listing of baleen whales under CMS followed in a similar manner. Appendix I listings of large cetaceans had been largely static since 1985 when only blue, humpback and right whales were included. In 2002, fin and sei whales were upgraded to Appendix I, while the minke whale was listed in Appendix II for the first time (Gillespie Reference Gillespie2005). As with CITES, the addition of minkes was particularly significant as they had earlier been declined Appendix II status, due to a suggestion that the IWC could give greater protection of this species. The CMS however, cognisant of that fact that it should ‘at the least, prohibit any taking that is not allowed under other agreements’, approved an Appendix II listing for the minke whale. For the time being, and as long as the moratorium is in place, the listing of baleen whales in both the CITES and CMS appendices ensures coordination with the IWC as mandated.
Unlike CITES and CMS, CCAMLR specifically provides for the conservation of marine resources of the Southern Ocean, not excluding harvesting carried out in a rational manner. Although CCAMLR specifically excludes whales from its convention, the Commission has a history of cooperating with IWC. The first crossover of issues concerned ecosystem considerations. In 1986, the IWC Scientific Committee responded to questions from CCAMLR on the suitability of whales as indicator species for krill availability (IWC 1987). Throughout the mid 1990s the links between the two secretariats strengthened over environmental concerns via a number of resolutions (IWC 1993, 1996, 1997) and in 1998, the IWC passed a resolution on ‘the Need for Research on the Environment and Whale Stocks in the Antarctic Region’ (IWC 1999). The resolution explicitly acknowledged a working group set up ‘to facilitate collaboration between the IWC and CCAMLR’ investigating aspects that related cetaceans to their habitat and to environmental change in the Antarctic. Accordingly, the two organizations have continued to work together on environmental issues. Of particular interest is recent work spearheaded by CCAMLR on the establishment of a network of marine protected areas, Antarctic specially protected areas, and Antarctic specially managed areas in the Antarctic Treaty area (IUCN 2005, SC-CCAMLR 2009). This work has focused on the recognition of protection to representative areas, scientific areas, and areas potentially vulnerable to impacts by human activities. Initial priority areas have been identified as part of bioregional assessment of environmental features and while these areas are not expected to become MPAs in their entirety, smaller areas within, but not limited to, the priority areas may be identified for designation as MPAs. Work on the establishment and prioritization of these given areas is ongoing.
Conservation management and the IWC
There is no doubt that the IWC has been repeatedly recognized as the primary authority for the management of whales in Antarctica, where management is limited to setting commercial catch quotas to zero i.e. through the moratorium and/or sanctuaries. The 1982 moratorium on commercial whaling is widely credited with saving many heavily-exploited whale populations from extinction and since it came into force it has allowed the limited recovery of some populations. In 1997, with an increasing awareness that whales should not be considered apart from the marine environment which they inhabit, and that detrimental changes may threaten whale stocks, the IWC decided that the IWC-SC should give priority to research on the effects of environmental changes on cetaceans. Since then, important topics addressed annually include pollutant and contaminant issues, physical and biological habitat degradation, the impact of noise and effects of fisheries.
The agenda of the IWC Commission however, has for decades been heavily weighted towards the ‘management of whale resources’ but in recent years the IWC has tried to broaden its mandate by establishing a ‘Conservation Committee’ to consider threats beyond the limited perspective of whaling (IWC 2004). The Conservation Committee was strongly backed by the pro-moratorium countries and just as strongly opposed by Japan and the countries supporting its views. The non-consensual nature of the Committee's establishment, illustrated quite clearly the divisive conflict that had developed in the IWC as to the scope of the Convention (Currie Reference Currie2007, Reeves Reference Reeves2009). The stated aim of the Conservation Committee was ‘to bring the IWC into the 21st century by transforming it from a traditional fishery management body to a modern conservation organization with a comprehensive agenda covering all aspects of the conservation of whales including protection from environmental threats’ (IWC 2004). In addition to helping to address these other threats in their own right, it was hoped that the broadening of the IWC's agenda would reduce the risk that its failure to achieve consensus on the regulation of exploitation (the RMS) would lead to the organization becoming dysfunctional to the detriment of whale conservation (Pew Environment Group 2009). Whaling countries argued that the central purpose of the ICRW was to regulate whaling, and contended that the objectives of the Conservation Committee were not in line with the dual objectives of the Convention, i.e. the conservation and management of whale resources. Other member nations argued that the ICRW should protect and restore the health and integrity of whale populations as part of the global oceans ecosystem; and furthermore that the Conservation Committee was fully consistent with the first objective of the ICRW. Despite the controversy, at its 2005 meeting the Committee agreed a Conservation Agenda that included two non-controversial though serious problems, ship strikes and ‘stinky’ grey whales harvested in Siberia (IWC 2006). As the IWC has broadened its mandate, a variety of IOs and IWC member nations have increased the pressure on the IWC to provide for action on emerging contemporary issues in the marine environment pertinent to whales.
Conservation management plans
At the same time as the development of an IWC Conservation Committee, various parties (including member nations of the IWC) have proposed the idea of management plans for whales in the Antarctic. The IWC has been called upon to develop comprehensive Management and Research Plans for the SOS.
Donovan et al. (Reference Donovan, Cañadas and Hammond2008) have proposed a methodology for developing effective conservation plans for cetaceans termed Conservation Action Plans (CAPs). As with the IWC's comprehensive assessments, this approach also highlights the necessity of establishing an in-depth understanding of species status and threats, yet also emphasizes the need for additional understanding of research needs, conservation and management targets, mitigation measures, administration requirements, in addition to feedback and monitoring. Reference is also made to the importance of cognisance of pressing regional conservation issues and current conservation actions as well as the relative importance of the given species and threat.
Building on the approach of Donovan et al. (Reference Donovan, Cañadas and Hammond2008) as well as addressing the issue of IWC oversight more directly, the Government of Australia has also proposed a management framework for conservation outcomes (Australia 2008). The stated impetus for proposing ‘Conservation Management Plans’ (CMPs) has been to ‘support the recovery of vulnerable cetacean species or regional populations and to address threats that affect multiple species’. The proposed framework, while not being specific on structure, lists numerous approaches that have been used successfully in wildlife management plans globally and that are applicable to whale management. Examples include recovery plans to improve the conservation status of threatened species, threat abatement plans to address key threatening processes, species action plans that prioritize management and research actions for conservation, and conservation plans for other taxa, values or protected areas. As with CAPs, CMPs are proposed as internationally-agreed, cooperative plans equipped to deal with all recognized threats to given whale populations, including small cetaceans. The main conservation outcomes would include 1) reduction of bycatch, 2) regulation of whale watching, 3) recovery of whale populations, and 4) the establishment of effective sanctuaries. The precept for CMPs is synchronization with other relevant international arrangements, strong support from member governments, and national adaptation of the more wide-reaching, regional CMPs (Australia 2008).
IWC synchronization with other relevant international arrangements has also been highlighted as crucial for whale conservation management in Antarctica by the Antarctic and Southern Ocean Coalition (ASOC). In a management plan submitted to the CCAMLR Commission meeting in 2008 (ASOC 2008a), they suggested that cooperation between the International Maritime Organization, CCAMLR, CBD, CMS and the Intergovernmental Oceanographic Commission of UNESCO would aid the IWC in management. In particular, the ASOC management plan proposed that the IWC instruct its SC to develop a comprehensive and non-lethal research programme to a) study and monitor the changes in the Southern Ocean ecosystem as they may affect whales, and b) to track the expected recovery of whale populations and the Antarctic ecosystem structure and properties since the moratorium and the designation of the SOS.
Current status of baleen whales in the Antarctic
Relatively little is known about the ecological role of baleen whales in the Antarctic marine ecosystem. Simply by virtue of their energetics, whales are important consumers. For example, Reilly et al. (Reference Reilly, Hedley, Borberg, Hewitt, Thiele, Watkins and Naganobu2004) estimated that even at current reduced densities, baleen whales in the South Atlantic sector of the Southern Ocean consume 4–6% of the biomass of krill in that area. Just how important the consumption of krill by baleen whales might be for Antarctic marine system however, is difficult to quantify for a number of reasons. Firstly, there are considerable uncertainties and assumptions in both data and models used to calculate consumption, especially for the larger baleen whales (Leaper & Lavigne Reference Leaper and Lavigne2007). Secondly, the Southern Ocean is not a single biome, but a suite of regional ecosystems where gradients in biological communities extend from the coast to the open ocean (Nicol et al. Reference Nicol, Worby and Leaper2008). Thirdly, there are considerable uncertainties in the estimates of krill stocks (Nicol et al. Reference Nicol, Worby and Leaper2008).
While a more recent body of work has increased our knowledge of baleen whale ecology, e.g. distribution in relation to prey abundance (Friedlaender et al. Reference Friedlaender, Halpin, Qian, Lawson, Wiebe, Thiele and Read2006, Santora et al. Reference Santora, Reiss, Loeb and Veit2010), resource partitioning (Friedlaender et al. Reference Friedlaender, Lawson and Halpin2009), feeding behaviour (Ware et al. Reference Ware, Friedlaender and Nowacek2010, Nowacek et al. Reference Nowacek, Friedlaender, Halpin, Hazen, Johnston, Read, Espinasse, Zhou and Zhu2011) and movement and migration (Stevick et al. Reference Stevick, Neves, Johansen, Engel, Allen, Marcondes and Carlson2010, Robbins et al. Reference Robbins, Dalla Rosa, Allen, Mattila, Secchi, Friedlaender, Stevick, Nowacek and Steel2011), most of what we know about Antarctic baleen whales, concerns numbers, for example historical abundances, population estimates and population trends (Kareiva et al. Reference Kareiva, Yuan-Farrell and O'Conner2006). This is not surprising given that the current IWC catch allocation approach, the RMP, requires abundance estimates as a key input. However, population estimates are also a key component of global conservation ‘assessments’ such as the listing of the risk status of species in the Red List maintained by the World Conservation Union (IUCN), established in 1948. Under the IUCN and in other conservation forums, a status listing and ranking system is often used to assess the probability that whales remain extant either in the present day or the near future (Table II). In this part of the review we detail what is currently known about Antarctic baleen whale population estimates and trends.
Population status
All six Antarctic whale species are migratory, and so population and estimates and trends may come from winter (breeding) or summer (feeding) grounds. Traditionally the IWC has divided the feeding grounds into six ‘Areas’ (I–VI, Fig. 1) for the purposes of management and population estimates and trends. Often population information is either estimated across management units or as part of management units and are scaled accordingly (Leaper et al. Reference Leaper, Zerbini, Bannister, Branch, Clapham, Donovan, Matsuoka and Reilly2008). Further complexity is added when estimates are reported from programmes using different survey designs. For some species, e.g. humpback and southern right whales information is also available for the low latitude breeding grounds, where a number of different ‘breeding stocks’ are recognized. The IWC assumes for the purpose of catch allocation, that the feeding grounds associated with each breeding stock can be defined. To date, stock boundaries have only been defined for humpbacks and are based on different ‘models’ according to different assumptions about mixing and sub-stock structure (IWC 2009a). However, when available, information from breeding stocks is an important complement to the estimates made for Antarctica and indicates the status of populations throughout their range.
Estimating the population sizes of whales before whaling has long served as a benchmark against which to evaluate status and recovery. The IWC set targets for whale population recovery based on the idea that a population below c. 54% of its pre-whaling level should be protected (Palumbi & Roman Reference Palumbi and Roman2006). However, it is difficult to reconstruct past populations accurately. Two main approaches have been used: those based on whaling removal data and those on genetic variability data. Demographic measures of past population sizes require 1) estimates of historical catch, 2) estimates of current abundance, and 3) a model relating mortality to population trajectory over time (Baker & Clapham Reference Baker and Clapham2004). Genetic measures of past population sizes require 1) estimates of genetic diversity corrected for gene flow, 2) estimates of generation time, and 3) estimates of mutation rates (Palumbi & Roman Reference Palumbi and Roman2006). Both whaling and genetic data are subject to uncertainty and rely on critical assumptions to make estimates. More importantly the methods often give conflicting results (e.g. see Palumbi & Roman Reference Palumbi and Roman2006) and confidence in any pre-whaling abundance estimate is probably premature, whichever method used. The only Antarctic whale species for which a genetic estimate is currently available is the minke whale (see Table III; Ruegg et al. Reference Ruegg, Anderson, Baker, Vant, Jackson and Palumbi2010), although there are published theta diversity values for southern right whales that would allow estimation of long-term abundance (see Jackson et al. Reference Jackson, Carroll, Smith, Patenaude and Baker2009).
Table III Demographic and genetic estimates of pre-whaling abundance.

N = no data are currently available. - = not applicable.
1Preliminary assessment not endorsed by the IWC.
2See Table IVb for sub-stock information. New Sabbatical reference case model.
3Preliminary assessment not endorsed by the IWC. Core reference case model.
4Preliminary assessment not endorsed by the IWC.
5It should be emphasized that this assessment is subject to many sources of uncertainty, particularly uncertainty in current abundance. For fin whales a previous estimate of 15 178 for (1979–88) instead of the most up to date estimated of Branch & Butterworth (Reference Branch and Butterworth2001). For sei whales an estimate of 11 000 (1979) has been used, which has not been endorsed by the IWC. In addition both estimates are made for the mature population only.
6Based on VPA modelling combined estimate for the Atlantic and Indian Ocean regions IWC Areas II, III & IV Pacific region IWC Areas V, VI & I (see Mori & Butterworth Reference Mori and Butterworth2006 for more details). Note that the estimate for current abundance used here has not been endorsed by the IWC.
7Long-term effective population size.
8Base case scenario for the ‘New Zealand + East Australia’ catch scenario, where all American pelagic whaling recorded east of 140°W and west of 140°E was included in the catch history. Coastal catches estimated from returns and exports in New South Wales and Tasmania (as described in Dawbin 1986) were included in addition to New Zealand coastal catches.
Humpback whales
Most populations of humpback whales have increased since the end of whaling, although there are several populations that remain small and for which no increase has yet been detected, i.e. the populations breeding near the South Pacific islands, Oceania sub-stocks E2, E3 & F. Humpbacks that include stocks A, B, C, D & E1 are currently listed as ‘Least Concern’ (Reilly et al. Reference Reilly, Bannister, Best, Brown, Brownell, Butterworth, Clapham, Cooke, Donovan, Urbán and Zerbini2008a), while the Oceania subpopulation is listed as ‘Endangered’ (Table II) (Childerhouse et al. Reference Childerhouse, Jackson, Baker, Gales, Clapham and Brownell2008). Feeding ground estimates for breeding stocks A, B and C are far lower than from the breeding grounds, while those for breeding stocks D, E and F are far higher. A combined breeding ground estimate equates to c. 65 500 whales and a feeding ground estimate c. 43 500. In the case of stocks A, B and C, it is widely agreed that the feeding ground estimates are likely to miss a substantial number of whales and therefore apply only to a portion of the population (see Zerbini et al. Reference Zerbini, Andriolo, Heide-Jørgensen, Pizzorno, Maia, Vanblaricom, Demaster, Simões-Lopes, Moreira and Bethlem2006b, Branch in press). For stocks D and E there has been some discussion as to whether firstly the breeding ground surveys cover the full distribution of these breeding stocks or secondly that a substantial number of whales do not migrate to the west and east coast of Australia each year (Branch in press). For stock F, data for the breeding areas is scarce (F1, Cook Islands and F2, French Polynesia), but a recent study by Robbins et al. (Reference Robbins, Dalla Rosa, Allen, Mattila, Secchi, Friedlaender, Stevick, Nowacek and Steel2011) reported a return movement of a humpback whale between the Antarctic Peninsula and American Samoa. There is no abundance estimate available for the Cook Islands (Hauser & Clapham Reference Hauser and Clapham2006), while the estimate for French Polynesia is based on only two out of 25 different islands where they have been sighted (Poole in press).
Breeding stocks C, D and E are by far the largest of the humpback sub-populations and in the Antarctic the highest abundances are found in IWC Areas IV and V. Interestingly humpback whales appear to be absent from the Ross Sea (Branch in press). According to the ‘comparable-areas’ estimates, circumpolar abundance estimates are increasing at 9.6% p.a. (Branch in press) while the rates of increase (available for four of the breeding stocks range) from 4.6–10.5% p.a. For stocks that are showing strong recovery, populations are 40–80% of their assumed pre-exploitation abundance, while stock A remains at only 20%. However, it would appear that for stock D, the population has recovered to such an extent that it now exceeds its pre-whaling abundance. The IWC has been unable to reconcile the current abundance of stock D with estimated historical levels using its demographic assessment models, and the issue is as yet unresolved (IWC 2007a). But despite the inherent difficulties in estimating past populations, it is reasonable to question whether pre-exploitation levels can be expected to provide a reasonable expectation of post-recovery carrying capacity.
Blue whales
There are currently fewer than 2000 blue whales in Antarctica and their numbers are still below 1% of their assumed pre-exploitation level (Branch et al. Reference Branch, Matsuoka and Miyashita2004). Accordingly blue whales are listed as ‘Critically Endangered’ by the IUCN and are considered one of the most at risk baleen whales in the Antarctic marine ecosystem (Reilly et al. Reference Reilly, Bannister, Best, Brown, Brownell, Butterworth, Clapham, Cooke, Donovan, Urbán and Zerbini2008b). A striking feature of the current distribution of blue whales is that modern sightings are aggregated close to the edge of the pack ice, while past catches extended further north. Whether this is due to retreat of the pack ice since the time of catching (de la Mare Reference De La Mare1997), or because the distribution of the species has contracted following exploitation, is unclear. For example, over 40 000 blue whales were caught in the waters around South Georgia, but the species is rare there now (Moore Reference Moore, Berrow, Jensen, Carr, Sears, Rowntree, Payne and Hamilton1999). Historical mark-recapture studies indicate that although blue whales are able to disperse entirely around the Antarctic, currently they remain restricted to a much narrower ring close to the pack ice and continental shelf and this restriction in range may increase their vulnerability (Branch et al. Reference Branch, Stafford, Palacios, Allison, Bannister, Burton, Cabrera, Carlson, Vernazzani, Gill, Hucke-Gaete, Jenner, Jenner, Matsuoka, Mikhalev, Miyashita, Morrice, Nishiwaki, Sturrock, Tormosov, Anderson, Baker, Best, Borsa, Brownell, Childerhouse, Findlay, Gerrodette, Ilangakoon, Joergensen, Kahn, Ljungblad, Maughan, McCauley, McKay, Norris, Rankin, Samaran, Thiele, van Waerebeek and Warneke2007). Until recently, there was little evidence for recovery in blue whales, but Branch (Reference Branch2007) has now estimated a ‘comparable-areas’ circumpolar rate of increase of 8.2% p.a. (Table IVa).
Table IVa Current estimates of Antarctic baleen whales on their feeding grounds adapted from Leaper et al. Reference Leaper, Zerbini, Bannister, Branch, Clapham, Donovan, Matsuoka and Reilly2008. N – no data are currently available. ‘–‘ not applicable.

1Naïve Model (IWC 2009a).
2The status of Antarctic minke whales is still currently under review within the IWC, although the IWC is nearing the end of a comprehensive review of their status. There are currently no agreed estimates and three have been presented here, based on different analysis methods. See IWC (2009b).
3Standard method (Branch Reference Branch2006).
4OK method (IWC 2009b).
5SPLINTR method (IWC 2009b).
6Scotia Sea grid of the SOWER 2000 survey (28.7–52.9°W).
7Antarctic Peninsula grid of the SOWER 2000 survey (49.5–72.1°W).
8Unsurveyed northern areas are taken into account to obtain estimates from ‘comparable areas’. The simple assumption employed by Branch & Butterworth (Reference Branch and Butterworth2001) and Branch (in press) is used here.
Fin whales
Fin whales are currently listed as ‘Endangered’ (Reilly et al. Reference Reilly, Bannister, Best, Brown, Brownell, Butterworth, Clapham, Cooke, Donovan, Urbán and Zerbini2008c) and in terms of total catch were the most heavily exploited whale in the Antarctic during the 20th century with over 718 000 animals taken (Fig. 3). For Antarctica (south of 60°S) the population is currently estimated to be less than 6000 (Branch & Butterworth Reference Branch and Butterworth2001) and just 2% of an assumed pre-exploitation abundance of about 325 000 whales (Reilly et al. Reference Reilly, Bannister, Best, Brown, Brownell, Butterworth, Clapham, Cooke, Donovan, Urbán and Zerbini2008c). Assessments of fin whale status have historically been problematic, mainly because a substantial proportion of the population is thought to range north of 60°S and surveyed areas do not represent their complete summer distributional range. The estimate of Branch & Butterworth (Reference Branch and Butterworth2001) therefore almost certainly represents an unknown fraction of their total abundance. However, it is not unreasonable to suppose that fin whales appear to be taking some time to recover (Branch & Butterworth Reference Branch and Butterworth2001).
Sei whales
Sei whales are the least known of the Antarctic baleen whales, and there are currently no agreed estimates for Antarctica. What is known is that with a total catch of over 125 000 animals between 1950s–70s, commercial whaling caused considerable declines throughout the species’ range. In the absence of dedicated surveys in sei whale habitat and resulting abundance estimates, it is not possible to assess whether there has been any increase in Southern Hemisphere sei whales since the cessation of whaling. The IUCN currently lists the sei whale as ‘Endangered’ based on (among other criteria) a population reduction over the period 1937–2007 of c. 75%, using data from IWC assessments conducted in the 1970s (Reilly et al. Reference Reilly, Bannister, Best, Brown, Brownell, Butterworth, Clapham, Cooke, Donovan, Urbán and Zerbini2008d).
Minke whale
Commercial whaling for minkes was not nearly as extensive as for other Antarctic baleen whales, and began much later in the 1970s (see Fig. 3). Since 1987, pelagic catching has continued under scientific permit - at a much reduced, but increasing, level. It is probable that the population size is in the hundreds of thousands, despite the fact there are no currently accepted estimates of current abundance for minke whales. Data analysed by standard methods (Branch Reference Branch2006) suggest a reduction of c. 60% through the 1978–91 (645 000) period and the 1991–2004 (338 000) period, but the IWC has been unable to determine whether the apparent decline is real or artefactual. If the decline is real its extent and causes are currently unknown, and it may still be continuing. Newer methods of analysing minke whale survey data, the OK method (Okamura & Kitakado Reference Okamura and Kitakado2009) and the SPLINTR (SPatial Line TRansect) (Bravington & Hedley Reference Bravington and Hedley2009) have yet to shed new light on the issue of current abundance as estimates differ significantly from each other (Table IVa). As a result, the IUCN has classified the minke whale as ‘Data Deficient’ until such time as IWC completes its minke whale comprehensive assessment (Reilly et al. Reference Reilly, Bannister, Best, Brown, Brownell, Butterworth, Clapham, Cooke, Donovan, Urbán and Zerbini2008e). The only study to calculate the long-term population size of minke whales (i.e. using genetic variability data) is that conducted by Ruegg et al. (Reference Ruegg, Anderson, Baker, Vant, Jackson and Palumbi2010). They estimated long-term abundance at 670 000 individuals, a value at least within the range of contemporary abundance estimates (Ruegg et al. Reference Ruegg, Anderson, Baker, Vant, Jackson and Palumbi2010).
Southern right whales
Southern right whales appear to making strong recoveries in some well-studied parts of the range, for example Argentina/Brazil, South Africa, and Australia (IWC 2001). Current estimates place the southern hemisphere population at about 7500 animals for which some sub-stocks have seen rates of increase between 7–8% p.a. (Table IVb). Although still scarce relative to historic abundance at only 12%, southern right whales are not considered under threat at the hemispheric level. Nonetheless, some breeding populations are still very small (e.g. Chile–Peru subpopulation), and data are insufficient to determine whether they are recovering. Right whales have been relatively slow to recover in the long-term, as compared to other species e.g. humpbacks, and many populations came perilously close to extinction during the late 19th and early 20th centuries (Jackson et al. Reference Jackson, Carroll, Smith, Patenaude and Baker2009). For the smallest extant stocks, it is not unreasonable to suppose that historically these populations suffered inverse dependent effects driven by factors such as loss of fitness a reduction in the benefits of sociality and demographic stochasticity (Courchamp et al. Reference Courchamp, Clutton-Brock and Grenfell1999). Like humpbacks, the IUCN has classified the southern right whale as ‘Least Concern’ (Reilly et al. Reference Reilly, Bannister, Best, Brown, Brownell, Butterworth, Clapham, Cooke, Donovan, Urbán and Zerbini2008f), but given the small size of the Chile/Peru subpopulation, it is currently listed as ‘Critically Endangered’ (Reilly et al. Reference Reilly, Bannister, Best, Brown, Brownell, Butterworth, Clapham, Cooke, Donovan, Urbán and Zerbini2008g).
Table IVb Current estimates of Antarctic baleen whales on their breeding grounds adapted from Leaper et al. Reference Leaper, Zerbini, Bannister, Branch, Clapham, Donovan, Matsuoka and Reilly2008. N – no data are currently available. ‘–‘ not applicable.

Recovery of baleen whales in the Antarctic
Baleen whales in present day Antarctica are for the most part characterized by small populations, at fractions of their assumed former abundance and often with restricted distribution. Recovery appears to be complex, occurring at different rates both temporally and regionally with the possibility that some populations may have even declined since the cessation of commercial whaling (although this remains controversial for some species such as minke whales). While there is no doubt that a number of humpback and southern right whale populations are showing strong recovery, other species populations and subpopulations continue to be of conservation concern. The long-term dynamics of Antarctic baleen whales will ultimately be affected by factors such as environmental change and density dependent limitations to growth. While baleen whales have developed life history strategies that keep them relatively buffered from interannual variability in environmental conditions (Wade Reference Wade2009), it is their response to longer-term environmental change that will be of primary importance to their long-term recovery and future status.
The ecosystems of the Southern Ocean are the product of the cumulative effects of harvesting as well as regional and global changes in the physical and biological environment (Nicol et al. Reference Nicol, Worby and Leaper2008). Sequential industrial overexploitation of many species other than whales occurred in the Southern Ocean throughout the 19th and 20th century. Seals and flightless seabirds were the initial targets of harvesting and as stocks of these were depleted, attention was focussed at lower trophic levels with fish, then krill and finally crabs and squid being taken (Nicol & Foster Reference Nicol and Robertson2003). In the physical environment, novel analyses of pre-satellite-era data have revealed increased mid-water ocean temperatures (Gille Reference Gille2003) and an overall 20–30% reduction in the extent of sea ice (Murphy et al. Reference Murphy, Clarke, Symon and Priddle1995, de la Mare Reference De La Mare1997, Reference De La Mare2009, Curran et al. Reference Curran, Ommen, Morgan, Phillips and Palmer2003). Since the late 1970s satellite remote sensing data combined with field and ship based observations have also detected changes in atmospheric circulation, including increasing and more southerly winds (Meredith et al. Reference Meredith, Murphy, Hawker, King and Wallace2008), regional differences in sea ice extent (Stammerjohn et al. Reference Stammerjohn, Martinson, Smith, Yuan and Rind2008, Turner et al. Reference Turner, Comiso, Marshall, Lachlan-Cope, Bracegirdle, Maksym, Meredith, Wang and Orr2009), changes in ocean state, e.g. freshening of the surface layer and deep water (Rintoul Reference Rintoul2007) and an increase in ocean acidification (Moy et al. Reference Moy, Howard, Bray and Trull2009).
With the removal of huge numbers of predators (including whales) from the Southern Ocean and the concomitant changes to the physical environment, it would be unrealistic to expect no change in the structure and functioning of the Antarctic marine ecosystem. It is also probable that the current carrying capacity is now different from that prior to pre-exploitation on both the feeding and breeding grounds (where environmental change may also have taken place). How this might affect a new status quo for whale populations however, is unknown, and will probably be further complicated by a multitude of threats that are not confined to directed take. Relatively little is known about how these threats may impact baleen whales in Antarctica, because (as far as we are aware) there has never been a comprehensive review for whales for this particular ecosystem. In the next part of the review we 1) identify and describe threats believed to have the potential to impact on Antarctic baleen whales, and 2) review the literature to identify which species are most likely to be impacted with respect to time frames and Antarctic areas (Table V). We also discuss which threats will probably have the greatest effect on Antarctic baleen whales and suggest key information needs for the future.
Table V Summary of threats likely to impact baleen whale populations in the Antarctic.

Threats to baleen whales
Table V summarizes threats likely to impact baleen whale populations with respect to species, time frames and Antarctic areas, and gives examples (from the literature where applicable) where impacts have been demonstrated. While commercial whaling is currently suspended, whales are still killed in scientific whaling operations in the Antarctic under Article VIII of the ICRW (Table V). The Japanese Whale Research Program Under Special Permit in the Antarctic (JARPA I and II) has been conducted every year from the 1987/88 to 2004/05 summer seasons (JARPA I) and every year since the 2005/06 summer season (JARPA II) and is currently the only programme to conduct scientific whaling in the Southern Ocean. Under JARPA II which began in the 2004/05 summer season there was a marked increase in the self-allocated quota by the Government of Japan. In both programmes minke and fin whales have been the target of harvest, although genetic monitoring surveys of Japanese market whale products (1993–2009) have detected tissue from 17 humpback whales, which suggests that at least this many may have been killed through entanglement or hunting (Steel et al. Reference Steel, Funahashi, Hamner and Baker2009).
The number of animals lethally sampled in the JARPA I and JARPA II programmes up to the 2008/09 summer and as reported to the IWC by the Government of Japan totalled 9136 whales, including 9122 minke whales and 14 fin whales. A large majority of the minke whales have been reported to be Antarctic minke whales although a relatively small number of dwarf minke whales have also thought to have been harvested. Given that there are no currently accepted estimates of current abundance for minke whales, it is difficult to assess the long-term effects of a continued and increasing catch of minke whales on the population. Apart from whaling under ‘Special Permit’, there appears no real prospect of large scale high seas commercial whaling operations resuming in the foreseeable future as whaling countries are never likely be able to gain the three-quarter majority of voting parties to overturn the moratorium. In addition discussions concerning the resumption of commercial whaling at the most recent IWC Commission meeting (held in Jersey in June 2011) failed. Apart from scientific whaling, the maintenance and operation of research stations and their associated logistics and scientific activities, tourism, and fisheries are the main human activities that currently take place in Antarctica (see e.g. Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno, Pfeiffer, Scott and Snape2009) that may impact Antarctic baleen whales. Threats to whales can also originate beyond Antarctica and include global (rather than local) problems such as climate change and ozone depletion and long-range marine pollution (see e.g. Aronsen et al. 2011).
Awareness of the threat of environmental contaminants to cetaceans is widespread but often difficult to disentangle from other anthropogenic impacts (Reijnders & Aguilar 2006). Pollution can arise from human activities that originate beyond Antarctica itself, for example from halogenated organic pollutants that are carried atmospherically from industrialized areas and then condense back into the ocean (Aguilar et al. Reference Aguilar, Borrell and Reijnders2002) or from chemical contamination and sewage disposal that occur as a result of the maintenance and operation of research stations and their associated logistics and scientific activities and tourism (Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno, Pfeiffer, Scott and Snape2009). Antarctic baleen whales can accumulate lipophilic compounds (e.g. halogenated hydrocarbons) and pesticides (e.g. dichloro-diphenyl-trichloroethane, DDT) in their blubber, as a result either of feeding on contaminated prey or inhalation in areas of high contaminant concentrations (e.g. regions of atmospheric deposition) (Barrie et al. Reference Barrie, Gregor, Hargrave, Lake, Muir, Shearer, Tracy and Bidleman1992, Wania & Mackay Reference Wania and Mackay1993). The range and degree of organic contaminants accumulated by minke whales biopsy sampled in IWC Management Areas IV and V has been described by Yasunaga et al. (Reference Yasunaga, Fujise, Zenitani, Tanabe and Kato2006) where on average, concentrations of these contaminants in minke whales were low relative to levels found in baleen whales in the Northern Hemisphere (Elfes et al. Reference Elfes, Vanblaricom, Boyd, Calambokidis, Clapham, Pearce, Robbins, Salinas, Straley, Wade and Krahn2010). Lower levels are not surprising since contaminants are at much lower concentrations in Antarctica than the industrialized Northern Hemisphere. Although contaminant levels can be readily measured if blubber biopsies are made available, the physiological consequences of persistent organic pollutants (POPs) body burdens are generally unknown in Antarctic baleen whales. In other regions some contaminants that are stored in blubber such as POPs can be mobilized metabolically during lactation (Aguilar & Borrell Reference Aguilar and Borrell1994). The result is that offspring receive a substantial inoculum of POPs as a transfer from their mother during nursing (Reijnders & Aguilar 2006).
Very little is known about the effects of other chemical contaminants on Antarctic baleen whales. Oil can damage skin, foul baleen, damage pulmonary and thoracic structures from inhalation of volatile components, and cause toxicity as a result of ingestion (Geraci & St Aubin 1990, Loughlin 1994). There have been two documented oil spill incidents in Antarctica in recent years - the Bahia Paraiso (Kennicutt et al. Reference Kennicutt, Sweet, Fraser, Stochton and Culver1991) and the MS Explorer (Republic of Liberia 2009), but there are no reported (or published) accounts of Antarctic baleen whales being affected.
The main human activities that contribute to ambient ocean noise in the Antarctic marine ecosystem are those associated with transportation, especially shipping and with seismic activity. While the numbers of ships in the Southern Ocean is still small in absolute terms there is a continuing upward trend from tour ships and from fishing vessels (ASOC 2008b). In many cases the tracks these ships use are limited and repetitive, as are those used by the Antarctic national operators undertaking resupply of the research stations, leading to a seasonal pattern of noise along distinct ‘highways’ with additional limited noise from short-term science activities elsewhere. This leaves much of the Southern Ocean largely free from ship noise. However, an understanding of the specific impacts of these sounds on Antarctic baleen whales is lacking. This is because few if any research programmes have looked specifically at noise impacts (but see Southall et al. Reference Southall, Bowles, Ellison, Finneran, Gentry, Greene, Kastak, Ketten, Miller, Nachtigall, Richardson, Thomas and Tyack2007 for other regions) despite increased concern from both the scientific community and a number of Parties to the Committee for Environmental Protection (CEP) (SCAR 2006). Despite an absence of hard data from Antarctica we do know that Antarctic baleen whales like many other marine animals rely on sound for short- and long-range communication, for orientation, and for locating prey (Tyack Reference Tyack2008) and they are particularly susceptible to the low-frequency sound (LFS - sounds at frequencies < 1000 Hz), the main frequencies used in shipping. Low frequency sounds can be a particular problem as they can travel great distances under water. Higher shipping traffic is of particular note for baleen whale communication and sound production give that the central frequency signal from shipping activity (the 20 to 200 Hz band) largely matches the frequencies used by baleen whales for some of their communication signals (Tyack Reference Tyack2008). Some seismic exploration is also ongoing in limited areas around the Antarctic continent, with a higher concentration on the eastern part of the continent, the Ross Sea and a small section of the Antarctic Peninsula (SCAR 2006).
While injuries and deaths resulting from ship collisions are a well documented threat to baleen whales (e.g. Best et al. Reference Best, Peddemors, Cockcroft and Rice2001, Tregenza et al. Reference Tregenza, Aguilar, Carrillo, Delgado and Diaz2002) it is only recently that efforts have been made to provide both a comprehensive and global assessment of such activities (see e.g. Laist et al. Reference Laist, Knowlton, Read, Collet and Podesta2001, Jensen & Silber Reference Jensen and Silber2004, Van Waerebeek & Leaper Reference Van Waerebeek and Leaper2007). For the Southern Hemisphere, especially the Antarctic marine ecosystem, information is particularly sparse where it is difficult to authenticate incidents reported from a wide range of sources, where there is a limited stranding response effort and a limited awareness of the problem. To date ship strike fatalities or injuries have been reported for only three animals in the western Antarctic Peninsula region; all humpback whales (Laist et al. Reference Laist, Knowlton, Read, Collet and Podesta2001, Jensen & Silber Reference Jensen and Silber2004, Van Waerebeek et al. Reference Van Waerebeek, Baker, Félix, Gedamke, Iñiguez, Sanino, Secchi, Sutaria, van Helden and Wang2007). Apart from certain species and areas there has been concern expressed about the inadequacy of information and statistics on ship strikes and there is a need for further data to be gathered so the extent of the problem can be assessed properly.
While wildlife tourism can build a valuable constituency out of a public interested in and sympathetic to marine mammals and wider conservation of the marine environment (Williams & Crosbie Reference Williams and Crosbie2007), there is also concern that tourist operations may also have a detrimental effect on whales (e.g. see pollution, noise and ship strikes above). In both the Ross Sea and the Peninsula region the majority of whale watching activity occurs in the summer between November and March. The most frequently sighted species of baleen whale are humpbacks, fins and minkes (O'Connor et al. Reference O'Connor, Campbell, Cortez and Knowles2009) where fin whales are often sighted near the continental shelves of the Peninsula and South Georgia, humpback and minke whales are most frequently found in the shallower, coastal waters. Encounters with blue whales and southern right whales can also occur on occasion (Williams & Crosbie Reference Williams and Crosbie2007). However, there have been few (if any) dedicated studies on the effects of whale watching on Antarctic baleen whales. In other regions tourist activities such as ship travel, small boat operations and landing operations can lead to a number of different potential impacts to baleen whales that include disruption to swimming or feeding activities, noise pollution, vessel strikes and habituation. Tourism to Antarctica has seen very substantial growth in visitor numbers since 1998 (over three-fold, IAATO 2010) which has translated into strong whale watching growth, but it is yet unknown how this may impact on baleen whales, if at all.
While fishing gear bycatch and entanglement are regarded as very serious threats to cetacean populations worldwide (Northridge Reference Northridge1991, Lewison et al. Reference Lewison, Freeman and Crowder2004, Read et al. Reference Read, Drinker and Northridge2006) there are virtually no reported incidents for Antarctica. The only report of a fatality (here a minke whale) was as a result of entanglement within the mainline of a longliner in the Ross Sea in 2004 (Kock et al. Reference Kock, Purves and Duhamel2006).
Some of the strongest signals of climate change have come from the Polar Regions, with for example, the most recent climate data showing that Antarctic Peninsula is one of the fastest warming places on earth (Vaughan et al. Reference Vaughan, Marshall, Connolley, Parkinson, Mulvaney, Hodgson, King, Pudsey and Turner2003). Interpretation of the responses of baleen whale populations to climate change however, are especially difficult to disentangle from the effects of exploitation, and may not be detected for some time given whales are such long-lived species. In the short-term, direct effects of temperature increases on baleen whales are unlikely because of their mobility and thermoregulatory ability (Castellini Reference Castellini2009). Instead, it is probable that climate change impacts will be mediated primarily through 1) changes in sea ice dynamics that alter habitat characteristics, and 2) changes in prey abundance and distribution (Moore Reference Moore2009). In the only published study to report on climate change effects, Leaper et al. (Reference Leaper, Cooke, Trathan, Reid, Rowntree and Payne2006) have shown that the breeding success of southern right whales feeding in South Georgia is driven by underlying relationships with the availability of krill, whose population fluctuations are correlated with changes in ocean climate, especially sea surface temperature (Trathan et al. Reference Trathan, Murphy, Forcada, Croxall, Reid and Thorpe2006).
Significance of threats
Because of limited or non-existent research it is not surprising that we know little about the threats (perhaps other than whaling) likely to impact baleen whales for the Antarctic marine ecosystem. However, it is possible to make some qualitative predictions about the ranking of threats even though they currently cannot be quantified. With the exception of scientific whaling in East Antarctica, where an annual quota of 850 ± 10% Antarctic minke whales and 10 fin whales are killed (IWC 2007b), it is probably reasonable to assume that of all the threats discussed above climate change will probably have the biggest impact on baleen whales. The IPCC AR4 (Solomon et al. Reference Solomon, Qin, Manning, Marquis, Averyt, Tignor, Miller and Chen2007) concludes that warming of the climate system is ‘unequivocal’ and that sea ice is projected to shrink in both the Arctic and Antarctic under all future emissions scenarios. Consequently, if the sea ice environment changes in future and if this is associated with changes in oceanic circulation, then this will undoubtedly affect the ecosystems on which predators such as baleen whales depend (Nicol et al. Reference Nicol, Worby and Leaper2008).
Pollution from local human sources occurs at a very small scale in the context of the 34.8 million km2 area of the Southern Ocean and the 18 000 km of Antarctic coastline (Aronson et al. 2011). In addition the implementation of Madrid Protocol in 1998 has raised the environmental standards across the Antarctic Treaty area (Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey, Moreno, Pfeiffer, Scott and Snape2009). Thus the effect of pollution is probably low to negligible for baleen whales, in comparison to other threats such as scientific whaling and climate change. Similarly, tourism, ship strikes and noise will probably present a fairly low threat to Antarctic baleen whales as procedures for mitigation should reduce impacts. For example, since its inception in 1991, the International Association of Antarctica Tour Operators (IAATO) (the body responsible for promoting, and practicing safe and environmentally responsible private sector travel, has grown to nearly 80 members), currently incorporating all but two Antarctic tour operators (Williams & Crosbie Reference Williams and Crosbie2007). Similarly, in 2005, the Conservation Committee of the International Whaling Commission established the Working Group on Ship Strikes to analyse the scientific and technical issues related to these events, recommend actions to mitigate impacts and coordinate the collaboration of institutions with competence in marine affairs (IWC 2005). In addition, at the 58th Session of the International Maritime Organisation Marine Environmental Protection Committee (IMO-MPEC) in 2008, the Committee agreed to the development of a guidance document for minimizing the risk of ship strikes with cetaceans (IMO-MEPC 2009). With respect to noise a recent review of the potential effects of scientific marine acoustic equipment on marine mammals by SCAR (2006) concluded that the risks were less than or comparable to shipping activities on their own.
One threat that may present a moderate risk to Antarctic baleen whales in the future comes from increased fishery activities, specifically the krill fishery. The krill fishery is small by comparison to other global fisheries but globally is one of the most underexploited fisheries i.e. less than 2% of the available catch limit was reached in 2010 (Nicol et al. Reference Nicol, Foster and Kawaguchi2011). The fishery has been carefully managed by CCAMLR through the setting of conservative and precautionary catch limits that take into account the needs of Antarctic predators that feed on krill. In 2010 and for the first time, trigger levels for catch limits were reached in some of the CCAMLR Management Areas and vessels were required to move into different areas to continue fishing (Nicol et al. Reference Nicol, Foster and Kawaguchi2011). While this is not an immediate concern for baleen whales, given that such a small proportion of the overall catch limit has been taken, the rapid expansion in 2010 (i.e. a 67% increase in catch in a single year) provides a strong signal that catches in the future are likely to increase. To date CCAMLR has faced little pressure to increase its precautionary catch limits, but with an increasing trend for demand for krill and possible decrease due to long-term ecological change in the region (Atkinson et al. Reference Atkinson, Siegel, Pakhomov and Rothery2004), an overall reduction in available krill could impact both recovering whale stocks and other dependent species.
Discussion
Inherent within many of the management frameworks, regulations and conservation tools for the Antarctic is the tendency to look towards individual species’ and populations, as well as at specific threats. Unfortunately this approach does not encompass the perspective of habitat and general biodiversity, with perhaps the notable exception of the work conducted on marine protected areas by CCAMLR. The active integration of multiple whale species each with differing status designations, exploitation and recovery trajectories, scientific understanding, as well as threats of differing time frames, severity and influence have yet to be incorporated into contemporary management. It is also difficult to know exactly how to galvanize member countries (of IWC or other IOs) to undertake the necessary science for a better understanding of threats. However, there are numerous potential avenues to enhance whale management within the Antarctic system and some key recommendations are discussed below.
Addressing threats and impacts
The Antarctic ecosystem is unique and remote. Relevant solutions to addressing threats and impacts to Antarctic baleen whales require a detailed and specialized understanding of the Antarctic ecosystem as a whole. Regional marine science and legislative experts are probably best placed to provide this perspective however, it is crucial that cetacean experts are consulted on pertinent characteristics of whale biology, life history and ecology to underpin any conservation measures. The immediate way to move this forward is to ensure that IWC-SC and CCAMLR are working in close collaboration. At present there is an open dialogue between the two organizations, yet for a more productive collaboration there must be clear instructions and objectives governing this association. Specific instructions might include: i) review of how current legislation, conventions and agreements apply to Antarctic baleen whale conservation and management, ii) global overview of cetacean threats (particularly with reference to Antarctic baleen whales) with relative level of concern and best practice mitigation strategies, and iii) in-depth analysis of distribution, diet, ecology and life-history of all Antarctic baleen whales.
This initial approach would ensure that there is no duplication of work, gaps in conservation measures are noted, and that all relevant measures (including particular bodies and instruments) are being applied and coordinated efficiently to aid whale conservation and management. The approach would also ensure that all species and all threats are being considered within regional management practices and initiatives. A longer-term plan might involve the negotiation of an Antarctic CMS agreement or the development of a comprehensive CMP for Antarctic baleen whales. These initiatives would also address a number of related issues.
Meaningful ‘measurables’ for whales
Current management of baleen whales primarily uses information on numbers, for example historical abundances, population estimates and population trends. However, (and by way of example) there are no currently accepted estimates of current abundance for minke whales. Newer methods of analysing minke whale survey data have yet to shed new light on the issue of current abundance, as estimates differ significantly from each other and the IWC-SC has worked on this issue without resolution for almost a decade. The lengthy examination of the SOWER/IDCR circumpolar cruise data for minke whales demonstrates clearly that it can be difficult to make a judgement on population size for management purposes, and problems such as these may not be restricted to this species alone. In the context of contemporary management of the Antarctic marine ecosystem it is perhaps timely to examine more closely what the specific targets for conservation and management should be, and how these might be quantified and assessed. For example, depending on the threats and possible mitigation measures, other attributes may be more appropriate to measure including habitat use, health and nutritional status. An essential factor in considering the appropriateness of any potential measurable is an evaluation of the ability to measure them and detect changes in them with reasonable resources and reliability in a reasonable timeframe. These issues are not only applicable to Antarctic baleen whales, but rather represent broader questions that should be asked in the context of management of other species also.
In the case of baleen whales it is often difficult to obtain robust information on abundance and it is not certain that a logical management strategy would be forthcoming even if agreed data were available. A broader perspective would be based on the best scientific information, taking into account the precautionary approach and socio-economic, cultural and ethical considerations. Measurables would also need to be flexible as well as biologically meaningful in terms of species, gender, sex, area, and significant in reducing threats. Implementation of such an approach would require additional discussion and consideration by (among others) animal ecologists, wildlife management specialists and theorists, implementing agencies and governments.
Characterization and integration of habitat in an effort to aid management
As this review has shown, (and see Leaper et al. Reference Leaper, Zerbini, Bannister, Branch, Clapham, Donovan, Matsuoka and Reilly2008) our understanding of the biology, status and habitat of Antarctic baleen whales is sadly incomplete. While there are numerous initiatives underway to investigate these, it is probable that this will be a lengthy procedure. One avenue for initiating management strategies in the near term is to focus on delineating distribution and range of Antarctic baleen whales. Investigation and characterization of temporal and spatial distribution ensures that i) threats are addressed and appropriately prioritized throughout the full range of a given species, ii) it is possible to be able to monitor the status of the ecosystem as a measurable of management, iii) modelling, planning and research efforts are cognisant of all species within the specified area of interest, and iv) it allows for the development of a marine protected area designation to enhance protection in a critical area. Networking of expert cetacean scientists and ecosystem modellers would be critical to meeting this objective and could most probably be facilitated through the IWC-SC as well as regional and international marine mammal conferences and working groups with other IOs.
Conclusions
The management of whale populations in the Southern Ocean has had a chequered history but has manifestly not succeeded in establishing a sustainable take. Since its implementation 25 years ago, the moratorium on whaling has saved many heavily-exploited populations from extinction, allowed some populations to recover and seen the development of a conservation agenda within the IWC. However, the moratorium has also been circumvented by continued whaling in Antarctica through the loophole of ‘Special Permit’ whaling, and more recently, by efforts to abandon a carefully developed scientific procedure in favour of the ad hoc setting of politically motivated catch limits. Given the protracted stalemate in the IWC (the primary global authority for the management of baleen whales), it is difficult to predict the future for whales and whaling in Antarctica especially in view of emerging environmental threats for which there is so far limited or non-existent research. For conservation outcomes one can only hope that the IWC supports 1) a scientific agenda that offers complete insight into multiple threats and species, 2) the continuation of protective measures to allow for both the full recovery of whale populations (including the SOS) and for the development of alternative, non-lethal uses, and 3) an understanding of other national, regional and international policy and legislation tools for species conservation management systems. Understanding the limitations on and opportunities for baleen whale recovery in the context of the contemporary Antarctic marine ecosystem will be vital for management and conservation not only of whales but for the Antarctic marine ecosystem as a whole. This task is not an easy one and therefore requires research, innovation and collaboration in a much more active and holistic approach than has been seen previously.
Acknowledgements
We thank Sue Fisher and Mark Simmonds for valuable comments on earlier drafts of this manuscript and two anonymous referees whose comments substantially improved the manuscript.












