Introduction
The great metabolic and physiological capabilities of extremophilic microorganisms adapted to the cold are widely recognized and supported by numerous scientific studies (Lo Giudice & Fani Reference Lo Giudice and Fani2015, Poli et al. Reference Poli, Finore, Romano, Gioiello, Lama and Nicolaus2017). Survival strategies in such harsh environments include the biosynthesis of extracellular polymeric substances (EPSs), adopted by a number of psychrophilic bacteria to cope with extremely low temperatures by protecting microbial cells from the direct contact with the surrounding cold environment (Poli et al. Reference Poli, Finore, Romano, Gioiello, Lama and Nicolaus2017).
EPS production falls within the scope of bioprospecting, where the investigation of new or scarcely explored biological matrices - which could be cold-adapted bacteria - is of fundamental importance. This is particularly true given the current extremely urgent need to find new and safety compounds with biotechnological relevance. EPS production by aquatic microorganisms has been investigated during recent years, due to the wide versatility, the considerable application possibilities in a number of industrial fields, and the ecological and protective roles that such molecules play in microorganisms (Carrion et al. Reference Carrion, Delgado and Mercade2015, Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). Nevertheless, cold-adapted marine bacteria from several Antarctic matrices (e.g. sediment, sea-ice and sponges) have seldom been explored as a source of EPSs (Lo Giudice & Rizzo Reference Lo Giudice and Rizzo2018), with most producers being Gammaproteobacteria (e.g. genera Colwellia, Halomonas, Pseudoalteromonas, Pseudomonas and Shewanella) (Mancuso Nichols et al. Reference Mancuso Nichols, Garron, Bowman, Raguénès and Guèzennec2004 & Reference Mancuso Nichols, Bowman and Guézennec2005a, Carrión et al. Reference Carrion, Delgado and Mercade2015, Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b) and Bacteroidetes (e.g. genera Winogradskyella, Flavobacterium, Polaribacter, and Olleya) (Mancuso Nichols et al. Reference Mancuso Nichols, Bowman and Guézennec2005b; Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a). To date, a sole Marinobacter isolate from a tropical climate has been reported as EPS producer (Bhaskar & Bhosle Reference Bhaskar and Bhosle2006), whereas no EPS has been described for cold-tolerant Marinobacter isolates. Among Gammaproteobacteria, the genus Marinobacter was first suggested by Gauthier et al. (Reference Gauthier, Lafay, Christen, Fernandez, Acquaviva, Bonin and Bertrand1992) for an extremely halotolerant, hydrocarbon-degrading, rod-shaped bacterium from Mediterranean seawater near a petroleum refinery (Liu et al. Reference Liu, Chen, Zhang, Yu, Liu and Li2012). Currently, the genus comprises 41 species (Chua et al. Reference Chua, Campen, Wahl, Grzymski and Mikucki2018) with most of them isolated from marine or related environments. According to Handley & Lloyd (Reference Handley and Lloyd2013), Marinobacter spp. are ubiquitous and possess good metabolic capabilities, but their role is underestimated.
Microbial EPS production could be affected by several environmental factors, as well as by the physiological state of the producer, nutrient availability and composition (Kumar et al. Reference Kumar, Mody and Jha2007), and physical external parameters (Mancuso Nichols et al. Reference Mancuso Nichols, Bowman and Guézennec2005a; Kumar et al. Reference Kumar, Mody and Jha2007). Moreover, bacterial strains can produce EPSs in different phases of their life cycles, generally with a higher yield during the exponential or stationary phase of growth. Even if some stressful conditions, such as a lack of nutrients, act as stimulatory variables, for many microorganisms the presence of rich substrates, as a carbon source, can increase biosynthetic activity. Furthermore, the presence of chemical precursors (such as sugars, phosphoenol pyruvates or acetyl CoA) seems to create a favourable environment. Finally, pH value, oxygen availability, temperature and salt concentration are among those parameters that can strongly affect microbial EPS production (Kumar et al. Reference Kumar, Mody and Jha2007). Research relating to the search for the optimal conditions for the microbial production of extracellular polymers possesses a wide scientific value. In addition to deepening the understanding of ecological aspects related to the functions performed by polymers, the discovery of new producers and the employment of large-scale production with minimal costs remain central targets of this field. Achieving such production can be only achieved with a fair knowledge of the optimal conditions.
In this context, this work is aimed at investigating the EPS production by a cold-adapted Marinobacter isolate, namely W1-16, from Antarctic surface seawater. Extracted EPSs are biochemically characterized and some potential EPS biotechnological application are discussed.
Material and methods
Bacterial strain isolation
Marinobacter sp. W1-16 was selected from several hundred isolates (Lo Giudice et al. Reference Lo Giudice, Bruni and Michaud2007) from Antarctic surface seawater (Terra Nova Bay, Ross Sea) due to its mucous phenotype during growth in liquid culture (marine broth (MB); Difco) and solidified medium (marine agar (MA); Difco) supplemented with glucose (0.6%, w/v). The strain was identified by 16S rRNA gene sequencing, as previously reported (Michaud et al. Reference Michaud, Di Cello, Brilli, Fani, Lo Giudice and Bruni2004). Marinobacter sp. W1-16 belongs at the Italian Collection of Antarctic Bacteria kept at the University of Messina (Italy).
The nucleotide sequence of Marinobacter sp. W1-16 has been deposited in the NCBI GenBank database under the accession number MH560355.
Phenotypic characterization of W1-16
The phenotypic characterization of W1-16 was performed according to Lo Giudice et al. (Reference Lo Giudice, Caruso, Mangano, Bruni, De Domenico and Michaud2012). The morphology and pigmentation of colonies were determined after growth on MA plates at 4°C, and the presence of flagella was verified by using the Bacto Flagella Stain (Difco). Gram reaction, motility and endospore presence were also analysed. Optimal temperature and pH ranges were detected by growing the strain in MB at different parameter values (4°C, 15°C, 20°C, 25°C, 30°C and 37°C for up to 4 weeks; pH 4, 5, 6, 7, 8 and 9). Growth in presence of 0–5% (w/v) NaCl concentration was tested on nutrient agar (NA). The presences of oxidase and catalase activities were determined. Hydrolysis of chitin, agar and starch and lipolytic activity were assayed. Growth on trypticase soy agar (TSA; Oxoid), TSA + 3% (w/v) NaCl, and thiosulfate-citrate-bile salts-sucrose (TCBS) agar (Difco) were also determined. Analytical profile index (API) tests (BioMerieux), including API 20E and API 20NE galleries, were used as additional biochemical and enzymatic tests. For tests carried out on solid and liquid media, cultures were incubated at 4°C for 21 days. All analyses were performed at least twice to confirm results. Susceptibilities to chloramphenicol (30 µg), tetracycline (30 µg), nalidixic acid (30 µg), penicillin G (10 µg), polymyxin B (30 µg), tobramycin (10 µg), and vibriostatic agent O/129 (10 µg) were assayed using the standard disk diffusion method, and expressed in terms of sensitivity or total resistance.
Optimization of the EPS production
Optimal growth conditions for EPS production by W1-16 were assayed by a step-by-step experiment, as previously reported (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). Bacterial cultures were performed by inoculating a bacterial pre-culture (10%, v/v) in the exponential phase in 300 ml of a minimal medium (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). Bacterial growth was spectrophotometrically monitored (UV-mini-1240, Shimadzu at λ 600nm (OD600)) at regular intervals during incubation at 4°C and/or 15°C, along with the EPS production, which was concomitantly determined through the phenol-sulphuric acid method. Glucose and sucrose (0.6%, w/v) were first evaluated at 4°C and 15°C as carbon sources. The effect of other variables was then evaluated in the following order: carbon source concentration (range 0.6–2%, w/v), pH (range 6–8) and salinity (range NaCl 1–5%, w/v).
EPS extraction from the culture medium
EPSs were extracted as previously reported in Caruso et al. (Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). To this purpose, different methods were opportunely merged. Briefly, a volume of cold ethanol was added to cell-free broth culture (1:1) obtained by centrifugation (8000 × g for 10 min at 4°C). A pellet precipitate, obtained after storing at −20°C overnight, was separated from the liquid phase by centrifugation at 10 000 × g for 30 min, and dissolved in hot water. The procedure was repeated twice. Dialysis was finally performed against tap water (48 h) and distilled water (24 h), and the raw extracts were freeze-dried and weighed.
EPS characterization
A preliminary chemical characterization of the EPSs extracted from W1-16 cultures was performed by colorimetric assays as specified in Caruso et al. (Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a). The Dubois method was used to establish the carbohydrate composition. Protein content was spectrophotometrically determined using Coomassie Brilliant Blue, while uronic acid was measured using the modified method described by Filisetti-Cozzi & Carpita (Reference Filisetti-Cozzi and Carpita1991).
For the determination of sugar composition, EPS hydrolysis was performed with a 2 M trifluoroacetic acid solution at 120°C for 2 h. Sugar components were identified by thin-layer chromatography and high-pressure anion-exchange pulsed amperometric detection (HPAE-PAD) with sugar standards for identification and calibration curves (Finore et al. Reference Finore, Orlando, Di Donato, Leone, Nicolaus and Poli2016).
The analysis of the major structural groups was carried out by Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance (NMR). In particular, the purified EPSs were subjected to FT-IR analysis for the detection of C = O bonds and O-H bonds (Mancuso Nichols et al. Reference Mancuso Nichols, Garron, Bowman, Raguénès and Guèzennec2004) in the range of 4000–400 cm−1, and in the range 1250–1050 cm−1 for the detection of sulphate content (Lijour et al. Reference Lijour, Gentric, Deslandes and Guezennec1994). EPS sample (10 mg ml–1 D2O) was analysed for 1H- and 13C-NMR spectra at the NMR Service of the Institute of Biomolecular Chemistry (ICB) of CNR. The sample was first exchanged twice with D2O with an intermediate lyophilization step and then dissolved in 500 μL of D2O. Chemical shifts were reported in parts per million (ppm) with reference to D2O and to CD3OD, for 1H and 13C spectra, respectively. The NMR analysis was performed using a Bruker AMX-600 MHz at 50°C to obtain 1H- and 13C-NMR spectra of polysaccharides (Yildiz et al. Reference Yildiz, Anzelmo, Ozer, Radchenkova, Genc and Di Donato2014).
Biotechnological potential of the EPSs
The biotechnological potentialities of the EPSs produced by W1-16 were explored in terms of emulsifying and cryoprotective actions, and heavy metal tolerance.
To test the emulsifying activity, an equal volume of lyophilized EPS solution (in distilled water; 0.5%, w/v) and standard hydrocarbons (hexane, (Baker); octane, hexadecane and tetradecane, (Sigma)) were vigorously shaken in glass tubes for 2 min, and the stable emulsion index (E24) was measured after 24 h, as described by Caruso et al. (Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a). Tween 80 (Biomedicals) and Triton X-100 (Sigma) were used as surfactant positive controls.
To test the cryoprotective action, bacterial biomass (1 ml), obtained by harvesting (10 000 × g for 20 min, 4°C) the liquid culture in the exponential phase of growth under optimal conditions, was subjected to four freeze-thaw cycles. Cell viability was evaluated spectrophotometrically and by seeding on solid media at the end of each thawing both in presence and absence of EPSs, in order to evaluate their eventual cryoprotective effect.
The isolate W1-16 was tested for heavy metal (i.e. cadmium, mercury, zinc and iron; range 10–10 000 ppm) tolerance both in presence and absence of sugar (0.6%, w/v) by the plate diffusion method. The method was applied as reported in Mangano et al. (Reference Mangano, Michaud, Caruso and Lo Giudice2014), by adding an aliquot of metal salt solution (0.5 ml; in sterile phosphate buffer saline (PBS)) to a central well on the solidified medium and sealing with soft agar (0.8% agar, w/v). Sterile PBS was used as a negative control. After pre-incubation at 37°C for 24 h, the strain was inoculated in duplicate in radial streaks and allowed to grow at 4°C for 21 days. Bacterial tolerance was expressed as the ratio between the length of growth in mm vs the length of total inoculated streak. Tolerance ranges were classified in complete (100% of growth), high (≥ 50–99% of growth), low (≥ 1–49% of growth) or absent (no growth; 0%) (Mangano et al. Reference Mangano, Michaud, Caruso and Lo Giudice2014). The influence of heavy metal concentration on EPS production was then evaluated during growth of W1-16 under previously determined optimal conditions, with addition of the same metals used for the tolerance test. EPS production was monitored in 300 ml cultures as described above.
Statistical analyses
Results were statistically analysed using MiniTab software (version 16.0). Results (mean values) from the EPS production enhancement were compared using one-way ANOVA and the Tukey test to individuate any significant differences among parameters and variables.
Results
Bacterial strain characterization
Marinobacter sp. W1-16 cells are Gram-negative, strictly aerobic with an oxidative metabolism, occur as asporogenic rods, and are motile. Colonies are non-pigmented, circular, convex, and shiny with entire edges, 1–2 mm in diameter on MA. Growth occurs between pH 4 and 9 (pH 7–8 optimum) and between 4 and 20°C (with an optimum range between 10 and 15°C), but not at 30°C or higher temperatures. The strain requires NaCl (1–5%; 3% optimum), grows on TSA and TSA + 3% NaCl, but does not grow on TCBS. It is not able to hydrolyse agar, chitin, starch, gelatin and tween 80, whereas aesculin is degraded. Acids from carbohydrates included in the API20E system are not produced. The following substances: arabinose, mannitol, N-acetyl-glucosamine, maltose, gluconate, caprate, adipate, malate, citrate and phenyl-acetate were not utilized by W1-16 strain as the sole carbon and energy source. Nitrate reduction is negative. H2S, indole and acetoin (Voges–Proskauer reaction) are not produced. It was positive for oxidase and negative for β-galactosidase, catalase, urease, ornithine and lysine decarboxylase, arginine dihydrolase and tryptophan deaminase activities. The strain was also tested for its ability to grow in the presence of different antibiotics and it was found to be sensitive to nalidixic acid, ampicillin, chloramphenicol, penicillin G, tetracycline, polymixyn B and tobramicin, whereas it was resistant to O/129.
Optimization of EPS production by Marinobacter sp. W1-16
Marinobacter sp. W1-16 produced EPSs when growing on glucose and sucrose at both 4°C and 15°C. Using glucose led to the production of a higher amount of exoproducts (Fig. 1), especially during incubation at 15°C (Fig. 1b), with a yield of 85.8 mg l−1 achieved after 192 h of incubation. However, the incubation at lower temperature (Fig. 1a) gave satisfactory yields, with a maximum of 69.8 mg l−1 of EPS after 192 h of growth. The addition of sucrose to the culture medium led to EPS yields up to 35.5 mg l−1 and 62.9 mg l−1 during incubation at 4°C (Fig. 2a) and 15°C (Fig. 2b), respectively. Continuous increases in growth were observed up to 240 h and 384 h of incubation at 15°C and 4°C, respectively, while EPS production generally increased until 192 h (exponential phase of growth). A correlation between EPS production and optical density values generally occurred during growth at both temperatures, detectable by the highest EPS yield found at the exponential phase of growth. This trend appeared more evident during incubation at 15°C (Figs 1b & 2b for glucose and sucrose, respectively). Based on these results, the combination of glucose as substrate and the incubation temperature of 15°C was retained for further analyses. Increased EPS yields were observed by shifting glucose concentration from 0.6% (68.03 mg l−1 after 144 h) to 1% and 2% (75.03 mg l−1 and 134.95 mg l−1, respectively, after 144 h and 96 h) when growing Marinobacter sp. W1-16 at 15°C.

Fig. 1. Influence of temperature on W1-16 growth (lines) and EPS production (histograms) during incubation at a. 4°C, and b. 15°C in presence of glucose (0.6%, w/v).

Fig. 2. Influence of temperature on W1-16 growth (lines) and EPS production (histograms), during incubation at a. 4°C, and b. 15°C in presence of sucrose (0.6%, w/v).
The effect of pH was evaluated by using glucose at a final concentration of 2% (w/v) and incubating W1-16 at 15°C. pH did not seem to severely affect the ability of Marinobacter sp. W1-16 to produce EPSs, even when pH 8 was chosen as optimal for EPS production (up to 131 mg l−1, Fig. 3a). Conversely, a weak influence on bacterial growth and EPS production was recorded by varying the NaCl concentration in the medium, with a better yield than was obtained at 3% NaCl (139 mg l−1 after 192 h of incubation) (Fig. 3b). The optimal conditions for EPS production by Marinobacter sp. W1-16 are summarized in Table I. Briefly, the strain produced up to 139 mg l−1 of EPS after 192 h incubation (exponential phase) when growing at 15°C and pH 8, in the presence of 2% (w/v) glucose and 3% (w/v) NaCl. No significant differences were observed in EPS production by the strain during incubation at different temperatures, pH levels and NaCl concentrations (P > 0.05), thus suggesting that there is no significant influence from these parameters. Conversely, glucose concentration strongly affected EPS production (P < 0.05). A positive correlation was observed between optical density and EPS yield during growth of Marinobacter sp. W1-16 under optimal conditions (r = 0.7 and P = 0.042, respectively).

Fig. 3. Influence of a. pH, and b. NaCl concentration on W1-16 growth (lines) and EPS production (histograms) during incubation at 15°C in presence of glucose (2%, w/v). NB: NaCl concentration was tested at pH 8.
Table I. Optimal conditions for EPS production by Marinobacter sp. W1-16 (in bold the optimal conditions that were determined by the step-by-step approach). EPSs amounts were determined as exoproducts obtained after lyophilisation.

EPS extraction and chemical characterization
Marinobacter sp. W1-16 was grown in batch culture under the optimal conditions reported in Table I. EPS extraction was performed in the phase of maximum production, determined spectrophotometrically, allowing a total yield of lyophilized exoproducts of 87 mg l−1. EPSs were highly powdery and soluble in water. Carbohydrates, proteins and uronic acids in purified EPSs, obtained after lyophilisation from each strain grown under optimal conditions, accounted for 38%, 2.7% and 7%, respectively, as determined by colorimetric assays.
The HPAE-PAD analysis revealed that the EPSs were constituted of Glc:Man:Gal:GalN:GalA:GlcA in relative molar proportions of 1:0.9:0.2:0.1:0.1:0.01. Estimated molecular weight was about 260 kDa. The analysis of the FT-IR spectrum in Fig. 4 reveals the presence of peaks between 1650–1050 cm−1, suggesting that the sample should be an exopolysaccharide. The band visible at about 1050 cm−1 represents the bonds C-O and C-O-C, typical of exopolysaccharides with an acidic nature. The FT-IR spectrum also shows the presence of amino sugars and proteins (1550 cm−1), and OH stretching (3300 cm−1), while a minor band at 2900 cm−1 indicates the presence of methyl groups C-H. Sulphate content was close to 8%.

Fig. 4. FT-IR spectra of EPSs produced by Marinobacter sp. W1-16.
The analysis of the 1H-NMR (Fig. 5, panel a) spectrum of the EPS confirmed the polysaccharide nature of the polymer, as already evidenced by its chemical analysis. Indeed, in the anomeric region of the spectrum, inter alia six main signals were found at δ 5.42 ppm, 5.29 ppm, 5.25 ppm, 5.22 ppm, 5.20 ppm and 5.19 ppm. The resonances are typical of monosaccharides in the alpha anomeric configuration and some of them are also compatible with those of sulphated sugars, whose presence was evidenced by the chemical investigation. The presence of substituting groups was also suggested by analysis of the 13C-NMR spectrum (Fig. 5, panel b) in which, besides the typical resonances of anomeric carbons (105–95 ppm) and ring carbons (from 80–55 ppm), the signals related to the C6 of uronic acids and to the methyl residues of acetyl substituent groups were found at 162.1 ppm and 21.0 ppm, respectively.
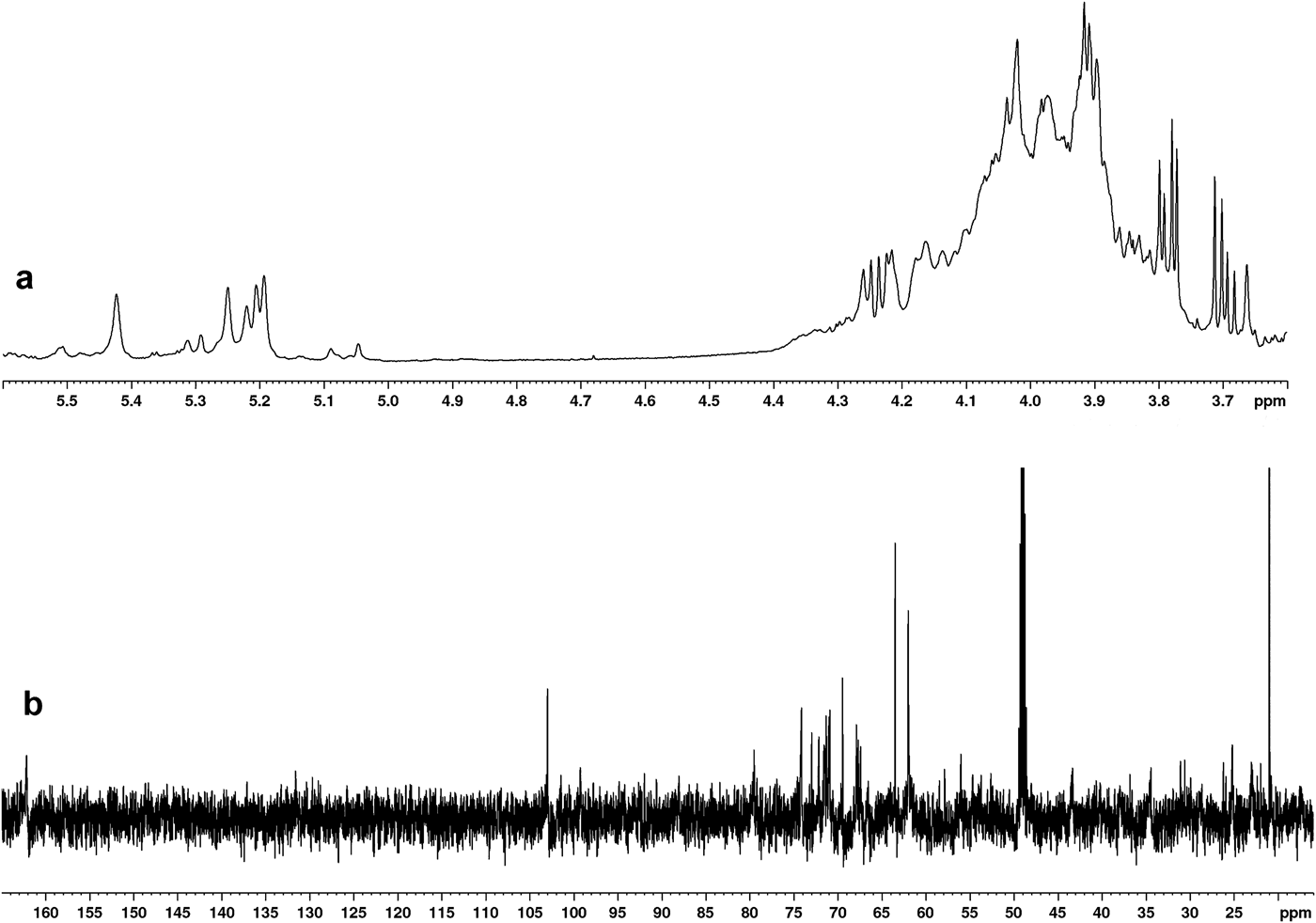
Fig. 5. NMR spectra of EPSs produced by Marinobacter sp. W1-16. a. 1H-NMR, and b. 13C-NMR spectra were registered in D2O at 50°C. Chemical shifts are reported in parts per million (ppm) with reference to D2O and to CD3OD, for 1H and 13C spectra respectively.
Emulsifying activity of EPSs
As it is shown in Table II, the E24 percentages obtained from cultures of Marinobacter sp. W1-16 in the presence of hydrocarbons were higher than those obtained by using synthetic surfactants (i.e. tween 80 and triton X-100 used as positive controls), with a maximum of 80% in presence of tetradecane.
Table II. Emulsifying activity of EPSs produced by Marinobacter sp. W1-16 (values higher than/equal to the controls are in bold).

EPSs as heavy metal chelating agents
Marinobacter sp. W1-16 showed a heavy metal tolerance that was in the order Hg < Cd < Zn < Cu < Fe, even though tolerances were always higher in the medium amended with glucose. The strain completely (100% of growth) tolerated Fe up to 10 000 ppm, Cu up to 2500 ppm, Cd and Zn up to 1,000 ppm, and Hg up to 10 ppm, respectively (Table III). In particular, the tolerance to Cd, Cu and Hg increased in the presence of the sugar in the medium.
Table III. Heavy metal tolerance in the presence and absence of EPS in the culture medium.

Legend: ✓ Complete growth (100%); ↑ High growth (> 50%); ↓ Low growth (< 50%); – Absent growth (0%)
A: 10 ppm; B: 50 ppm; C: 100 ppm; D: 500 ppm; E: 1000 ppm; F: 2500 ppm; G: 5000 ppm; H: 7500 ppm; I: 10000 ppm.
EPSs as cryoprotective agents
The study of EPS effects on cell survival ratio showed no evident differences in bacterial growth between cultures that were (EPS+) or were not (EPS -) stimulated for the production of EPSs after the first and second freezing/thawing cycles. Conversely, a cryoprotective effect was observed after the third and fourth cycles (Fig. 6).

Fig. 6. Growth of the EPS-producing Marinobacter sp. W1-16 after four freezing/thawing cycles. The horizontal black line indicates OD600 values of the culture medium inoculated with untreated bacteria (unfrozen).
Discussion
Marinobacter sp. W1-16 showed the best growth and enhanced mucoid morphology in the presence of sugars, and was selected from several hundred isolates from diverse Antarctic matrices (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b; Lo Giudice et al. Reference Lo Giudice, Bruni and Michaud2007, Reference Lo Giudice, Caruso, Mangano, Bruni, De Domenico and Michaud2012, Reference Lo Giudice, Casella, Bruni and Michaud2013) for investigation on EPS production. Cold-adapted Marinobacter from Antarctica have been poorly reported, although two novel halotolerant species from Antarctic sediments, i.e. Marinobacter antarcticus Liu et al. (Liu et al. Reference Liu, Chen, Zhang, Yu, Liu and Li2012) and Marinobacter guineae Montes et al. (Montes et al. Reference Montes, Bozal and Mercade2008), and brines (Chua et al. Reference Chua, Campen, Wahl, Grzymski and Mikucki2018) have been described. Further, at the time of writing, no report exists on EPS-producing cold-adapted Marinobacter isolates. Conversely, other EPS-producing bacterial isolates from marine polar environments have been described, as is shown in Table IV.
Table IV. Chemical composition and properties of EPSs produced by polar marine bacterial species (data are from Casillo et al. 2018).

*Monosaccharide abbreviations: Glc, glucose; Gal, galactose; Rha, rhamnose; Fuc, fucose; GalA, galacturonic acid; Man, mannose; GlcA, glucuronic acid; GlcNAc, N-acetyl glucosamine; GalNAc, N-acetyl galactosamine.
**n.d. not described.
One of the principal issues concerning the discovery and use of new bioactive compounds lies in the optimization of biosynthesis conditions, as well as in finding molecules with new molecular structures, key elements for the detection of possible innovative applications. Accordingly, the exploration of new sources should be accompanied by a careful and deep examination of the factors favouring enhanced production. In this study, EPS production was monitored over time by varying the carbon source and its concentration, temperature, pH and NaCl concentration, in order to individuate the optimal growth conditions. In line with previous observations on Antarctic marine bacteria, Marinobacter sp. W1-16 produced the highest amount of exoproducts during the exponential phase (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a)
As is well known, the availability and typology of the carbon source are among the main parameters that strongly affect EPS yield and chemical composition. In this study, good yields of EPSs were achieved by Marinobacter sp. W1-16 in the presence of both glucose and sucrose, although glucose was found to be the optimal substrate in terms of EPS yield. Conversely, previous investigations on cold-adapted bacteria from Antarctic sponges (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a) and seawater (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b) showed that sucrose was the most suitable sugar substrate. One exception was a Winogradskyella isolate that produced the highest EPS yield when growing on glucose. As was previously observed for other Antarctic isolates (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b), the EPS biosynthesis process by W1-16 was mainly affected by the carbon source concentration rather than by its chemical nature. Indeed, the increase in the concentration of the optimal carbon source allowed the polymer production by the strain to double, thereby confirming the important role of C/N ratio in bacterial EPS production (Kumar et al. Reference Kumar, Mody and Jha2007). The biosynthesis strategies can involve the use of different chemical precursors, from which EPSs with different chemical structures could derive (Nicolaus et al. Reference Nicolaus, Panico, Manca, Lama, Gambacorta and Maugeri2000). Thus, the discovery of strains that prefer different starting compounds could be consequently interpreted as an indicator of the production of different polymers, with different functional properties.
The incubation temperature also influences the EPS production yield over time, and it is strictly correlated to the intrinsic physiological needs of the strain. The psychrotrophic Marinobacter sp. W1-16 more efficiently produced EPSs at 15°C than at 4°C. An indirect effect of temperature on EPS production by W1-16 could be hypothesized as higher optical density (OD) values for bacterial growth, corresponding to higher EPS yields. Such results are in contrast with those previously obtained for other cold-adapted bacteria, which showed more effective EPS production at sub-optimal incubation temperatures (Mancuso Nichols et al. Reference Mancuso Nichols, Bowman and Guézennec2005a, c; Marx et al. Reference Marx, Carpenter and Deming2009; Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). However, the EPS production capacity of W1-16 at low temperatures (both 4°C and 15°C) confirmed once again the possible cryoprotective role played by these molecules in cold environments, as previously suggested (Marx et al. Reference Marx, Carpenter and Deming2009, Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). Finally, pH did not seem to affect the biosynthetic activity by W1-16, whereas a weak influence on both bacterial growth and EPS production was recorded by varying the NaCl concentration in the medium. This finding could be related to the halophilic nature of Marinobacter isolates.
The ecological roles played by bacterial EPSs in natural environments could find applications in diverse biotechnological fields. Bacterial EPS production originates from the cellular need to develop survival strategies, which can assist the cells by playing a protective role against environmental adversities (Costa et al. Reference Costa, Raaijmakers and Kuramae2018). A cryoprotective action was observed for the EPSs produced by Marinobacter sp. W1-16 after the third and fourth freezing–thawing cycles. They showed a more marked effect than those observed for Winogradskyella spp. strains from Antarctic sponges (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a), and for Pseudoalteromonas sp. MER 144 from Antarctic seawater (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b), but a lower protective action when compared with the freeze–thaw survival ratio reported for Colwellia sp. GW185 and Shewanella sp. CAL606 from Antarctic sponges (Caruso et al. 2018). However, such results further confirmed the ability of cold-adapted bacteria ability to tolerate repeated freezing–thawing cycles (Marx et al. Reference Marx, Carpenter and Deming2009), and the potential role as cryoprotective agents for EPSs of polar origin. The Marinobacter sp. W1-16 capacity to produce EPSs also at suboptimal temperature (i.e. 4°C) further corroborated its possible cryoprotectant role.
Some EPS moieties could easily transform into ionic forms, thus supporting the chelating action of EPSs towards cations, such as heavy metals. EPS chelation activity was proved by Qin et al. (Reference Qin, Zhu, Chen, Wang and Zhang2007) and Sand & Gehrke (Reference Sand and Gehrke2006) in binding and concentrating metallic elements necessary for cell survival (i.e. zinc, cobalt and iron). As well as for beneficial metals, EPSs could also sequestrate toxic metallic compounds, thus acting as protective agent against environmental pollution. In this study, Marinobacter sp. W1-16 displayed a heavy metal resistance pattern that was similar to those previously reported for Antarctic marine bacteria (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). In particular, it showed a good tolerance level towards metals that are considered essential for microbial life, but it slightly tolerated more toxic metals, some of which occur at low concentrations in Antarctic environments (i.e. mercury and cadmium; Bargagli et al. Reference Bargagli, Nelli, Ancora and Focardi1996). The increased tolerance shown by the strain in the presence of a factor stimulating the EPS production, i.e. the carbohydratic substrate, could prove that in presence of a stressful condition, such as metal pollution, the production of agents able to chelate free ions could reduce the toxicity of such contaminants, by reducing their concentration in the surrounding environments (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Finore2018a, Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). These assumptions are supported by previous results describing the more efficient bacterial EPS production in presence of metals (Ozturk & Aslim Reference Ozturk and Aslim2008). Several authors have widely described the heavy metal chelating property of bacterial EPSs. Among them, Gupta & Diwan (Reference Gupta and Diwan2017) deeply explored the different heavy metal sorption strategies in the function of the polymer characterization. Adsorbing properties of EPSs from Marinobacter isolates were described towards copper and lead under neutral pH (Bhaskar & Bhosle Reference Bhaskar and Bhosle2006), but investigations on this topic about this genus remain scant. In addition to the chelating action, good emulsifying activities of Marinobacter sp. W1-16 EPS were also detected, thus suggesting a possible role in the removal of pollutants other than metals (Banat et al. Reference Banat, Makkar and Cameotra2000).
Chemical analyses performed on the EPSs produced by Marinobacter sp. W1-16 under optimal conditions indicated a exopolysaccharidic nature, with a monosaccharidic composition (Glc:Man:Gal:GalN:GalA:GlcA), a molecular weight of 260 kDa and a sulphate content of 8%. The molecular mass is in the typical range detected for bacterial EPSs, generally between 20 and 2000 kDa (Arias et al. Reference Arias, Moral, Ferrer, Tallon, Quesada and Bejar2003, Manzoni & Rollini, Reference Manzoni and Rollini2001), and it is in line with that reported for Pseudoalteromonas sp. MER144 (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b). Bacterial EPS monosaccharidic composition is very variable, although in cold-adapted bacteria it is usually characterized by the presence of mannose and galactosamine (Mancuso Nichols et al. Reference Mancuso Nichols, Garron, Bowman, Raguénès and Guèzennec2004; Mancuso Nichols et al. Reference Mancuso Nichols, Garon Lardiere, Bowman, Nichols, Gibson and Guézennec2005c) in addition to the most common glucose and galactose (Carrion et al. Reference Carrion, Delgado and Mercade2015). All these residues were detected in this study of the EPSs extracted from Marinobacter sp. W1-16. Information about the chemical structure of bacterial EPSs is helpful for understanding their functions and properties. Indeed, the sulphate levels and the uronic acid content, just reported for EPSs from cold-adapted bacteria, are generally considered to be valid parameters for gauging the beneficial emulsifying and heavy metal chelating properties of EPSs (Mancuso Nichols et al. Reference Mancuso Nichols, Garron, Bowman, Raguénès and Guèzennec2004). Such chemical residues are ionizable, and they could easily interact with cationic metals, or other insoluble compounds, with their negative charges (Qin et al. Reference Qin, Zhu, Chen, Wang and Zhang2007). The carbohydrate content of EPSs from Marinobacter sp. W1-16 was higher than that reported for an Antarctic Pseudoalteromonas isolate (Caruso et al. Reference Caruso, Rizzo, Mangano, Poli, Di Donato and Nicolaus2018b), while levels of protein and uronic acid content were in line with previous reports. Although EPSs have a common basic chemical structure, they can undergo or present structural variations. These include chain length, the presence – as well as the combinations and arrangements – of different functional groups, substituents and bonding. Such variations depend on external parameters, such as temperature, pH and carbon source conditions (Sheng et al. Reference Sheng, Yu and Li2010). Different molecular organizations are responsible for different properties of EPSs. Keeping in mind all the possible implications that some EPS properties could have, the results presented here acquire great ecological value, especially considering the important role these substances play in biogeochemical cycling of metals, pollutant removal capacity and microbial resistance to the cold.
In conclusion, this study enlarges our existing, and still limited, knowledge on cold-adapted EPS-producing bacteria, with a focus on the genus Marinobacter. Interesting chemical properties and applications (i.e. emulsifying and cryoprotective activities, and heavy metal chelation) have been proven for the EPSs produced by W1-16, thus encouraging us towards the development of strategies addressed to the exploitation of these natural polymers in several biotechnological fields.
Acknowledgments
This research was supported by grants from PNRA (Programma Nazionale di Ricerche in Antartide), Italian Ministry of Education and Research (Research Projects PNRA 2003/1.5 and PNRA 2004/1.6), and from MNA (Museo Nazionale dell'Antartide). The authors would like to thank the anonymous reviewers for their careful reading and valuable suggestions.
Author contribution
C.C., L.M. and A.L. conceived the idea and planned the research. C.C. and S.M. carried out the microbiological experiments and processed the experimental data. A.P., B.N. and G.D. designed the chemical characterization analysis, P.D. and I.F. carried out the experiments for chemical analysis and processed the results. C.R. performed the statistical analysis and graphical elaboration. C.R. and A.L. wrote the manuscript with inputs from all authors.
Details of data deposit
The nucleotide sequence of the strain has been deposited in the NCBI GenBank database.












