Introduction
Many seabirds breed on remote islands in the absence of mammalian predators and are particularly vulnerable to predation by introduced mammals, such as rats (Rattus spp.) and cats (Felis catus L.) (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012). Few oceanic islands have escaped invasion by mammals, and invasive rodents are likely to be responsible for the greatest number of bird extinctions from islands (Howald et al. Reference Howald, Donlan, Galvan, Russell, Parkes, Samaniego, Wang, Veitch, Genovesi, Pascal, Saunders and Tershy2007).
Gough Island (40°82'S, 9°85'W) in the south Atlantic Ocean is an important breeding ground for 23 species of seabird (several of which are globally threatened) and has long been considered one of the world’s most important seabird breeding islands (Swales Reference Swales1965). It is home to at least 13 species of burrowing petrels, including virtually the entire global population of the Endangered Atlantic petrel (Pterodroma incerta Schlegel). It also hosts significant global populations of the Near-threatened grey petrel (Procellaria cinerea Gmelin), two shearwaters (great shearwater (Puffinus gravis O’Reilly) and little shearwater (Puffinus assimilis Gould)), soft-plumaged petrel (Pterodroma mollis Gould), Kerguelen petrel (Aphrodroma brevirostris Lesson), broad-billed prion (Pachyptila vittata Forster), common diving petrel (Pelecanoides urinatrix Gmelin) and at least three species of storm petrel (white-faced storm petrels (Pelagodroma marina Latham), grey-backed storm petrel (Garrodia nereis Gould) and Fregetta spp.; Brooke Reference Brooke2004). The breeding success of these burrowing petrels on Gough Island appears to be very poor, with exceptionally low burrow occupancy and breeding success in four species studied from 2009–11 (Cuthbert et al. Reference Cuthbert, Louw, Lurling, Parker, Rexer-Huber, Sommer, Visser and Ryan2013b). A second population of prions was recently discovered breeding in the summer on Gough Island, morphologically similar to MacGillivray’s prion (Pachyptila macgillivrayi Mathews) from Amsterdam and St Paul islands in the temperate Indian Ocean (Ryan et al. Reference Ryan, Bourgeois, Dromzée and Dilley2014). Blue petrels (Halobaena caerula Gmelin) were also found breeding on Gough Island for the first time in 2014 (Ryan et al. Reference Ryan, Dilley, Jones and Bond2015).
House mice (Mus musculus L.) were brought to the island by sealers in the 19th century and were thought to have little impact on the island’s birds, being regarded as ‘probably harmless’ (Elliott Reference Elliott1953). The impact of house mice on Gough Island’s seabird populations has received particular attention since 2001 when mouse predation was identified as the most probable cause of the high chick mortality of Tristan albatross (Diomedea dabbenena Mathews) (Cuthbert & Hilton Reference Cuthbert and Hilton2004). Breeding success was less than half that of Diomedea spp. albatrosses breeding at other locations (Tickell Reference Tickell2000), including those with introduced rats (Possession Island; Weimerskirch Reference Weimerskirch1992) and mice (Marion Island; Nel et al. Reference Nel, Taylor, Ryan and Cooper2003). Island-wide Tristan albatross chick production fell below 10% for the first time in 2014 and is almost exclusively due to predation by introduced house mice (Wanless et al. Reference Wanless, Ryan, Altwegg, Angel, Cooper, Cuthbert and Hilton2009, Davies et al. Reference Davies, Dilley, Bond, Cuthbert and Ryan2015). Recent observations of mouse-injured Atlantic yellow-nosed (Thalassarche chlororhynchos Gmelin) and sooty (Phoebetria fusca Hilsenberg) albatross chicks are also a cause for concern (Cuthbert et al. Reference Cuthbert, Louw, Parker, Rexer-Huber and Visser2013a), especially given that both species are listed by the International Union for Conservation of Nature as Endangered.
The hundreds of thousands of petrels that breed in burrows and caves on Gough Island are also affected by mice (Cuthbert & Hilton Reference Cuthbert and Hilton2004, Cuthbert et al. Reference Cuthbert, Louw, Lurling, Parker, Rexer-Huber, Sommer, Visser and Ryan2013b). To date, direct evidence of mouse predation on burrowing petrels has been recorded for Atlantic petrel and great shearwater chicks (Wanless Reference Wanless2007). While it is probable that mice have a negative impact on all burrow-nesting petrels breeding on Gough Island, when compared to the number of recorded chick failures, relatively few chicks have been observed showing wounds characteristic of mouse attacks (Wanless Reference Wanless2007). It is not known how quickly or frequently mice kill petrel chicks, or if they kill them outright or weaken them to the point where they die from their injuries. Mice are quick to scavenge dead chicks in burrows, leaving few clues to determine the cause of death. By understanding the prevalence, nature and speed of mouse attacks these assumptions and high chick failure rates can be qualified.
We hypothesized that mice may have far more severe effects on burrow-nesting birds than has previously been recognized, and suspected that mice attack and kill burrowing petrel chicks very quickly and throughout the year. An array of cameras was used to investigate the mechanisms and frequency of mouse predations on Gough Island.
Methods
Fieldwork
Fieldwork was conducted from October 2013 to January 2015, covering two breeding seasons for MacGillivray’s prion and one breeding season for six other study species. Regular nest checks were made with a burrowscope to record breeding success, and infra-red video cameras were installed at a sub-sample of burrows to record activity inside the nest chambers. Video cameras were used to film activity in the nest chamber from hatching to when the chicks were about half-grown for great shearwaters (December–February), common diving petrel (December–February), soft-plumaged petrels (February–April) and MacGillivray’s prions (January–February) over the summer, and for Atlantic petrels (August–September) over the winter (see Table I for details). For grey petrels and broad-billed prions, regular nest checks were performed using a burrowscope to estimate breeding success and monitor chicks for mouse wounds.
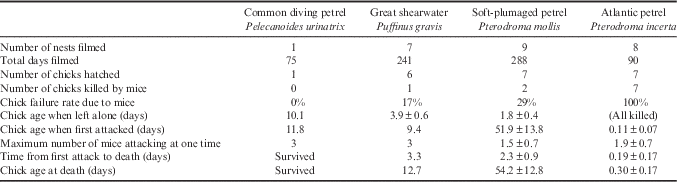
Table I Summary of the video surveillance results from filming the nests of four petrel species on Gough Island in 2014. The motion activated infra-red cameras were installed into the burrow chamber at roughly mid-incubation and connected to a video surveillance system which enabled a live feed and footage was recorded onto a computer. Values are expressed as mean±standard deviation.
Filming nests with video surveillance cameras
There were sufficient great shearwater, soft-plumaged and Atlantic petrel burrows within 100 m of the meteorological station at Transvaal Bay to monitor activity with small digital video recorders (DVRs). Petrel nests were chosen at random within range of the 200 m video cables. Each DVR camera (B/W low light mini camera, code E-25B-B36, 1/3" CCD) was housed in 40 mm PVC piping to keep it dry and secure from mouse damage, fitted with a wide angle lens (2.1 mm board lens, covering 120°) and accompanied by a ring of 12 infra-red light-emitting diodes (LEDs). Inspection hatches were dug through the roof of the burrow passage to gain access to the nest chamber. Each camera was then secured to a metal angle-iron pole and positioned 20–30 cm away from the incubating bird. The eight motion activated cameras were connected to a video surveillance system (SuperDVR software) which enabled a live feed and footage was recorded onto a computer. Despite extensive searches in a 200 m radius from the station, only two common diving petrel burrows were found. Both burrows had new nest material in the chamber with an adult present, but only one pair laid an egg and this burrow was monitored with a DVR camera. No storm petrel nests were found, despite reasonably large numbers of white-faced storm petrels active around the station at night.
Cameras were installed into the burrow chamber at roughly mid-incubation, when the occupants would be unlikely to abandon their nest due to the disturbance. Camera installation took <10 minutes and did not result in any immediate nest failures. We suspected that the mice would depredate newly hatched and newly independent chicks; therefore, it was important to have the cameras in situ before hatching started.
Prions breed in burrows and natural rock cavities, but there were no active prion burrows within range of the DVR camera system. Therefore, we chose to monitor birds in Prion Cave (40°21.161'S, 9°53.114'W), an accessible cave where MacGillivray’s prions breed (Ryan et al. Reference Ryan, Bourgeois, Dromzée and Dilley2014; previously reported as broad-billed prions by Cuthbert et al. Reference Cuthbert, Louw, Lurling, Parker, Rexer-Huber, Sommer, Visser and Ryan2013b). Two nests with wounded chicks, found at approximately 28 days old, were filmed with a GoPro camera and an external 12V red light to record mouse–chick interactions.
Breeding success
Atlantic petrel (n=92), soft-plumaged petrel (n=42) and great shearwater (n=147) burrows were monitored (Table II) along established monitoring transects (Cuthbert et al. Reference Cuthbert, Louw, Lurling, Parker, Rexer-Huber, Sommer, Visser and Ryan2013b). These were supplemented with additional nests located closer to the station. All burrows were checked with a burrowscope to determine contents, as relying on other cues or responses can overestimate occupancy, and thereby underestimate breeding success (Rexer-Huber et al. Reference Rexer-Huber, Parker, Ryan and Cuthbert2014). The burrowscope was custom-made using a high resolution conical pinhole camera, LED torch and a 7" colour monitor, producing a clear image of the inside of the burrow. Burrows were checked during early incubation, and again when chicks were predicted to be hatching and fledging.
Table II Burrowing petrel nests monitored on Gough Island in 2014 at laying, hatching and fledging to estimate the breeding success.

* Some early chick failures may have been missed.
To estimate the breeding success of summer-breeding MacGillivray’s prions, 60 nests were monitored in Prion Cave. Nests were checked every 5 days from pre-laying to hatching, and then every 10 days until chicks fledged. Broad-billed prions breed in late winter and seven nests in a rock cave at Snoekgat (40°20.88'S, 9°52.72'W), and 11 nests in burrows above the Golden Highway (40°20.52'S, 9°53.27'W) were checked at mid-incubation and again at the small chick stage, although some early chick failures may have been missed.
Grey petrels breeding in Gonydale were monitored (n=41 burrows) from laying to fledging. Burrows were fitted with observation hatches to allow a direct view of the nest chamber with a burrowscope, and were checked every 4 days from pre-laying to hatching and then every 7–10 days until the chicks fledged.
Relating chick size at hatching to chick survival
Broad-billed prion, MacGillivray’s prion and grey petrel eggs were measured (length, L, and maximum breadth, B) to the nearest 0.1 mm using Vernier callipers. The fresh mass of eggs (g) was estimated from the relationship: mass=Kw x LB2 (Hoyt Reference Hoyt1979), where L and B are in cm and the constant Kw=0.51 for all species (Warham Reference Warham1990). Egg masses for other species were obtained from the literature (Table III). Chick mass at hatching was estimated at approximately two-thirds of the fresh egg mass, as this is typical of petrels (Payne & Prince Reference Payne and Prince1979, Schramm Reference Schramm1983, Booth et al. Reference Booth, Minot, Imber and Fordham2000).
Table III Summary of the hatching period (listed in succession from spring–summer–winter) and fresh egg mass in relation to chick survival in 2014 (previous years in parenthesis from Cuthbert et al. Reference Cuthbert, Louw, Parker, Rexer-Huber and Visser2013a) for a selection of burrow-nesting petrels and the endemic Gough bunting on Gough Island.

Data analysis
The video files recorded a date and time stamp which enabled us to record a detailed sequence of activity for each filmed nest, including hatching date, frequency of mouse attacks, age of the chick when it was first left alone, and the time of death (for chicks that died before fledging). For all species, hatching success was calculated as the proportion of eggs that produced live chicks; this was a maximum estimate as not all eggs were monitored from laying. Fledging success was calculated as the proportion of hatched chicks that survived to fledge, and the total breeding success as the proportion of eggs laid that produced fledged chicks. Since individual mice could not be identified in the footage, the maximum number of mice involved in an attack was recorded as the maximum number of mice in the frame at one time. Regression analyses were conducted in the R 3.1.2 (R Core Team 2014) where a binomial generalized linear model run in package lme4 was used (Bates et al. Reference Bates, Machler, Bolker and Walker2014). Data are presented as mean±standard deviation (SD).
Results
Video cameras captured the first confirmed records of fatal attacks by mice on soft-plumaged petrel and MacGivillray’s prion chicks. Attacks were also recorded on great shearwater and Atlantic petrel chicks, adding further evidence of attacks on these species since the first records from 2004. In addition, live broad-billed prion and grey petrel chicks were found with wounds typical of those inflicted by mice (see Table III). Video recordings showed the speed with which mice kill chicks and that mice have severe effects on burrow-nesting birds. Our results show that mice affect burrowing petrels year round.
Broad-billed prion
Despite extensive searches, only 18 broad-billed prion nests with an incubating bird were located by mid-September 2014: seven nests in Snoekgat cave and 11 nests in burrows on the path to the Golden Highway. By 28 September 2014 all of the nests at Snoekgat cave had failed with evidence of mouse incisor marks on freshly broken egg shells (Fig. 1) and no evidence of any eggs having hatched. Only two of the 11 burrows on the path to the Golden Highway contained chicks by 15 October 2014, and both had failed by 6 November 2014 (18% hatching success and 0% breeding success). On 29 October 2013, a small prion chick (~2 weeks old) was found alive, but with severe mouse wounds, in its burrow on the north-east slope of 960 Hill (Fig. 2).

Fig. 1 Evidence of mouse incisor marks on a freshly broken broad-billed prion egg shell at Snoekgat cave in September 2014 (photo Ben Dilley).

Fig. 2 A broad-billed prion chick (~2 weeks old) found alive, but with severe mouse wounds, in its burrow on the north-east slope of 960 Hill, Gough Island, in October 2013 (photo Ben Dilley).
Common diving petrel
Only two common diving petrel burrows were found within 200 m of the station in November 2013. Both burrows had new nest material in the chamber with an adult present, but only one pair laid an egg. This burrow was monitored with a camera from 27 November 2013 to 6 February 2014. Mice frequently passed through the burrow, but the incubating adult did not react to their presence. On 13 December 2013 at 17h30 GMT the parent left the burrow, leaving the egg unattended. Within 2 hours a pair of mice had discovered the egg, but they could not break the shell before the parent returned to resume incubation at 20h12. The chick hatched on 18 December 2014, was brooded for 10.1 days and fledged on 6 February 2014 (age 49 days). Despite being repeatedly agitated by mice (Fig. 3), this chick was not wounded or attacked.

Fig. 3 Despite being repeatedly investigated by mice, this common diving petrel chick (here 15 days old) was never wounded or attacked (photo Ben Dilley).
Great shearwater
Seven great shearwater nests were filmed for 241 days from mid–late incubation (mid-December) until chicks were medium-sized (mid-February; Table I) when the cameras were removed to install into the soft-plumaged petrel burrows at late incubation. In one nest, during the last week of incubation, the parent abandoned its egg after 23 days without relief from its partner, and 48 minutes later a mouse entered the burrow and attempted unsuccessfully to gnaw into the egg. At 59 minutes after the adult departed, a larger mouse arrived and made a hole through the pointed end of the egg, and within 74 minutes of the egg being abandoned it had been reduced to a small fragment of egg shell with mouse bite marks. An adult great shearwater (presumably a parent) entered the burrow 130 minutes after the egg was abandoned and settled on the nest mound. The bird was joined by another adult 2 days later, before both birds abandoned the burrow.
The chicks hatched in the remaining six filmed nests and were left alone after 3.9±0.6 days (range 3.1–4.5 days). One chick was wounded on the lower rump by a single mouse 9.4 days after hatching. Following repeated attacks over 3.3 days by up to three mice at a time, the chick died (Fig. 4). When first attacked, this chick appeared in good health and had been fed by a parent on two occasions since being left alone at 4.3 days old. Although the other five chicks were frequently visited and occasionally agitated by mice, none was wounded and all survived to fledge (nests were monitored with a burrowscope after the cameras were removed). Great shearwater fledgling success was 60% and breeding success was 44% in 2014 (n=147 nests, Table II).

Fig. 4 This great shearwater chick (here 10 days old) died 3.3 days after first being attacked by a mouse (photo Ben Dilley).
MacGillivray’s prion
Eggs were laid in Prion Cave from approximately 23 November to 3 December 2013 (n=60 nests), and chicks hatched in the first week of January 2014 (85% hatching success). Chicks were brooded for 5–10 days after hatching. Chick survival was very low, with 18% of chicks surviving to fledge in late-February 2014, giving an overall breeding success of 15% (Table II). Almost all of the chick failures (93%, n=42) occurred in the first 10 days of February 2014 when chicks were >20 days old. GoPro footage of an injured chick showed two mice gnawing at its neck wound (Fig. 5), with more mice and two Gough moorhens (Gallinula comeri Allen) feeding off dead chicks in the cave. In December 2014, 60 nests were again monitored in Prion Cave. Further video evidence of mice attacking and killing chicks was recorded, and by the first week of February 2015 all chicks had died, giving an average breeding success over both years of 7%.

Fig. 5 Mice attacking a MacGillivray’s prion chick (here ~20 days old) in Prion Cave (photo Ben Dilley).
Soft-plumaged petrel
Of the nine soft-plumaged petrel burrows filmed, seven chicks hatched. Two nests failed when the eggs were left unattended and were eaten by mice. One egg was abandoned by the parent 7 days after an incubation shift change and within 16 minutes two mice appeared in the burrow and ate the egg, which appeared to contain a well-developed chick. This burrow remained empty for a further 4 nights before an adult occupied the burrow overnight. The other egg was left alone for 4 days after an incubation shift change and was eaten by a single mouse after 3.1 hours. Two days later an adult returned to the burrow. The seven chicks that hatched were left alone after 1.8±0.4 days (range 1.4–2.3 days), and these small chicks appeared to be extremely vulnerable to mouse predation (being of a similar body size to an adult mouse). All seven chicks were frequently visited and agitated by mice, which appeared to lick the chicks’ down, presumably feeding on food spilt when the parents fed their chick. However, fatal chick attacks by mice did not occur until April when the chicks were much larger (n=2, age 42 and 61 days; Table I). No wounds were seen on the five chicks that survived to fledge. The chick survival rate in 2014 was 63% (n=42 nests), with a 45% breeding success (Table II).
Grey petrel
Grey petrels breeding in Gonydale were checked from laying to fledging (n=41 burrows). Hatching success was 85% and 40% of chicks survived to fledge. Overall breeding success was 34% (Table II). Four grey petrel chicks were found alive with mouse wounds on the lower rump (Fig. 6). Of these chicks, three were dead within a week and one survived. For the remaining chick failures, three were killed by brown skuas (Stercorarius antarcticus Lesson) that dug up their burrows, three died from unknown causes, and 12 were found dead and partly mouse-eaten in their burrows. These chicks were almost certainly killed by mice because in all cases the chicks appeared in good health on the previous visit 7–10 days previous. Therefore, it is probable that mice were responsible for 71% of the chick failures.

Fig. 6 A grey petrel chick (2 weeks old) with a mouse injury (photo Ben Dilley).
Atlantic petrel
Cameras were placed in eight Atlantic petrel burrows at late incubation and monitored for a total of 90 days. One egg was abandoned on 24 September 2014 after prolonged incubation and when inspected the egg was found to be addled. The other seven eggs hatched between 23 August and 13 September 2014. All seven chicks were attacked by mice within 2.7±1.7 hours of hatching (range 0–4.8 hours), and were killed by mice within 7.2±4.0 hours of hatching (range 3.1–15.1 hours; Table IV). In all cases, the chicks were still being brooded and the initial attack was by a single mouse which was not deterred by the presence of the adult petrel. The mouse would grasp the chick with its front feet while standing on its hind legs and gnaw at one spot until the chicks’ skin was broken (Fig. 7). Mice appeared to attack whichever part of the small chick was exposed, starting with the rump (n=4), top of the head (n=2) or back of the neck (n=1). The mice would then expose a large wound on the lower rump, characteristic of wounds seen on freshly dead chicks commonly found in burrows by fieldworkers since 2004. On average 1–3 mice would attack at one time (mean 1.9±0.7 mice) and kill the chick within 4.5±4.0 hours (range 1.5–13.1 hours). In one nest, the chick was hatching when a mouse pulled off the cracked eggshell, attacked the wet chick and killed it within 4 hours. Some adults dropped their wings to better cover the newly hatched chick, but the mice pushed underneath the wing, eventually causing the adult to move aside. All attacks were initiated at night, but in two nests mice returned during the day to kill the injured chicks (Table IV). A one minute video of an Atlantic petrel chick being attacked by a mouse is available at https://youtu.be/VVehgRcfO98.

Fig. 7 A mouse attacking a newly hatched Atlantic petrel chick with the parent sitting alongside (photo Ben Dilley).
Table IV The speed with which mice killed seven newly hatched Atlantic petrel chicks. ‘-’ represents an egg being incubated, ‘H’ indicates hatching, the numbers represent the hourly maximum number of mice attacking a chick at one time, ‘X’ indicates a dead chick.

Hatching success was 90% at 92 monitored burrows, but chick survival was 13% (11/83). The timing of chick failures followed a similar pattern to the camera-monitored nests, with most failures occurring shortly after hatching.
Factors affecting chick survival
Although there was a trend for larger chicks to have higher survival (Fig. 8), the relationship was not significant (r 2 =0.198, F1,4=0.991, P=0.376). This was largely due to high chick survival of soft-plumaged petrels. The likelihood of a chick surviving to fledge appears to be related to the time of hatching (season) and its mass at hatching, with both prion species having the lowest chick survival rates and all winter breeders having low chick survival rates.

Fig. 8 Relationship between the estimated chick mass at hatching (g) and the chick survival (%) for burrowing petrels in 2014–15. Solid diamonds indicate summer breeders and open diamonds indicate winter breeders. Species abbreviations are (from top): soft-plumaged petrel (Pt. mollis), great shearwater (Pu. gravis), grey petrel (Pr. cinerea), MacGillivray’s prion (Pa. MacGillivray), Atlantic petrel (Pt. incerta) and broad-billed prion (Pa. vittata).
Discussion
This study shows that mice kill chicks of all species of burrowing petrels studied on Gough Island. The impact of mice on chicks of surface-nesting albatrosses has been well documented, as these species are readily observed and are therefore easier to monitor (Cuthbert & Hilton Reference Cuthbert and Hilton2004, Wanless et al. Reference Wanless, Ryan, Altwegg, Angel, Cooper, Cuthbert and Hilton2009, Davies et al. Reference Davies, Dilley, Bond, Cuthbert and Ryan2015). In 2004, video cameras recorded fatal attacks by mice on burrowing petrel chicks (Wanless Reference Wanless2007), but since 2004 there have been few direct records of mouse interactions with burrow-nesting petrels because of the technical difficulties in observing inside burrows (Brooke Reference Brooke2004). Once killed, a chick carcass is usually completely consumed by mice or removed from the burrow by moorhens, leaving little evidence for the nest failure or if the chick even hatched. Atlantic petrel chicks were killed within hours of hatching and the carcasses were consumed quickly, which explains why so few mouse-injured chicks have been found during routine nest checks relative to the total number of chick failures. Atlantic petrel breeding success in 2014 was lower than any other year monitored to date (range 36–69%; Wanless et al. Reference Wanless, Ratcliffe, Angel, Bowie, Cita, Hilton, Kritzinger, Ryan and Slabber2012, Cuthbert et al. Reference Cuthbert, Louw, Lurling, Parker, Rexer-Huber, Sommer, Visser and Ryan2013b). With the exception of one mouse-injured grey petrel chick which recovered, all mouse-injured chicks died from their injuries.
In winter, mice have limited food resources (Cuthbert et al. unpublished) and the winter-breeding petrels were worst affected by mice, with chicks hatching in early winter (grey petrels) having a higher chick survival rate than chicks hatching in mid-winter (Atlantic petrels) or late winter (broad-billed prions, Fig. 8). Other winter-breeding species have not been studied because of difficulty locating their burrows, but late winter breeders, such as little shearwaters and great-winged petrels (Pterodroma macroptera Smith) (Table III), are probably also severely affected by mouse predation. Little shearwaters have become rare around the station on Gough Island over the last 30 years (Ryan, personal observation), and fieldworkers have been unable to locate any great-winged petrels. Great-winged petrels occurred in ‘large numbers’ and little shearwaters were ‘extremely abundant’ around The Glen on the east coast of the island in 1955 (Swales Reference Swales1965).
Previous research has shown smaller seabirds are more vulnerable to rodent predation (Jones et al. Reference Jones, Tershy, Zavaleta, Croll, Keitt, Finkelstein and Howald2008) and our inability to locate any storm petrel nests suggests that small species have higher mortalities on Gough Island. Video footage from inside the 25 monitored burrows (Table I) showed that incubating birds often left their burrows for a short period (<10 minutes), usually in the early evening, and some individuals more frequently than others. In some cases, birds were absent for a few hours or even days, allowing mice to eat their egg (Fig. 9). Temporary egg desertion has been documented for many procellariiforms, and eggs may still hatch despite being neglected for up to 2 days (Boersma & Wheelwright Reference Boersma and Wheelwright1979). Campos & Granadeiro (Reference Campos and Granadeiro1999) recorded white-faced storm petrels on Selvagem Grande Island leaving their eggs for 1–6 days, resulting in 17/35 (48.6%) eggs failing due to mice predation, 12 of which were eaten by mice within 24 hours of being left alone. Temporary egg desertion is also frequent in blue petrels (Ancel et al. Reference Ancel, Petter and Groscolas1998), a species recently found breeding on Gough Island in the summer of 2014 (Ryan et al. Reference Ryan, Dilley, Jones and Bond2015).

Fig. 9 A mouse predating on a temporarily neglected great shearwater egg (photo Ben Dilley).
Gough Island mice are 50–60% heavier than those from any other island (mean: 35 g; Cuthbert et al. unpublished). Peak mouse densities are also among the highest recorded for island populations (266 mice ha-1) with relatively low seasonal variations (4–5-fold) driven primarily by an absence of other mammalian predators and an abundance of seabird chicks as a winter food source (Cuthbert et al. unpublished). Larger mice are better able to bite into seabird eggs, and presumably have an advantage when attacking seabird chicks. Given that chicks of the two largest burrowing petrels on Gough Island, grey petrel and great shearwater, are both killed, it is probable that all species are impacted.
Mice have been present on Gough Island for more than a century. How have these petrels managed to maintain their populations in the face of this predation? Firstly, apart from 1957, there are few detailed records on Gough Island’s seabirds prior to 2000 when year-round seabird monitoring and research began; therefore, it is not known how long mice have been affecting chick survival. There are few early records on burrowing petrel populations, but Tristan albatross are better documented and attacks on chicks have almost certainly been happening since the 1970s and have probably contributed significantly to an estimated 50% decrease in the breeding population over 50 years (cf. Wanless et al. Reference Wanless, Ryan, Altwegg, Angel, Cooper, Cuthbert and Hilton2009). Secondly, mice target eggs and chicks, reducing petrel reproductive success, but adult survival is not directly affected and this is more important for maintaining their populations (Le Corre Reference Le Corre2008). Although burrowing petrels have long lifespans and low reproductive rates, some natal recruitment would be required to maintain adult populations. The MacGillivray’s prion and blue petrel have recently been discovered breeding on Gough Island, but it is not known if these populations were overlooked or if they recently colonized the island.
Elsewhere, introduced rodents coexist with burrow-nesting seabirds, but seabird populations are either supplemented by immigration from other colonies, or predation is lower due to smaller rodent populations (Quillfeldt et al. Reference Quillfeldt, Schenk, McGill, Strange, Masello, Gladbach, Roesch and Furness2008, Brooke et al. Reference Brooke, O’Connell, Wingate, Madeiros, Hilton and Ratcliffe2010). In other cases, however, seabird populations are too large to census accurately, and the effects of introduced rodents may not be immediately recognized (Major et al. Reference Major, Bond, Jones and Eggleston2013). The nearest potential sources of immigrants to Gough Island are Inaccessible and Nightingale islands, which are both 400 km away (Ryan Reference Ryan2007), making inter-island movement of petrels unlikely (Brooke Reference Brooke2004, Buxton et al. Reference Buxton, Jones, Moller and Towns2014). We believe that given the relatively recent arrival of mice (<200 years), their strong initial bottleneck (Gray et al. Reference Gray, Wegmann, Haasl, White, Gabriel, Searle, Cuthbert, Ryan and Payseur2014), the generally high adult survival and longevity of petrels (Brooke Reference Brooke2004), and initially large populations of petrels (Swales Reference Swales1965) have all combined to result in the persistence of petrels on Gough Island. Their continued persistence, though, is perilous in the face of the intense mouse predation reported here.
Our estimates of breeding success in 2014 were similar to or higher than recent estimates for summer-hatching species (Cuthbert et al. Reference Cuthbert, Louw, Lurling, Parker, Rexer-Huber, Sommer, Visser and Ryan2013b), but in 2014 Atlantic petrels had the lowest breeding success recorded, as was the case for Tristan albatrosses (<10%; Davies et al. Reference Davies, Dilley, Bond, Cuthbert and Ryan2015). The low breeding success of Atlantic petrels is of particular concern since virtually the entire population breeds on Gough Island. The species has not been recorded breeding on the main island of Tristan da Cunha for 40 years and is probably extinct there (Ryan Reference Ryan2007), but small numbers may breed on Inaccessible Island (Ryan personal observation). Of equal concern is the recently discovered population of MacGillivray’s prion on Gough Island (Ryan et al. Reference Ryan, Bourgeois, Dromzée and Dilley2014). This species is extinct on Amsterdam Island, and only a relict population of a few hundred birds breed on La Quille, a stack off St Paul Island (Worthy & Jouventin Reference Worthy and Jouventin1999). If, as seems likely, the Gough Island population is part of this species (Ryan unpublished data), the island supports virtually the entire world population. MacGillivray’s prion chick survival in Prion Cave was low in both 2014 (18%) and 2015 (0%). This is much lower than the 60–70% chick survival rate by prions breeding at predator-free islands (Liddle Reference Liddle1994).
Broad-billed prions had the worst breeding success of all species monitored in 2014. In addition, a substantial search effort was needed to find burrows containing incubating adults, despite their being the most common petrel seen at night around the station. Most nests failed at the egg or early chick stage (16/18 eggs laid) and the two small chicks recorded also disappeared, resulting in 0% breeding success. These results are similar to previous years when small samples of nests gave breeding success estimates of 0–9% (Cuthbert et al. Reference Cuthbert, Louw, Lurling, Parker, Rexer-Huber, Sommer, Visser and Ryan2013b).
Our study confirms that house mice are significant predators of petrel eggs and chicks on Gough Island, and that all species are likely to be impacted. Video footage showed that mice can be very effective predators of burrowing petrels, killing chicks within hours of hatching while still brooded by their parents, and also tackling large chicks of many times their body size. Gough Island is the highest priority island for introduced vertebrate eradication in the UK Overseas Territories (Dawson et al. Reference Dawson, Oppel, Cuthbert, Holmes, Bird, Butchart, Spatz and Tershy2015), and urgent action is needed if prospects for seabirds on Gough Island are to be improved. Petrels, particularly the smaller and rarer species, are likely to be extirpated from Gough Island if mice are not eradicated in the near future. Preparations for such an operation are complex, but are ongoing (Broome & Garden Reference Broome and Garden2013).
Acknowledgements
We thank the fieldworkers on Gough Island for their monitoring efforts, in particular R. Wanless, R. Cuthbert, C. Jones, M. Risi and W. Kuntz. J. Bradley found the diving petrel nest and R. Meyer helped to develop the system of burrow cameras and LED lighting. Permission to undertake the work on Gough Island was obtained from the Tristan Conservation Department. Logistical and financial support was provided by the South African Department of Environmental Affairs, through the South African National Antarctic Programme, the National Research Foundation, the University of Cape Town and the Royal Society for the Protection of Birds (RSPB). Long-term monitoring on Gough Island was established with a grant from the UK Foreign and Commonwealth Office with further support over the years from the UK Government’s Overseas Territories Environment Programme, the RSPB, and the Agreement on the Conservation of Albatrosses and Petrels. We thank two anonymous reviewers for their useful comments which improved previous drafts.
Author contributions
BJD and DD conducted the field work and preliminary analyses; BJD developed the burrow cameras and wrote the first draft. PGR and ALB supervised the research, assisted in the field and advised on manuscript preparation.
Supplemental material
A supplemental appendix will be found at http://dx.doi.org/10.1017/S0954102015000255.
















