Introduction
Dibutyl phthalate (DBP) is a widely used plasticizer with stability, flowability, low volatility and good low-temperature resistance (Jaworek Reference Jaworek2013). It is not chemically bound to a polymeric matrix and can migrate from the plastic (Staples et al. Reference Staples, Adams, Parkerton, Gorsuch, Biddinger and Reinert1997). The degradation half-life of DBP is over 20 years (Bajt et al. Reference Bajt, Mailhot and Bolte2001). The slow degradation, low volatility, high bioaccumulation and toxicity characteristics of DBP may adversely affect organisms (Guo et al. Reference Guo, Chen, Jiang, Ma and Zheng2014).
The effects of DBP on aquatic algae and submerged macrophytes include growth inhibition (Phaeodactylum tricornutum Bohlin and Chlorella vulgaris Beyerinck (Beijerinck)), cell division inhibition (Scenedesmus obliguus (Turpin) Kützing), physiological and biochemical indices alteration (Ceratophyllum demersum L., Vallisneria spiralis L. and Potamogeton maackianus A.Benn.) (Kuang et al. Reference Kuang, Zhao and Cheng2003, Chi et al. Reference Chi, Liu, Li and Huang2006, Li et al. Reference Li, Guo, Mu, Wang and Yin2006, Yang & Duan Reference Yang and Duan2010). Furthermore, DBP may lead to metabolic abnormalities in abalone (Haliotis diversicolor supertexta Lischke) (Zhou et al. Reference Zhou, Chen and Cai2015). The effects of DBP on arthropods include rapid maturation, reduced spawning (Drosophila melanogaster Meigen), increased mortality (Folsomia fimetaria L.) and reduced movement (Gammarus pulex L.) (Thurén & Woin Reference Thurén and Woin1991, Jensen et al. Reference Jensen, van Langevelde, Pritzl and Krogh2001, Atli Reference Atli2010). It also poisons fish and amphibians causing organ damage (male zebrafish Danio rerio (F. Hamilton)) and interfering with gonad differentiation (frog Rana rugosa Temminck and Schlegel) (Ohtani et al. Reference Ohtani, Miura and Ichikawa2000, Xu et al. Reference Xu, Chen, Liu, Zeng, Zhou and Li2014). The effects of DBP on mammals (mainly mice and rats) include severe reproductive toxicity, reproductive abnormalities, embryo toxicity, organ damage and even neurotoxicity (Murakami et al. Reference Murakami, Nishiyama and Higuti1986, Salazar et al. Reference Salazar, Castillo, Ariznavarreta, Campón and Tresguerres2004).
Though it has been classified as a priority controlled pollutant by the US Environmental Protection Agency (Xu et al. Reference Xu, Li and Wang2008), studies have determined that DBP remains a ubiquitous environment contaminant that can be found in food packaging materials, household items, soils, freshwater, marine water, organisms and even air (Fatoki & Ogunfowokan Reference Fatoki and Ogunfowokan1993, Staples et al. Reference Staples, Adams, Parkerton, Gorsuch, Biddinger and Reinert1997, Lu et al. Reference Lu, Xue, Shao, Gu, Zeng and Luo2016). Unfortunately, information on DBP concentrations in Antarctic krill is still scarce.
Antarctic krill live in groups (Kokubun et al. Reference Kokubun, Choy, Kim and Takahashi2015). This species makes up an estimated biomass of 400–1550 million tons, which may be the largest biomass of a single multicellular organism (Gigliotti et al. Reference Gigliotti, Davenport, Beamer, Tou and Jaczynski2011). The aim of this study was to determine if there were phthalate esters in Antarctic krill and to discuss their potential sources and adverse effects.
Materials and methods
Samples, reagents and instruments
Antarctic krill were purchased frozen whole from the Liaoning Fishery Group (Dalian, Liaoning, China). This batch (35–55 mm in body length) was caught in the first quarter of 2016 from the waters surrounding the Chinese Great Wall Antarctic Station. Dimethyl phthalate (DMP), diethyl phthalate (DEP), DBP, dioctyl phthalate (DEHP) standards (≥ 99%) were obtained from Aladdin (Shanghai, China). All other reagents used in the study were of analytical reagent grade. A FUD-1200 freeze dryer (Rikakikai Tokyo, Japan), a Kun Shan Ultrasonic Instruments cleaning machine (Jiangsu, China), a Rotavapor-3 rotating evaporator (Buchi, Flawil, Switzerland) were obtained. Pre-coated silica gel GF254 high-performance thin layer chromatography (HPTLC) plates (10×10 cm2) were obtained from Haiyang Chemical (Qingdao, China), a development chamber (10×12×5 cm3, consisting of a double-groove glass chamber) was obtained from Shanghai Xinyi Instrument (Shanghai, China). A Thin Layer Chromatography Scanner 3 equipped with Wincats 1.4.1 software was purchased from CAMAG (Muttenz, Switzerland). None of the experimental instruments or materials contained plastic products.
Preparation of sample solutions and standard solutions
The middle sections of whole frozen Antarctic krill (1000 g) were freeze-dried, leaving 200 g of dried krill. The dried krill was divided into eight equal samples (25 g per sample). Each sample was extracted separately using the following method. One sample was put into a glass beaker and dipped with three times the volume of cyclohexane (purified by distillation before use) and then extracted for 0.5 h by ultrasonic treatment at 25°C, 300 w and 40 kHz. The cyclohexane extract was filtered through a qualitative filter (after flushing with cyclohexane). The above extraction procedure was repeated three times until the extraction solution was close to colourless. All the filtrates were combined and concentrated at 60°C using a rotating evaporator to remove the cyclohexane. The residue (1.56 g) was dissolved in cyclohexane and its volume was adjusted to 5 ml (samples 1–8).
A standard solution was prepared as a mixture of DMP, DEP, DBP and DEHP dissolved in cyclohexane. The standard solution contained 1 mg ml-1 concentrations of DMP, DEP, DBP and DEHP standards.
High-performance thin layer chromatography
The HPTLC was performed according to the previously reported method (Chen et al. Reference Chen, Wang and Zhu2006). The standard solution, sample 1 and a cyclohexane (blank) solution were spotted onto the same GF254 plate, and developed with a developing solvent that was a mixture of ethyl acetate, ethyl ether and isooctane (1:4:15 v/v/v). Double wavelength reflection absorption flying-spot scanning (λ S 275 nm, λ R 340 nm) was performed to detect the plate. Quality analysis was according to the Rf value of the spot and quantitative analysis was employed by comparison to the external standard.
Repeated trials
The eight sample solutions were detected using one GF254 plate (3 μl each sample). The DBP content was quantified by HPTLC. Peak areas were recorded and the DBP content in Antarctic krill was calculated.
Gas chromatography-mass spectrometry
The conditions of gas chromatography-mass spectrometry (GC-MS) were those of Lahimer et al. (Reference Lahimer, Ayed, Horriche and Belgaied2013).
Results
High-performance thin layer chromatography
The HPTLC clearly separated the four phthalate esters, and the results of double wavelength reflection absorption flying-spot scanning of the standard solution (λ S 275 nm, λ R 340 nm) are shown in Fig. 1. The results of the scan of sample 1 are shown in Fig. 2. Only DBP was found to be present. The scan of the blank solution indicated that there was no phthalate ester present in the cyclohexane solution (Fig. 3).
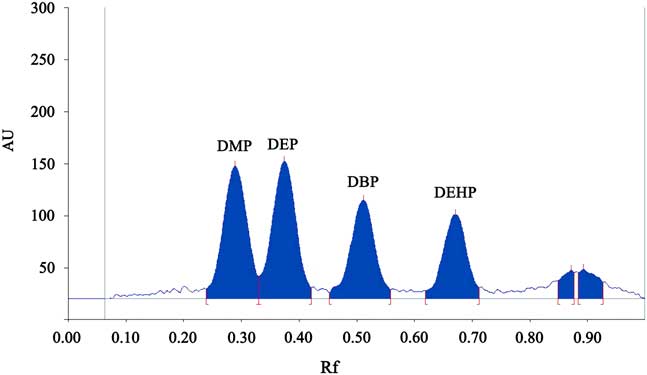
Fig. 1 The chromatogram of the standard solution showing the average peak areas for dimethyl phthalate (DMP) diethyl phthalate (DEP), dibutyl phthalate (DBP) and dioctyl phthalate (DEHP). AU=absorbance unit.
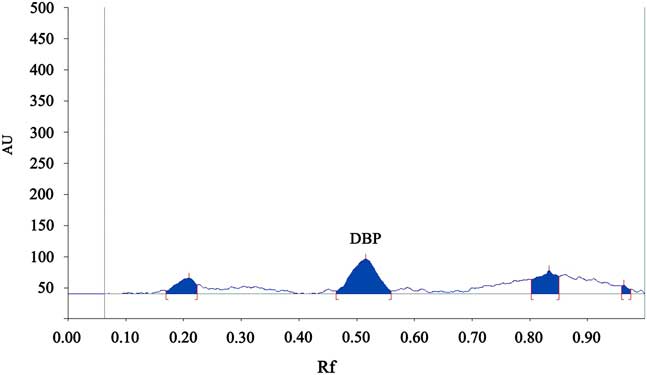
Fig. 2 The chromatogram of sample 1. AU=absorbance unit.
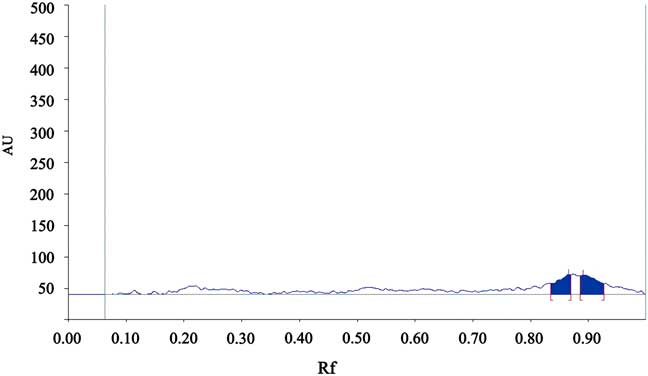
Fig. 3 The chromatogram of the blank solution. AU=absorbance unit.
Linearity and repeated trials
The DBP content was calculated using the external standard method. The linear equation of DBP and the linear range (Table I) was determined as the following:
with a standard deviation of 1.94%. The results for the eight sample solutions according to the linear equation are shown in Table II. The mean content of DBP in freeze-dried Antarctic krill was 0.1043±0.0005 mg g-1 (104.3±0.05 mg kg-1).
Table I Average peak areas corresponding to different amounts of dibutyl phthalate (DBP).

AU = absorbance unit.
Table II The peak areas and dibutyl phthalate (DBP) concentrations for repeated sampling of freeze-dried Antarctic krill.

AU = absorbance unit, SD = standard deviation.
Gas chromatography-mass spectrometry
The result of the GC-MS for sample 1 is shown in Fig. 4. The retention time of DBP was 10.915 min.
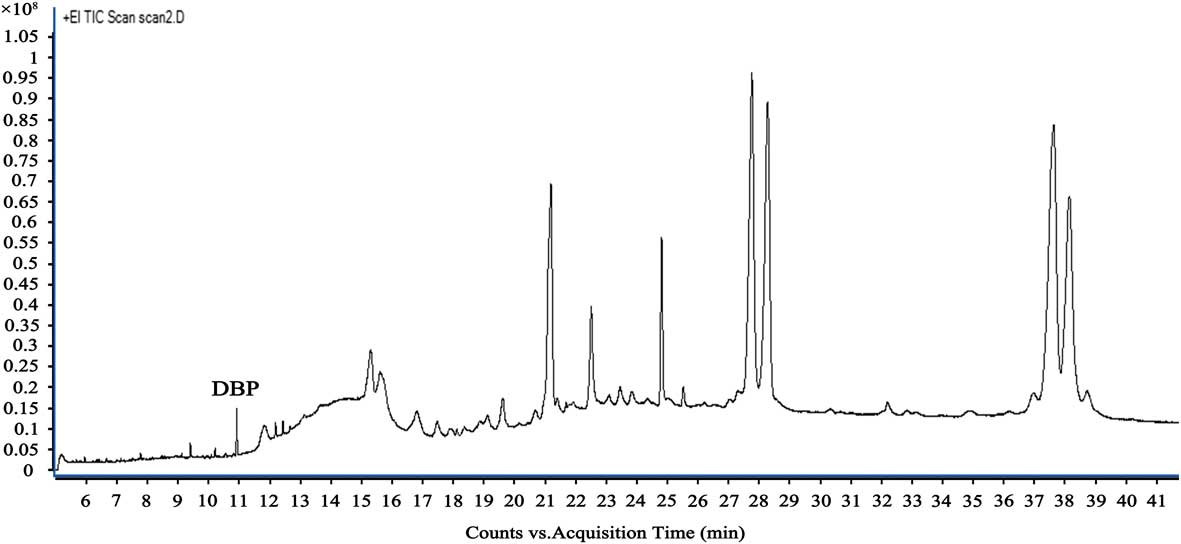
Fig. 4 The retention time of dibutyl phthalate (DBP).
The mass spectrograms of sample 1 and the standard solution are shown in Figs 5 & 6. This mass spectrogram confirms the presence of DBP in Antarctic krill.
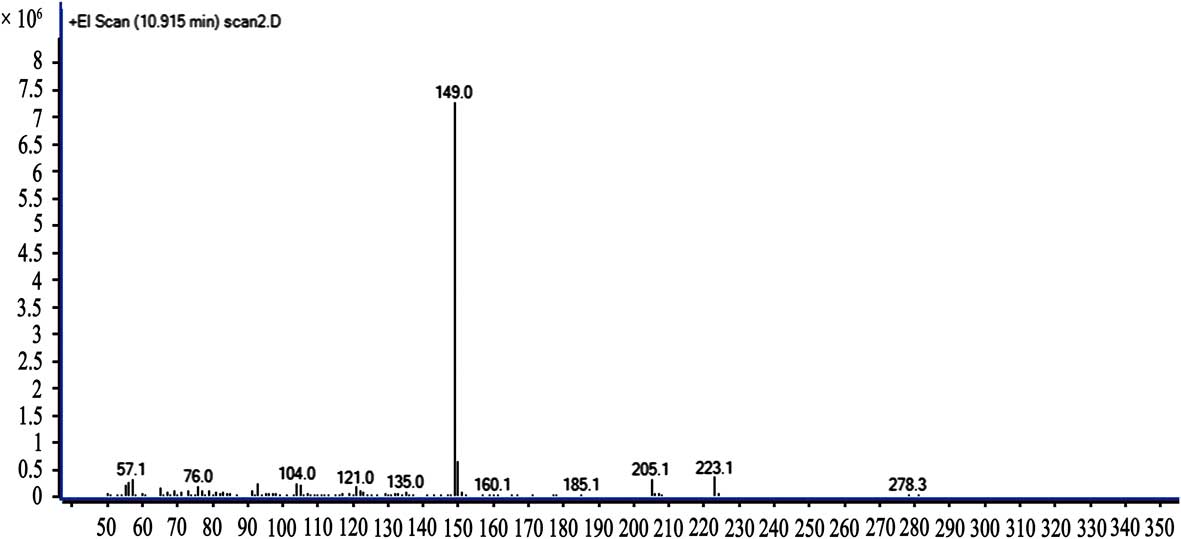
Fig. 5 The mass spectrogram of sample 1 with a retention time of 10.915 min.
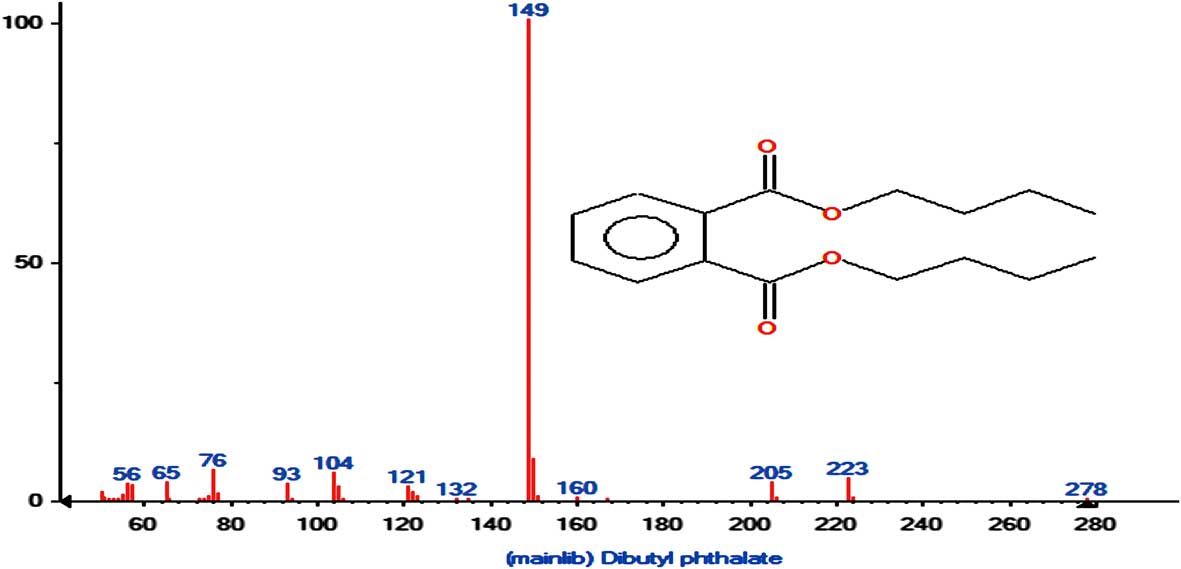
Fig. 6 The mass spectrogram of the dibutyl phthalate (DBP) standard solution.
Discussion
The levels of four phthalate esters were measured in Antarctic krill using HPTLC. Only DBP was observed. This is the first report of DBP in Antarctic krill. The structure of DBP determined by GC-MS confirmed its presence. The DBP content was 0.1043±0.0005 mg g-1 (104.3±0.05 mg kg-1).
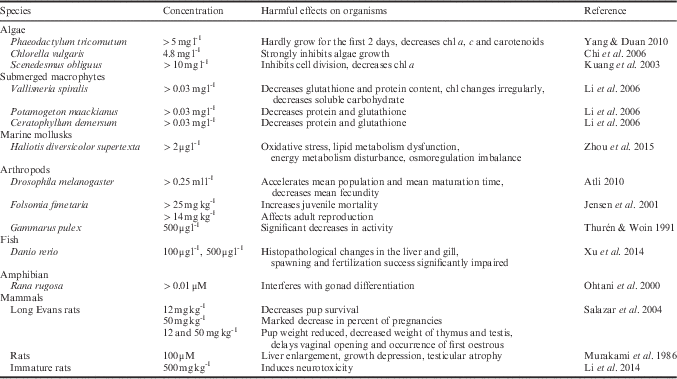
The concentrations of DBP that are toxic to organisms and the effects of DBP toxicity are summarized in Table III. Exposure to DBP has marked effects on organisms, with significant effects on reproduction. The concentrations associated with DBP toxicity range from 0.2 μg l-1 to 500 mg kg-1. Therefore, the 104.3±0.05 mg kg-1 level found in Antarctic krill (20.86±0.05 mg kg-1 in fresh Antarctic krill accounting for 80% water content) is high. Although the effects of DBP on Antarctic krill have not been determined, based on the effects on other species this level of DBP may pose a serious risk to the Antarctic krill. Antarctic krill is a key part of the marine ecosystem, and the accumulation of DBP could lead to poisoning of other animals in the food chain; thereby, seriously affecting the Antarctic ecosystem.
Table III Harmful effects of dibutyl phthalate (DBP) on organisms and their related concentrations.
There may be multiple pathways by which DBP may accumulate in Antarctic krill. One source of DBP could be their food (which could produce DBP and absorb DBP from marine water) (Chi et al. Reference Chi, Liu, Li and Huang2006, Namikoshi et al. Reference Namikoshi, Fujiwara, Nishikawa and Ukai2006). Chitin adsorption may also be a possible source of DBP (McKay et al. Reference McKay, Blair and Gardner1982). Ge et al. (Reference Ge, Li, Lin, Zhao and Han2012) reported hat fishes could absorb DBP from water; therefore, Antarctic krill may also directly absorb DBP from marine water. The source of DBP in krill requires further research.
Information on the sources and concentrations of DBP in Antarctic biota is still absent. It may reach Antarctica through long-range atmospheric and hydrospheric transport. Plastics discarded by human activities may also be a source of DBP as human activities have increased in recent years (Stark et al. Reference Stark, Snape and Riddle2006). Migratory species (such as seabirds) may introduce DBP into the local food web and may bio-transport DBP into the Antarctic (Mwangi et al. Reference Mwangi, Lee, Wang, Sung, Fang, Lee and Chang-Chien2016). However, the true source of DBP in the Antarctic needs further investigation.
Fresh Antarctic krill, Antarctic krill powder and Antarctic krill oil (the main krill products used by humans) are exported and widely consumed all over the world. The maximum allowable daily level of DBP for humans is 8.7 μg day-1 (Kokubun et al. Reference Kokubun, Choy, Kim and Takahashi2015). The observed concentration of 104.3±0.05 mg kg-1 DBP in the freeze-dried Antarctic krill is high and is a serious concern for human consumption.
Acknowledgements
The experimental raw materials used in this study were supported by the Liaoyu Fishery Group. This study was funded by the scientific & technological project of Shandong Province (grant number: gg10002088). The authors declare that they have no conflicts of interest.
Author contribution
DL designed the research. XH performed the experiments and analysed the data. XH and DL contributed to writing the manuscript.












