Introduction
Antarctica remains a largely untouched region of the planet. However, even here, areas have been contaminated by petroleum hydrocarbons, notably diesel oil, associated with anthropogenic activities through national governmental research stations as well as the rise in tourism in recent decades, both primarily supported by ships (Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey and Moreno2009, Hughes Reference Hughes and Cowan2014). Currently, over 100 facilities are established on the continent, primarily supporting research activities (Hughes Reference Hughes and Cowan2014). Petroleum hydrocarbons represent the major source of energy used to run facilities in Antarctica, and they are the primary source of hydrocarbon pollution in this region (Ruberto et al. Reference Ruberto, Vazquez, Lobalbo and Mac Cormack2005). Diesel oil, the most commonly used hydrocarbon fuel in the Antarctic region, consists of a complex mixture of aliphatic and aromatic hydrocarbons and is made by the fractional distillation of petroleum (Gallego et al. Reference Gallego, Loredo, Llamas, Vázquez and Sánchez2001). It also contains toxic compounds such as phenolics that tend to persist in contaminated environments, along with heavy metals including cadmium (Cd), chromium (Cr) and lead (Pb) (Lee et al. Reference Lee, Hagwall, Delfino and Raotvg1992). Heavy metals have been reported to alter the activities of Antarctic microbial communities and to affect the enzymes involved in various metabolic processes, including the impairment of microbial diesel degradation. Oil spillage as a result of accidental discharge during transportation and leakage from storage tanks and pipelines has become a serious and persistent threat in Antarctica (Aislabie et al. Reference Aislabie, Balks, Foght and Waterhouse2004, Hughes Reference Hughes and Cowan2014), particularly affecting the seasonally melted active layer of the soil profile overlying permafrost. Hydrocarbon contamination of Antarctic soils can alter the microbial community's structure and function and affect the abundance of small invertebrate organisms. Spilt oil tends to persist for a long time in the Antarctic environment owing to the chronically low temperatures, limited nutrient availability and dry conditions of the environment, which slow the rate of microbial processes and abiotic degradation (Aislabie et al. Reference Aislabie, Saul and Foght2006).
The use of microorganisms in order to remediate contaminated soil has increased significantly in recent years, surpassing conventional methods as they have proved to be ecologically friendly, simple to apply, cheap and effective (Aislabie et al. Reference Aislabie, Saul and Foght2006, Ahmad et al. Reference Ahmad, Asokan, Yasid, Nawawi, Subramaniam and Zakaria2018). However, in Antarctica, the use of indigenous Antarctic microorganisms for such activities is required, as the importation and release of non-native biota are not permitted under the Environmental Protocol to the Antarctic Treaty (Aislabie et al. Reference Aislabie, Foght and Saul2000). Various studies have confirmed the ability of native microbes to break down petroleum hydrocarbon contaminants in the harsh environmental conditions of Antarctica (Hughes et al. Reference Hughes, Bridge and Clark2007). The most frequently isolated hydrocarbon-degrading bacteria from contaminated Antarctic soils belong to the genera Acinetobacter, Pseudomonas, Rhodococcus and Sphingomonas genera (Ruberto et al. Reference Ruberto, Vazquez, Lobalbo and Mac Cormack2005, Aislabie et al. Reference Aislabie, Saul and Foght2006) and are known to be capable of degrading alkanes (Shukor et al. Reference Shukor, Hassan, Jusoh, Perumal, Shamaan and MacCormack2009) and aromatics (Aislabie et al. Reference Aislabie, Foght and Saul2000).
One of the most significant characteristics of petroleum hydrocarbon-degrading bacteria is their ability to emulsify hydrocarbons in an aqueous solution by producing surface active molecules that cause a reduction in surface and interfacial tension in aqueous solutions and hydrocarbon mixtures (Ibrahim et al. Reference Ibrahim, Ijah, Manga, Bilbis and Umar2013). Biosurfactants are used extensively in bioremediation as they can improve microbial growth on hydrophobic surfaces and also increase the nutrient intake of hydrophobic substrates, thereby making the hydrocarbon pollutant accessible to microorganisms (Ibrahim et al. Reference Ibrahim, Ijah, Manga, Bilbis and Umar2013). The use of microbes capable of degrading hydrocarbons as well as of producing biosurfactants can speed up the bioremediation of hydrocarbon-polluted sites. The utilization of hydrocarbons by bacteria has been reported to depend strongly on environmental variables, including temperature, pH, salinity and nitrogen sources (Habib et al. Reference Habib, Ahmad, Wan Johori, Shukor, Alias and Khalil2018). Thus, these environmental variables are important factors affecting the efficiency of diesel biodegradation. Optimization of the conditions for diesel degradation by microorganisms is essential in order to ensure successful treatment of diesel. Optimization means establishing the foremost solution, representing the best compromise amidst several varying demands subject to predefined requirements called restrictions (Karamba et al. Reference Karamba, Ahmad, Zulkharnain, Syed, Khalil and Shamaan2016). Optimization is very significant in biotechnological processes. Optimum conditions for the rapid growth of a microorganism must be attained in order to achieve desirable responses. Optimizing the variables required for biological processes is crucial to overcoming any difficulties that are identified. The application of optimization in a biotechnological process tends to make such processes efficient and achievable (Ibrahim et al. Reference Ibrahim, Shukor, Abdul Khalil, Halmi, Syed and Ahmad2015, Karamba et al. Reference Karamba, Ahmad, Zulkharnain, Syed, Khalil and Shamaan2016). Traditionally, optimization has been carried by examining the effects of one factor at a time (OFAT) on an experimental response. However, one major drawback of this approach is that it does not study the interaction effects between the examined variables. Previous studies have successfully used the traditional OFAT approach in order to determine the optimal conditions for substrate degradation (Shukor et al. Reference Shukor, Hassan, Jusoh, Perumal, Shamaan and MacCormack2009, Habib et al. Reference Habib, Ahmad, Wan Johori, Shukor, Alias and Khalil2018, Lee et al. Reference Lee, Ahmad, Yasid, Zulkharnain, Convey and Johari2018). Therefore, in order to achieve the maximum rate of diesel degradation, it is important to optimize bacterial growth and degradation. This study was conducted in order to determine the optimum conditions for diesel degradation by the known Antarctic soil bacterial strains Arthrobacter spp. strains AQ5-05 and AQ5-06 using the traditional OFAT method.
Materials and methods
Samples and media
The isolates of Arthrobacter spp. strains AQ5-05 and AQ5-06 used for this study were originally isolated from Antarctic soil obtained on King George Island, South Shetland Islands (Lee et al. Reference Lee, Ahmad, Yasid, Zulkharnain, Convey and Johari2018). Arthrobacter spp. strains AQ5-05 (ref. KX946130-KX946131) and AQ5-06 (ref. KX946127) nucleotide sequences are deposited in the National Center for Biotechnology Information (NCBI) database. The diesel fuel used for the experiment was obtained from a Petronas filling station in Selangor, Malaysia, and was used as the sole source of carbon in all of the experiments. Bushnell–Haas (BH) broth was used for screening and growth optimisation of diesel-degrading bacteria (Bushnell & Haas Reference Bushnell and Haas1941). The medium used was composed of MgSO4 (0.2 g l-1), CaCl2 (0.02 g l-1), K2HPO4 (1.0 g l-1), NH4NO3 (1.0 g l-1) and FeCl3 (0.05 g l-1). The pH of the medium was adjusted to 7.0 ± 0.2 at 25°C prior to sterilization. In order to ascertain the ability of the strains to use diesel oil as the only source of carbon, a non-inoculated BH broth was used as a control. All of the experiments were performed in triplicate.
Screening for diesel-degrading ability
Strains were pre-cultured in nutrient broth medium for 24 h in a shaking incubator at 150 rpm and 10°C. Following incubation, cultures were centrifuged at 5000 rpm for 10 min, and the resulting pellets were washed twice using 1× phosphate-buffered saline (137 mM NaCl and 2.7 mM KCl in 10 mM phosphate buffer, pH 7.4) and then re-suspended to an OD600 of 1 in BH broth containing 0.5% (v/v) diesel as the sole source of carbon and energy. The diesel medium was incubated at 10°C for 168 h in a shaking incubator at 150 rpm. The 10°C incubation temperature was chosen in order to mimic typical summer soil surface temperatures at King George Island (Lee et al. Reference Lee, Lim and Yoom2016). The diesel-degrading activity of each bacterial strain was observed at intervals of 24 h, with bacterial growth determined by measuring the optical density of the medium (OD600). The residual hydrocarbon in diesel oil from each culture was extracted every 12 h using a solvent extraction method by destructive sampling from a triplicate flask containing culture (50 ml of BH media in a 250 ml conical flask). n-Hexane (1:1 media to n-hexane) was used in order to separate the cellular material. After evaporation, the remaining residual hydrocarbon in diesel was quantified gravimetrically (Patowary et al. Reference Patowary, Patowary, Kalita and Deka2017). The diesel degradation percentage was calculated as in Eq. (1):
where X is the original mass of diesel and Y is the mass of residual diesel oil in the test sample.
After 7 days of culture, the amount of degraded hydrocarbon was analysed in the extracted diesel oil samples.
Furthermore, in order to confirm the degradation ability of both strains, the n-hexane-separated samples of treated diesel oil and the abiotic control were further analysed using a gas chromatograph mass spectrometer (GC/MS-QP2010 Ultra, Shimadzu, Japan) equipped with an auto-injector (GC2010). The GC programme was optimized and all of the analyses were performed with a 10:1 split ratio. Helium was used as the carrier gas with a flow rate of 0.8 ml min-1, maintaining an injection temperature of 250°C. The column oven temperature was set at 50°C with a hold time of 10 min and was subsequently increased to 300°C with a ramp of 8°C min-1, with a final hold time of 37 min. The mass spectrometric data were acquired in electron ionization mode (70 eV). The ion source temperature and interface temperature for MS were set at 200°C and 250°C, respectively. The mass range (m/z) was selected as 40–700 for the entire analysis. The chromatograms were analysed with GC/MS solution software, the compound identification was carried out using the NIST 11 library database and alkane standard solution (C8–C20) was used as an internal standard.
Diesel hydrocarbon degradation kinetics
The degradation of diesel was evaluated using the first-order reaction kinetics expressed in Eq. (2):
where lncr is the concentration of the residual diesel oil in the medium at any given time (mg l-1), lnco is the initial concentration of diesel oil, k signifies the rate constant representing the rate of degradation and t represents time (days). The half-life of diesel oil (t 1/2) was evaluated using Eq. (3):
where k denotes the degradation rate constant (days-1).
OFAT optimization of diesel degradation and growth conditions
The growth of bacteria and diesel degradation activity were optimized using the OFAT method based on the following selected parameters: temperature, nitrogen source, initial pH, salinity and initial substrate concentration. The influence of the selected parameters was assessed in 50 ml of BH media contained in a 250 ml conical flask shaken at 150 rpm and 10°C. Each tested parameter was optimized while keeping all other parameters at a constant level for the traditional scaling-up approach. Optimization was performed sequentially (pH, temperature, nitrogen source, nitrogen concentration, salinity and substrate concentration (diesel)). Thus, each subsequent parameter was examined after considering the previously optimized parameters. The influence of temperature was examined at 5°C, 10°C, 15°C, 20°C and 30°C, while the effects of pH were optimized using acetate buffer (pH 5.0–6.0), phosphate buffer (pH 6.0–7.5) and Tris-HCl buffer (pH 7.0–8.0). Salt tolerance was studied by adding various concentrations of sodium chloride (w/v) to the medium (0%, 1%, 2%, 3%, 4% and 5%). The influence of initial diesel concentration was evaluated at 0.5%, 1.0%, 1.5%, 2.0%, 3.0%, 4.0%, 5.0%, 6.0% and 7.0% (v/v). Nitrogen sources evaluated included ammonium nitrate, glutamate, ammonium sulphate, ammonium carbonate, sodium nitrate, ammonium chloride, leucine and aspartic acid. After selection of the most effective source, it was tested at concentrations from 0 to 1.4 g l-1. All experiments were conducted in triplicate, including the negative control. Prior to these experiments, bacterial cell suspensions were prepared by growing the isolates in 100 ml of nutrient broth for 24 h at 10°C and 150 rpm. Bacterial cells were then centrifuged at 500 g for 10 min and washed twice by re-suspending in 1× phosphate buffer solution. Finally, the cell suspension was adjusted to an absorbance of 1 (OD600), equivalent to 2.75 × 107 colony-forming units ml-1. Bacterial growth and diesel degradation were evaluated after 7 days of incubation.
Microbial adhesion to hydrocarbon assay
The cellular hydrophobicity of both isolates was measured as previously described by Rosenberg (Reference Rosenberg1984). Briefly, 24 h grown bacterial culture was collected, washed and rinsed twice with saline. The bacteria were then re-suspended to an absorbance of 1 (OD600) measured on a spectrophotometer (UV mini 1240, Shimazdu, Japan). A total of 300 ml each of hexadecane and tetrahexadecane were added to 5 ml of bacterial cell suspension in a clean borosilicate round-bottom glass tube. The mixture in the tube was vortexed for 2 min, and then was set to stand for 15 min to allow hexadecane and tetrahexadecane to separate from the aqueous phase. The sample was carefully removed from the aqueous phase and absorbance was measured at 600 nm. Bacterial adhesion to the hydrocarbon was calculated using Eq. (4):
Screening assays for biosurfactant production
Bacterial strains were grown in nutrient broth medium at 10°C and 150 rpm for 24 h. Following incubation, cultures were centrifuged (5000 rpm, 10 min), rinsed twice and then adjusted to an absorbance of 1 (OD600) in BH medium containing Na2HPO4 (2.2 g l-1), FeSO4.7H2O (0.01 g l-1), NaCl (0.05 g l-1), K2HPO4 (1.4 g l-1), CaCl2 (0.02 g l-1) and MgSO4.7H2O (0.6 g l-1). The BH medium was supplemented with 0.5 ml of filtered-sterilized trace element solution containing: H3BO3 (0.56 g l-1), ZnSO4 (0.29 g l-1), CoCl2.6H2O (0.42 g l-1), CUSO4.5H2O (1.78 g l-1) and MnSO4 (0.39 g l-1) and 3% (v/v) of sterilized diesel (polytetrafluoroethylene 0.4 μm) as the sole carbon source (Sriram et al. Reference Sriram, Gayathiri, Gnanaselvi, Jenifer, Mohan and Gurunathan2011). The medium pH was adjusted to 7.2, and it was sterilized at 121°C for 15 min. Bacterial suspensions were inoculated into 100 ml medium in 250 ml flasks and incubated at 10°C for 7 days at 150 rpm.
Following incubation, bacterial cultures were centrifuged at 3000 rpm for 5 min. Pellets were then re-suspended in phosphate buffer. The resulting pellets and supernatants were used for biosurfactant screening, oil displacement testing, drop collapse testing and emulsification testing (Sriram et al. Reference Sriram, Gayathiri, Gnanaselvi, Jenifer, Mohan and Gurunathan2011). All of the experiments were conducted in triplicate, and sterile distilled water served as a control.
Drop collapse test
A drop collapse test was conducted using mineral oil as the hydrocarbon substrate, as described by Youssef et al. (Reference Youssef, Duncan, Nagle, Savage, Knapp and McInerney2004). Approximately 10 μl of mineral oil was set on a grease-free glass slide, followed by drops of each of supernatant or pellet being placed onto the centre of the oil film. Drop collapse activity was assessed by examining the oil drop shape after 1 min. A positive outcome was recorded if the shape of the oil drop collapsed or flattened, while negative outcome was recorded if the shape remained rounded.
Oil displacement test
Oil displacement assessment was performed in triplicate. A total of 100 μl of used engine oil was added to Petri dishes containing 50 ml of distilled water. Then, 10 μl each of cell-free culture supernatant, pellets or water (control) was carefully added to the oil surface. Following 30 min of incubation, the diameter of the clear zone was measured by comparing it to the negative control (Rodrigues et al. Reference Rodrigues, Banat, Teixeira and Oliveira2006).
Emulsification test
The emulsification assay was performed as previously described by Cooper & Goldenberg (Reference Cooper and Goldenberg1987). A total of 4 ml of each supernatant and pellet suspension was added to an equal amount of hexadecane and diesel. The resulting mixture was then vortexed for 5 min. The height of the emulsion layer was then measured after 24 h. The emulsification activity index was calculated as the ratio of the height of the emulsion layer to the total height of the liquid as in Eq. (5):
where E24 is the emulsification activity index after 24 h, h emulsion is the emulsion layer height and h total is the height of the total liquid.
Biofilm formation test
The biofilm formation test relies on the ability of bacterial strains to form biofilms on plastics, typically polypropylene (PPE) and polystyrene (PLS) (Tribelli et al. Reference Tribelli, Martino, Lopez and Raiger2012). Bacterial strains were cultivated in Luria–Bertani (LB) broth and incubated at 10°C for 24 h at 150 rpm. Following incubation, the overnight-grown bacterial culture was readjusted to an optical density of 0.3 (OD600) in the same LB broth. Several 96 well plates of PPE and PLS containing 3 μl of bacterial culture, 15 μl of sterilized hydrocarbon (3% v/v; diesel and hexadecane) as the sole source of carbon and 285 μl of minimal medium were prepared in triplicate. As the negative control, 3 μl of sterile LB medium was used. The plates were then sealed with parafilm, wrapped in aluminium foil and then incubated for 7 days at 10°C in static conditions as described by Tribelli et al. (Reference Tribelli, Martino, Lopez and Raiger2012), with modification of the incubation temperature in order to mimic the maximum Antarctic summer temperature. After incubation, bacterial growth was estimated by measuring the absorbance at 600 nm (absorbance of bacterial growth; ABG). Then, the PLS and PPE plates were aspirated with 100 μl of 10 mM MgSO4 for 20 min. The wells were then washed and stained with crystal violet solution for another 20 min. The dye was then washed by rinsing with water and extracted with 96% ethanol. The absorbance of crystal violet (ACV) was then measured at 550 nm. The adherence indices were estimated using Eq. (6):
Statistical analysis
All of the experiments were performed in triplicate. The data obtained are presented as the mean ± standard deviation. One-way analysis of variance (ANOVA) was used to compare data within treatment groups, and Turkey's test was carried out in order to conduct post hoc pairwise analyses if a significant difference was observed.
Results
Evaluation of diesel degradation ability
The ability of Arthrobacter spp. strains AQ5-05 and AQ5-06 to metabolize diesel oil was evaluated by incubating both strains in BH medium supplemented with 0.5% (v/v) diesel as the sole carbon source at 10°C and 150 rpm. Strain AQ5-06 showed greater growth (OD600 = 0.956) over the 168 h incubation period than did AQ5-05 (OD600 = 0.758) (Fig. 1). Gravimetric analysis showed that strain AQ5-06 degraded 37.5% of the diesel provided, while strain AQ5-05 degraded 34.5% of the diesel provided. The GC/MS analyses of the residual diesel oil extracted from strain AQ5-05 and AQ5-06 cultures were carried out after 7 days and compared with an abiotic control under the same conditions. Figure 2 displays the results of the obtained chromatograms. Based on the chromatograms, it was observed that the samples treated with Arthrobacter spp. strains AQ5-05 and AQ5-06 clearly reduced diesel oil as compared to the abiotic control sample. Short- and medium-chain alkanes were removed more efficiently than long-chain alkanes by both strains, and the rate of removal decreased as the carbon chain length increased. The highest degradation abilities of both isolates were observed for C8 and C10 (45–50%), lower degradation abilities were observed for C13 and C22 (15–30%) and the lowest degradation abilities were observed for > C25. Removal of aromatic compound was also observed. The length of the n-alkane chain influenced its degradation significantly, as after 7 days of incubation C8 and C10 were reduced to 50% by both strains. Similarly, C13 was reduced by 15–30%, whereas C25 was only reduced by 10%. In addition, reductions in aromatic compounds were also observed. These results therefore validate the gravimetric analysis results, as well as suggesting the significant effectiveness of both bacterial strains for degrading various components of hydrocarbons. The specific degradation rates of AQ5-05 and AQ5-06 were 0.023 and 0.029 days-1, respectively. The degradation rate constant was higher in AQ5-06 (k = 0.047 days-1, t 1/2 = 14.7 days-1) than in AQ5-05 (k = 0.031 days-1, t 1/2 = 22.3 days-1), which is again consistent with a faster degradation process.

Fig. 1. Growth of strains AQ5-05 (empty circles) and AQ5-06 (empty squares) in Bushnell–Haas media containing 0.5% (v/v) diesel as the sole carbon source at 10°C and diesel degradation by strains AQ5-05 (filled circles) and AQ5-06 (filled squares) based on gravimetric analysis (n = 3). Error bars represent the mean ± standard deviation for the triplicates. Non-inoculated samples serve as controls.

Fig. 2. Gas chromatography/mass spectrometry profile of diesel oil extracted from the aqueous phase of the medium after 7 days of incubation with and without strains AQ5-05 and AQ5-06. a. Chromatograph of abiotic control. b. Chromatograph of diesel oil treated with strain AQ5-05. c. Chromatograph of diesel oil treated with strain AQ5-06.
Growth optimization using OFAT
The effects of the various selected environmental parameters of pH, temperature, NaCl concentration, nitrogen source and substrate concentration were evaluated using the OFAT technique. Figure 3 shows the influence of initial pH on Arthrobacter spp. strains AQ5-05 and AQ5-06, indicating that pH 7.5 supported the optimum growth of both strains. Significant differences between pH treatment groups overall were detected by ANOVA for growth (F(8, 18) = 188.640, P < 0.001 and F(8, 18) = 796.937, P < 0.001, respectively) and substrate degradation (F(8, 18) = 206.123, P < 0.001 and F(8, 18) = 156.608, P < 0.001, respectively) in both strains, with the optimum being at pH 7.5. Post hoc tests comparing growth and degradation at pH 7.5 and the other tested pH values indicated that the mean growth and degradation values were significantly greater at the optimum pH for both strains. As the pH level increased towards alkaline pH 8–9, a rapid decrease in growth was apparent for both strains.

Fig. 3. Effects of initial pH on growth of strains AQ5-05 and AQ5-06 in diesel oil medium and diesel degradation by strains AQ5-05 (filled squares) and AQ5-06 (filled circles) at 10°C and with 1.0 g l-1 (NH4)2SO4. Error bars represent mean ± standard deviation for the triplicates.
The effects of temperature on growth and degradation achieved by strains AQ5-05 and AQ5-06 are illustrated in Fig. 4. Both strains showed optimum growth between 10°C and 15°C, with growth decreasing at higher temperatures. Strain AQ5-05 showed optimal growth and the highest degradation at 10°C, with significant differences between culture temperatures (F(5, 12) = 2092.797, P < 0.001 and F(5, 12) = 82.789, P < 0.001, respectively), whereas AQ5-06 attained maximum growth and the highest diesel reduction at 15°C, again with significant differences overall (F (5, 12) = 165.485, P < 0.001 and F(5, 12) = 121.376, P < 0.008, respectively). Post hoc comparisons showed that there were significant differences between the mean value at 10°C (M = 1.7140, SD = 0.010) and the other tested temperatures for AQ5-05 and at 15°C (M = 1.066, SD = 0.055) and the other tested temperatures for AQ5-06.

Fig. 4. Effects of temperature on the growth of strains AQ5-05 and AQ5-06 in diesel medium at 10°C and diesel degradation by strains AQ5-05 (filled squares) and AQ5-06 (filled circles) at 7.5 pH and with 1.0 g l-1 (NH4)2SO4. Error bars represent the mean ± standard deviation for the triplicates.
The influences of various nitrogen sources on the growth and degradation achieved by strains AQ5-05 and AQ5-06 are illustrated in Fig. 5a. Ammonium nitrate and ammonium sulphate gave the greatest enhancement in the growth and degradation of both strains, demonstrating overall significant differences between the difference nitrogen sources (F(8, 9) = 42.899, P < 0.001 and F(8, 9) = 47.556, P < 0.001, respectively). Post hoc analysis showed no significant differences between the two best sources. Due to its availability and widespread use as a cheap nitrogen source for bioremediation, ammonium sulphate was therefore selected as the primary source of nitrogen for the next stage of this study. The impacts of various ammonium sulphate concentrations on the growth and degradation achieved by strains AQ5-05 and AQ5-06 are presented in Fig. 5b, with both bacterial strains showing optimum growth at 0.4 g l-1 ammonium sulphate. Analyses of variance again identified significant differences in growth (F(7, 16) = 66.673, P < 0.001 and F(7, 16) = 71.648, P < 0.001, respectively) and diesel degradation (F(7, 16) = 775.906, P < 0.007 and F(7, 16) = 78.712, P < 0.001, respectively) between the various ammonium sulphate concentrations. Post hoc tests showed that the mean values at 0.4 g l-1 were significantly greater than those at the other tested concentrations in both strains.

Fig. 5. a. Effects of various nitrogen sources on the growth of strains AQ5-05 (empty bars) and AQ5-06 (striped bars) and diesel degradation by strains AQ5-05 (dark bars) and AQ5-06 (dotted bars) at 7.5 pH, 10°C and 15°C and with 1.0 g l-1 (NH4)2SO4. Error bars represent mean ± standard deviation for the triplicates. b. Effects of various ammonium concentrations on the growth of strains AQ5-05 and AQ5-06 in diesel medium and diesel degradation by strains AQ5-05 (filled squares) and AQ5-06 (filled circles) at 7.5 pH and 10°C and 15°C. Error bars represent mean ± standard deviation for the triplicates.
The impacts of various salt concentrations on the growth and degradation achieved by strains AQ5-05 and AQ5-06 are illustrated in Fig. 6. Growth of both strains was best at zero- or low-salt concentrations (0% and 1% w/v). As salt concentration increased above this level, there was a corresponding decrease in bacterial cell growth. Analysis of variance identified significant overall differences in growth achieved by strains AQ5-05 and AQ5-06 at various salt concentrations (F(5, 12) = 120.263, P < 0.001 and F(5, 12) = 139.795, P < 0.001, respectively), as well as in degradation (F(5, 12) = 701.790, P < 0.002 and F(5, 12) = 102.532, P < 0.001, respectively). Pairwise analyses confirmed that there were also significant differences at 0% and 1% (w/v) salt concentrations in both growth and degradation for both strains. The mean values achieved at the optimum salt concentration (1% w/v) were significantly greater than those achieved with the other tested concentrations.
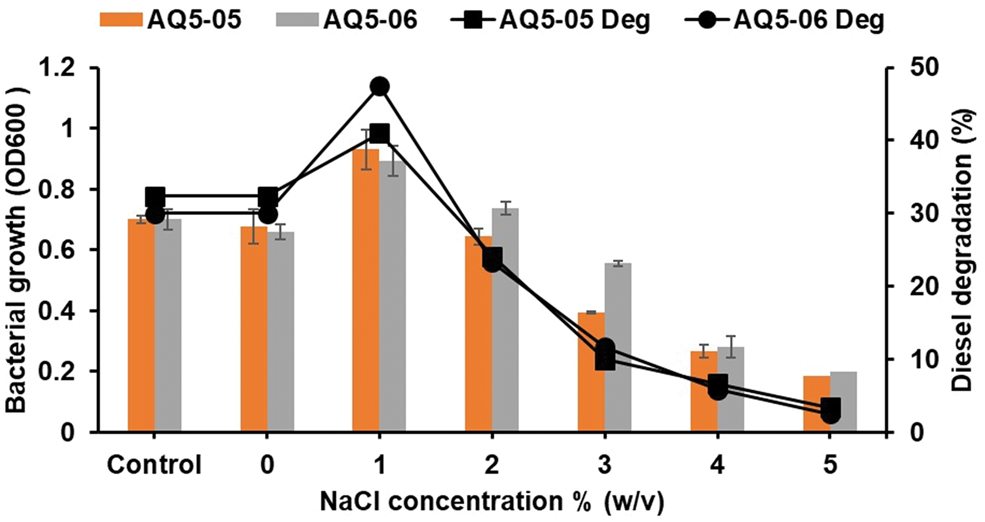
Fig. 6. Effects of various salt concentrations on the growth of strains AQ5-05 and AQ5-06 in diesel medium at 10°C and diesel degradation by strains AQ5-05 (filled squares) and AQ5-06 (filled circles) at 7.5 pH, 10°C and 15°C and 0.4 g l-1 (NH4)2SO4. Error bars represent mean ± standard deviation for the triplicates.
The effects of initial diesel concentration on the growth and degradation achieved by strains AQ5-05 and AQ5-06 are illustrated in Fig. 7. The optimum initial diesel concentration supporting the growth of both isolates was between 2% and 3% (v/v). The differences in growth achieved at various initial concentrations were significant in both strains (F(8, 18) = 207.284, Pp < 0.001 and F(8, 18) = 190.391, P < 0.001, respectively), as well as for degradation (F(8, 18) = 323.757, P < 0.001 and F(8, 18) = 273.105, P < 0.015, respectively). Pairwise tests confirmed that there were no significant differences in growth or degradation between 2& and 3% initial concentrations. However, significant differences were apparent between the optimum initial diesel concentration (2% and 3% v/v) and the other tested concentrations.

Fig. 7. Effects of various diesel concentrations on the growth of strains AQ5-05 and AQ5-06 and diesel degradation by strains AQ5-05 (filled squares) and AQ5-06 (filled circles) at 7.5 pH, 10°C and 15°C and with 0.4 g l-1 (NH4)2SO4 and 1% (w/v) NaCl. Error bars represent mean ± standard deviation for the triplicates.
Microbial adhesion to hydrocarbon assay
The effects of hydrocarbons on bacterial growth are presented in Table I. Both strains showed good hydrophobicity characteristics. Following extraction with hexadecane and tetrahexadecane, strain AQ5-05 demonstrated cellular hydrophobicity levels of 73.0% and 60.5% to hexadecane and tetrahexadecane, respectively. Slightly higher values (81.5% and 70.5%, respectively) were obtained for strain AQ5-06. These data indicate that the bacterial cells tend to float easily in hydrocarbon oils, a vital property in petroleum hydrocarbon degradation.
Table I. Microbial adhesion to hydrocarbons in strains AQ5-05 and AQ5-06.

Screening assay for biosurfactant production
The results of the biosurfactant production test are summarized in Table II. Both the pellet suspensions and the culture supernatants of strains AQ5-05 and AQ5-06 showed positive results in the drop collapse test. The mineral oil drop became flattened within 1 min, strongly suggesting the presence of biosurfactants. Similarly, in the oil displacement test, strain AQ5-06 culture supernatants showed a clear halo zone with a diameter > 1 cm, while strain AQ5-05 culture supernatants generated a halo of 0.9 cm. Both strains exhibited good emulsification activity. The two strains demonstrated the strongest emulsification in hexadecane (> 50%), whereas in diesel both strains demonstrated relatively low emulsification activities.
Table II. Results of the biosurfactant test using supernatant (SPT) and pellet suspension (PLT).

+ = positive results for drop fattening, ++ = strongly positive results for drop fattening, SLY = slight layer.
Biofilm formation test
Both strains demonstrated greater biofilm adherence in the presence of hexadecane and diesel than in the control conditions (Table III). Strain AQ5-06 displayed the greatest tendency to form a biofilm in the presence of hexadecane, 15–30 times greater than the control in PPE plates and 17–40 times greater than the control in PLS plates.
Table III. Biofilm formation indices of strains AQ5-05 and AQ5-06 on polystyrene (PLS) and polypropylene (PPE) plates in hexadecane and diesel (OD600/OD550).

Effects of heavy metals on diesel metabolic activity of AQ5-05 and AQ5-06
The effects of various heavy metals on growth and diesel degradation activity were evaluated by growing strains AQ5-05 and AQ5-06 in BH medium containing diesel as the sole carbon source, with a 1 ppm concentration of various heavy metal ions. Mercury (Hg) and silver (Ag) greatly impeded the growth and diesel degradation activity of both strains (Fig. 8a & b). The growth (F(9, 10) = 72029.644, P < 0.001 and F(9, 20) = 3335.543, P < 0.001, respectively) and degradation activity (F(9, 10) = 105.21, P < 0.001 and F(9, 20) = 246.021, P < 0.001, respectively) of both strains were significantly influenced by the various heavy metals trialled. Post hoc tests identified no significant differences between the effects of Hg and Ag for both strains. In the absence of either Hg or Ag, strains AQ5-05 and AQ5-06 degraded 37.3% and 46.6% diesel, respectively, which reduced to ~4% of the initial diesel amount in the presence of either heavy metal ion.

Fig. 8. Effects of various heavy metals on the growth and degradation activity of a. AQ5-06 and b. AQ5-05. Error bars represent mean ± standard deviation for the triplicates.
Discussion
Antarctica is a remote and dry continent. Its chronically low temperatures are a challenge for microbial metabolic activity. However, human activities in the southern polar region are associated with the risk of pollution events. King George Island is one of the largest islands in the South Shetlands archipelago, and it is subjected to some of the most intense levels of human activity in Antarctica, with multiple heavily populated research stations run by various national operators within the Antarctic Treaty System (ATS) and several of the most visited tourist sites in the region (Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey and Moreno2009, Eduardo et al. Reference Eduardo, Padeiro, de Ferro, Mota, Leppe and Verkulich2015). Human activity on the island is inevitably associated with the use of fossil fuels and therefore the occurrence of accidental fuel spills (Hughes et al. Reference Hughes, Bridge and Clark2007). Fuel spills may both provide nutrients supporting specific microbial growth and produce harmful effects on other microbial growth and activity.
Persistent hydrocarbon spills are now evident on King George Island, specifically in areas close to several research stations near the Fildes Peninsula. Leakage from fuel storage tanks is to date the primary source of petroleum hydrocarbon pollution here, a consequence of noncompliance with ATS regulations and poor waste management by the operators responsible (Braun et al. Reference Braun, Hertel, Mustafa, Nordt, Pfeiffer, Peter, Tin, Liggett, Maher and Lamers2014). These hydrocarbons enter the local soils and sediments. Remediation of polluted sites is an urgent challenge facing the Antarctic Treaty Parties generally, and specifically those countries operating the affected stations (Hughes & Convey Reference Hughes and Convey2014). Bioremediation requires the use of appropriate microorganisms, and as the introduction of non-native microbes is prohibited under the Environmental Protocol to the Antarctic Treaty, the identification and development of protocols using local Antarctic microbes are important.
Various studies have documented the existence of Antarctic hydrocarbon-degrading bacteria and fungi (e.g. Aislabie et al. Reference Aislabie, Foght and Saul2000, Hughes et al. Reference Hughes, Bridge and Clark2007). Bacteria belonging to the genera Pseudomonas, Acinetobacter, Sphingomonas (Gram-negative) and Rhodococcus and Arthrobacter (Gram-positive) are the most commonly encountered taxa present in hydrocarbon-polluted sites (Whyte et al. Reference Whyte, Schultz, van Beilen, Luz, Pellizari and Labbé2002, Shukor et al. Reference Shukor, Hassan, Jusoh, Perumal, Shamaan and MacCormack2009, Habib et al. Reference Habib, Ahmad, Wan Johori, Shukor, Alias and Khalil2018). Arthrobacter spp. strains AQ5-05 and AQ5-06 are cold-tolerant strains isolated from chronically polluted Antarctic soil that can utilize diesel as their sole source of carbon and energy, and as shown here, they are capable of significant diesel degradation. After an initial acclimatization period of 24 h, both strains achieved maximum diesel oil degradation within 7 days. Aliphatic hydrocarbon degradation by microbes is initiated by oxidation of the terminal methyl group to form a primary alcohol, which is then oxidized to produce aldehyde and lastly fatty acid derivatives. Nonetheless, at times, ɷ-hydroxy fatty acids are produced when both ends of the alkane molecules are included in the oxidation process. The ɷ-hydroxy fatty acids produced are then transformed into dicarboxylic acids via β-oxidation. Secondary alcohols are formed by sub-terminal oxidation, and these are further converted to ketone and afterwards oxidized to an ester by Baeyer–Villiger monooxygenase. Hydroxylation of esters to produce alcohols and fatty acids is facilitated by an esterase enzyme. The broad ranges of alkanes (C8–C33) comprising the short-chain, medium-chain and long-chain alkanes, as well as aromatic compounds existing in the abiotic control sample, were reduced by both bacterial strains. Both strains tended to degrade short- and medium-chain alkanes more efficiently. However, reduction of long-chain alkanes and aromatic compounds was evident. Generally, alkanes of lower molecular weight tended to degrade faster than those of greater molecular weight due to presence of shorter carbon chains. Habib et al. (Reference Habib, Ahmad, Wan Johori, Shukor, Alias and Khalil2018) also reported high rates of degradation of short-chain alkanes with Antarctic bacteria. Alkanes ranging from C9 to C14 are the most commonly detected in Antarctic soil, and this aliphatic fraction represents the major sources of pollution near Antarctic stations. Thus, microbial populations with the competence to degrade C12–C18 are needed for the biodegradation and bioremoval of oil spillages around Antarctic research station, as diesel oil typically consists of n-alkanes in this range. Whyte et al. (Reference Whyte, Schultz, van Beilen, Luz, Pellizari and Labbé2002) reported the detection and characterization of alkane-degrading (alkB and alkM) genotypes in both hydrocarbon-contaminated and hydrocarbon-non-contaminated Antarctic soils. Whole-genome sequencing of the bacterial strain AQ5-05 revealed that this strain harbours alkB and alkM and napthalene dioxygenase (ndoB) genes, which are responsible for the degradation of a wide range of alkanes (C8–C33) and aromatic compounds.
Previous studies have reported species of Enterobacter, Rhodococcus, Bacillus, Pseudomonas and Arthrobacter to be diesel degraders (Yousaf et al. Reference Yousaf, Andria, Reichenauer, Smalla and Sessitsch2010). Other studies (e.g. Dussán & Numpaque Reference Dussán and Numpaque2012) have reported non-diesel-degrading strains such as Arthrobacter sp. PQ II. The genera Arthrobacter and Rhodococcus have been identified among the major hydrocarbon-degrading bacteria (Whyte et al. Reference Whyte, Schultz, van Beilen, Luz, Pellizari and Labbé2002). Studies of non-polluted soils have also revealed psychrophilic or cold-tolerant members of the Arthrobacter genus to be important members of bacterial communities in Antarctic soils (Dsouza et al. Reference Dsouza, Taylor, Turner and Aislabie2015). Margesin et al. (Reference Margesin, Moertelmaier and Mair2013) and Lee et al. (Reference Lee, Ahmad, Yasid, Zulkharnain, Convey and Johari2018) also reported Arthrobacter spp. strains capable of degrading petroleum hydrocarbons (n-alkanes and phenol) at low temperatures.
Temperature is a significant parameter controlling microbial growth and enzymatic activity, and it greatly affects the rate of bacterial hydrocarbon degradation. The increased viscosity of spilled oil at low temperatures reduces the volatilization of short-chain alkanes, thereby increasing their toxicity and delaying the onset of microbial degradation (Margesin & Schinner Reference Margesin and Schinner1999). Reaction rates generally follow an Arrhenius relationship, meaning that as temperature decreases, there is a corresponding decrease in biodegradation. Antarctic soil bacteria are predominantly psychrotolerant rather than psychrophilic, which is consistent with the temperature variation characteristic of terrestrial environments in this region (Convey et al. Reference Convey, Coulson, Worland and Sjoblom2018). Therefore, bioremediation is normally planned and performed in the summer period when soils are unfrozen soils and liquid water is available. The temperature optimization experiments carried out here confirmed that both strains were psychrotolerant, growing best at temperatures between 10°C and 15°C. This concurs with the findings of Shukor et al. (Reference Shukor, Hassan, Jusoh, Perumal, Shamaan and MacCormack2009), Margesin et al. (Reference Margesin, Moertelmaier and Mair2013) and Lee et al. (Reference Lee, Ahmad, Yasid, Zulkharnain, Convey and Johari2018).
Both bacterial strains grew most effectively on diesel at initial pH values of 7.0–7.5, demonstrating a strong affinity towards phosphate buffer. Much less growth was observed at pH values of 6.0 and 8.0, demonstrating that both bacterial strains are pH sensitive. Several studies have reported optimal growth in diesel-degrading bacteria at near-neutral pH (Habib et al. Reference Habib, Ahmad, Wan Johori, Shukor, Alias and Khalil2018).
Salinity can also have a significant influence on bacterial growth (Karamba et al. Reference Karamba, Ahmad, Zulkharnain, Syed, Khalil and Shamaan2016). High salt concentrations can inhibit bacterial metabolic activity due to the increase in osmotic pressure affecting the solubility and movement of essential ions. Both strains studied here were intolerant of high salt concentrations. However, salt-resistant strains are potentially advantageous in the decontamination of hydrocarbon pollution in marine and coastal polluted areas (Durán & Esposito Reference Durán and Esposito2000). The composition and content of salts vary considerably in Antarctic soils, most notably with proximity to the coast or to dense colonies of marine vertebrates, as well as with the age of the soil in some areas (Convey et al. Reference Convey, Chown, Clarke, Barnes, Bokhorst and Cummings2014). A number of diesel-degrading bacteria have been reported to demonstrate peak diesel degradation activities at low salt concentrations, in accordance with the data obtained here (Habib et al. Reference Habib, Ahmad, Wan Johori, Shukor, Alias and Khalil2018).
The availability of a suitable nitrogen source is an essential factor controlling bioremediation, although bacterial growth can be hindered by higher concentrations of nitrogen. Ammonium sulphate and ammonium nitrate best supported the growth of the two studied strains. Ammonium sulphate was selected for further study as it is readily and cheaply available and is the most used source of nitrogen by bacteria (Shukor et al. Reference Shukor, Hassan, Jusoh, Perumal, Shamaan and MacCormack2009). Various diesel-degrading bacteria have been reported to use ammonium sulphate as their sole nitrogen source (Shukor et al. Reference Shukor, Hassan, Jusoh, Perumal, Shamaan and MacCormack2009). In the natural environment in Antarctica, ammonium dispersed from vertebrate colonies has been shown to be an important driver of local terrestrial diversity (Bokhorst et al. Reference Bokhorst, Convey and Aerts2019).
The influence of substrate concentration on bacterial growth has been documented in experiments using a range of substrate concentrations (Ruberto et al. Reference Ruberto, Vazquez, Lobalbo and Mac Cormack2005). Most studies conducted on diesel degradation have used relatively low diesel concentrations (between 0.5% and 1.5%) due to the toxicological effect of diesel on bacterial membranes (Shukor et al. Reference Shukor, Hassan, Jusoh, Perumal, Shamaan and MacCormack2009). The two bacterial strains studied here showed some resistance to diesel concentration, with best growth observed at 3% (v/v). Shukor et al. (Reference Shukor, Hassan, Jusoh, Perumal, Shamaan and MacCormack2009) also reported a diesel-degrading Pseudomonas strain isolated from Antarctic soil that grew optimally at 3.5% (v/v) diesel concentration. Kwapisz et al. (Reference Kwapisz, Wszelaka, Marchut and Bielecki2008) even reported degradation at 6% (v/v) diesel, but this required the addition of glucose (0.2% w/v) and yeast extract (0.1% w/v). After optimization of all of the growth parameters via the OFAT method, strains AQ5-05 and AQ5-06 demonstrated enhanced diesel degradation efficiency, withstanding up to 3.0% diesel (v/v) and breaking down 41.0% and 47.5% diesel, respectively, as confirmed by gravimetric analysis and validated by GC/MS analysis.
The production of biosurfactants and biofilm formation are crucial for the successful and effective application of diesel-degrading bacterial strains in polluted soil. The formation of biofilms stabilizes the soil substrate, while biosurfactants produced by bacterial cells significantly increase pollutant bioavailability (Tribelli et al. Reference Tribelli, Martino, Lopez and Raiger2012). Both bacterial strains studied here showed biosurfactant production. Ibrahim et al. (Reference Ibrahim, Ijah, Manga, Bilbis and Umar2013) reported 50–90% emulsification, a positive result for the drop collapse assay and a 20–51 mm oil displacement test for strains of bacteria isolated from crude oil-polluted soil. Patowary et al. (Reference Patowary, Patowary, Kalita and Deka2017) reported a positive drop collapse assay, a surface tension reduction from 51.8 to 29.6 mN m-1 and 81.8% hydrocarbon degradation by biosurfactant-producing Pseudomonas aeruginosa PG1. Similarly, Kumari et al. (Reference Kumari, Singh and Singh2012) reported 60.6% and 49.5% petroleum hydrocarbon degradation by biosurfactant-producing Pseudomonas sp. BP10 and Rhodococcus sp. NJ2, respectively. These results are consistent with our findings. When biosurfactant-producing bacteria are exposed to polluted substrates, the improved biodegradation achieved is attained through mobilization, solubilization or emulsification of the oil (Ibrahim et al. Reference Ibrahim, Ijah, Manga, Bilbis and Umar2013). Biosurfactants boost microbial hydrocarbon degradation, making the substrate accessible by increasing its water solubility through emulsification and enabling bacterial cell contact with hydrophobic substrates, thus reducing bacterial cell surface hydrophobicity (Kumari et al. Reference Kumari, Singh and Singh2012).
Emulsification activity is one of the most regularly and extensively employed techniques for testing for the presence of biosurfactants, although the results may differ considerably depending on the molecular weight of the hydrocarbons emulsified (Tribelli et al. Reference Tribelli, Martino, Lopez and Raiger2012, Ibrahim et al. Reference Ibrahim, Ijah, Manga, Bilbis and Umar2013). The use of hexadecane and diesel here showed that diesel cannot be easily emulsified as it is heavier than hexadecane and comprises a combination of complex compounds. Both isolates displayed poor formation of biofilms in diesel, in contrast with their performance with hexadecane. Bacterial strains capable of forming biofilms may become attached to diesel-containing substrates, giving them greater access compared to planktonic communities. In addition, cells in biofilms typically have improved metabolic competence and greater cell density than planktonic cells (Ibrahim et al. Reference Ibrahim, Ijah, Manga, Bilbis and Umar2013), which can therefore lead to enhanced and more effective degradation.
Strains AQ5-05 and AQ5-06 demonstrated significant maximum degradation competence, degrading 41.0% and 47.5% of diesel in the test system over 7 days, respectively. Zhang et al. (Reference Zhang, Wang, Li, Xiang and Achal2014) reported 30–60% diesel degradation using various surfactant-producing bacterial isolates. Other studies have also reported effective diesel-degrading bacterial strains obtained from Antarctic soils (Aislabie et al. Reference Aislabie, Balks, Foght and Waterhouse2004, Habib et al. Reference Habib, Ahmad, Wan Johori, Shukor, Alias and Khalil2018). Strain AQ5-06 displayed the greatest degradation effectiveness and demonstrated a good biofilm adherence index, along with the production of biosurfactants.
In summary, the two psychrotolerant diesel-degrading Antarctic bacterial strains studied here proved to be competent at degrading diesel up to 3% (v/v) within 7 days at 10°C. Optimization of physical environmental factors including temperature, substrate concentration, pH, salt concentration and nitrogen source significantly enhanced the diesel degradation activity of these strains. Both AQ5-05 and AQ5-06 achieved maximum diesel degradation at between 10°C and 15°C, confirming that the Antarctic summer soil environment provides appropriate environmental conditions for diesel remediation by these strains. The presence of features such as biofilm formation, biosurfactant production and emulsification activity may also help in boosting the diesel degradation activity of these strains. Further optimization through statistical response surface methodology, combined with the purification and characterization of the biosurfactants produced and the identification of the pathway used for diesel degradation, will assist in the development and application of these two strains in field bioremediation approaches.
Author contributions
S.A. Ahmad and M.Y. Shukor carried out the research. M. Abdulrasheed and N.N. Zakaria conducted the research and drafted the manuscript. A.F. Ahmad Rosle and A. Zulkharnain carried out the statistical data analyses. S. Napis and P. Convey edited and reviewed the manuscript. G. Gonzalez-Rocha and S.A. Alias collected the soil samples used for the bacterial isolation.
Financial support
This project was financially supported by the research grants attached to S.A. Ahmad (GP-Matching Grant/2017/9300436, GPM-2018/9660000 and GPM-2019/9678900) disbursed by Universiti Putra Malaysia (UPM). The authors thank the Tertiary Education Trust Fund (TETFUND) through Gombe State University, Gombe, Nigeria, for financial sponsorship of M. Abdulrasheed. P. Convey is supported by NERC core funding to the British Antarctic Survey's ‘Biodiversity, Evolution and Adaptation’ Team. We also thank the Public Service Department of Malaysia (JPA) for granting a master programme scholarship to A.F. Ahmad Rosle.
Details of data deposit
The isolates of Arthrobacter spp. strains AQ5-05 and AQ5-06 used for this study were originally isolated from Antarctic soil obtained on King George Island, South Shetland Islands (Lee et al. Reference Lee, Ahmad, Yasid, Zulkharnain, Convey and Johari2018). Arthrobacter spp. strains AQ5-05 (ref. KX946130-KX946131) and AQ5-06 (ref. KX946127) nucleotide sequences are deposited in the NCBI database.














