Introduction
In today's world of more than 7 billion people, more than 50% of them already live in urban areas, and this proportion keeps increasing (Satterthwaite et al., Reference Satterthwaite, McGranahan and Tacoli2010; Bloom, Reference Bloom2011). The urban population needs food, and with an increasing middle-income class, the demand for animal-source foods is increasing (Rae, Reference Rae1998). The provisioning of fresh food is challenging, especially in low- and middle-income countries where infrastructure is poor and it may be impossible to maintain cold chains, forcing slaughter to occur closer to the urban consumers. This creates an increased market for urban produced animal-source foods and an economic incentive for urban livestock keeping (Schiere and van der Hoek, Reference Schiere and van der Hoek2001; van Veenhuizen and Danso, Reference van Veenhuizen and Danso2007). Also, due to high land prices and the general shortage of space in cities, production of perishable products, including animal products, with high market values and less demand of land are often preferred (De Zeeuw and Lock, Reference De Zeeuw and Lock2000; Nugent, Reference Nugent and Bakker2000; Midmore and Jansen, Reference Midmore and Jansen2003). Animals can give a high turnover on a limited area, and the scavenging behavior of many animals, such as pigs and chickens, make them possible to keep even without land (Schiere and van der Hoek, Reference Schiere and van der Hoek2001; van Veenhuizen and Danso, Reference van Veenhuizen and Danso2007).
Even though urban livestock keeping is providing food and livelihood to urban farmers and consumers, as well as different value chain actors, there are downsides to it, such as the public health risks. Livestock keeping in dense urban areas cause sanitary problems with the disposal of manure, attracts disease vectors and scavengers and may serve as reservoirs for pathogens and can therefore constitute an increased risk for emergence and transmission of zoonoses (De Zeeuw and Lock, Reference De Zeeuw and Lock2000; Schiere and van der Hoek, Reference Schiere and van der Hoek2001).
In addition to the urban livestock, cities contain a high density of pets, including feral domestic pets such as stray dogs and cats, as well as wildlife, for which cities may particularly provide ample opportunities for peridomestic scavengers. Urban areas create specific ecosystems with higher temperatures, ‘urban heat islands’, and less seasonal changes, which coupled with the establishment of anthropophilic mosquitoes in urban centers, particularly in tropical regions, may contribute to the emergence of vector-borne diseases (Gubler, Reference Gubler1996; Shochat et al., Reference Shochat, Warren, Faeth, McIntyre and Hope2006; Bradley and Altizer, Reference Bradley and Altizer2007; Saxena et al., Reference Saxena, Tiwari, Saxena, Mathur, Nair and Růžek2011; Lindahl et al., Reference Lindahl, Ståhl, Chirico, Boqvist, Thu and Magnusson2013). The movements of humans and animals in and out of high-density cities, mean that zoonotic spill-over events, that previously could have caused isolated outbreaks in remote rural areas, now have increased risks of being spread within urban areas, and thereby increasing the potential for continued human-to-human transmission (Morse, Reference Morse1995; Cutler et al., Reference Cutler, Fooks and Van Der Poel2010). In fact, most of the emerging infectious diseases in humans are zoonotic (Taylor et al., Reference Taylor, Latham and Woolhouse2000; Jones et al., Reference Jones, Patel, Levy, Storeygard, Balk, Gittleman and Daszak2008), making the cities crowded with both animals and people likely hotspots for future disease emergence.
Urban livestock keeping is of increasing importance for providing highly nutritious animal-source foods, livelihoods and public health. However, most research on the risks associated with livestock and zoonotic pathogen transmission are still conducted with a rural focus. This review was conducted to screen the available literature on zoonoses in urban animals, in order to identify knowledge gaps in this field, critical for public health interventions, particularly with respect to geographical distribution of the studies and pathogens studied.
Material and methods
This review used a systematic approach to obtain thorough and unbiased results that are transparent and replicable (Protocol in supplementary material 1), but did not follow all the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009) and the protocol was not peer-reviewed. In addition, because the scope was so wide, no attempt was made to conduct a meta-analysis, and because the aim of the review did not contain any research questions regarding an intervention, it was not applicable to have all PICOS (participants, intervention, control, outcome, study design). The retrieval of literature for this study was conducted in four steps.
First, for the literature search in scholarly databases, keywords were chosen and search strings were defined (Supplementary material 1). More specifically, 28 keywords were selected to create 26 search strings, which were then used to search the three databases PubMed, CAB Direct and Web of Science. The inclusion criteria were that the abstract was in the English language, literature was published from 2003 to 2013 (until November 2013), research was performed in urban areas, it was original research on animals and at least one zoonotic pathogen was studied. Second, citations were downloaded into the reference manager, Mendeley (Mendeley Ltd), and duplicates were removed. The total publications retrieved from all three searches were 876 and after the duplicates were deleted, the final number of references was 398. In addition, 11 ‘gray literature’ papers were found by searching institutional databases (Table 1); all of these were however excluded after screening because they did not fulfill the inclusion criteria.
Table 1. Institutional databases screened for studies on zoonoses in urban animals during 2003–2013, and the number of studies found using search terms (hits) and how many were imported to be screened

Third, abstracts were reviewed by the two authors independently. When there was disagreement between reviewers, the abstracts were re-reviewed by one of the reviewers and accepted if at least one reviewer was considering that the paper should be included; in total 140 abstracts were included at this stage. For these abstracts, the full papers were sought and downloaded if available.
Fourth, abstracts and full papers, when available, were then reviewed, and data were extracted from all papers and abstracts fulfilling the inclusion criteria listed above. This study used the criteria for poor quality as reported by Alonso et al. (Reference Alonso, Lindahl, Roesel, Traore, Yobouet, Ndour, Carron and Grace2016), in short, poor quality was defined as methods not clear or incomplete, inappropriate methods or data analyses, biased or potentially biased selection that is not acknowledged, and reported results that are incomplete, unclear, or inaccurate.
Classifications of extracted data
A template was created in MS Excel to extract relevant data (Supplementary material 2). A journal was classified as being an ISI journal if it is listed in Thomson Reuters Web of Science (http://ip-science.thomsonreuters.com/mjl/). A study was considered to study multiple pathogens if the pathogens belonged to different genera. A study including multiple species of Brucella or Echinococcus, respectively, would therefore not be considered a multispecies study. Type of study (a prevalence study, a study to evaluate risk factors, or other studies) was classified depending on what seemed the main purpose of the paper. In many cases however, a paper reported both prevalence data and risk factors analyzed.
The studies were classified as being urban, rural, or peri-urban, based on what was stated in the paper, or how the area was described. One problem with studies on urban agriculture has been the lack of common definitions of what constitutes urban or peri-urban (Mougeot, Reference Mougeot and Bakker2000; Satterthwaite et al., Reference Satterthwaite, McGranahan and Tacoli2010). For this purpose, each paper was judged on if it provided some kind of definition used in the paper, or a description of the study areas that would help comparisons with other studies.
Probabilistic sampling was concluded when the paper reported random sampling, and was considered explained if there was any methodology at all describing the method. For the purpose of this paper, the use of road kills or traps without a specified randomization protocol is considered as convenience sampling (Anderson, Reference Anderson2001).
Results
Papers included
After screening of the abstracts, 140 papers were included. At this stage, it was attempted to retrieve the full documents of these, and data were extracted. An additional 47 papers were excluded when full papers were reviewed. The most common reasons for exclusion were that it could not be verified that the study was conducted in urban or peri-urban areas (7), the paper did not actually study a zoonosis (12), the paper did not include a study in animals, but only in human beings (14), soil (5) or food products (1), or other reasons (8). Five papers studying knowledge, attitudes and practice about zoonoses were retained, even though they did not include animal studies per se. One additional paper also studied knowledge, attitudes and practices in addition to looking at prevalence in animals. The final number of included papers from which data were extracted were 80 full papers and 13 abstracts (Fig. 1). For some of the abstracts evaluated, there were full papers available online but in other languages, most commonly Spanish and Portuguese. Five papers were not published in ISI journals; three of these were published as conference proceedings.

Fig. 1. Flowchart of the review process.
The included papers were published between 2004 and 2013, and in 64 (69%) papers it was possible to find out when the study had been conducted or ended, in cases of longitudinal studies. According to the papers that provided this information, studies were conducted between 2001 and 2012, and there was an average of 2.8 (range 1–10) years between the stated year of ending a study and the year of publication.
Sixty of the 80 full papers were considered of acceptable quality, whereas 20 papers were judged to be of poor quality because there were no data on sample selection or collection, an unclear randomization process, inaccuracies in data, difficulties in understanding methodology, results or how results were obtained and unsubstantiated conclusions. Two of the papers judged to be of poor quality were published as proceedings for conferences.
Geographical distribution and types of urban animals
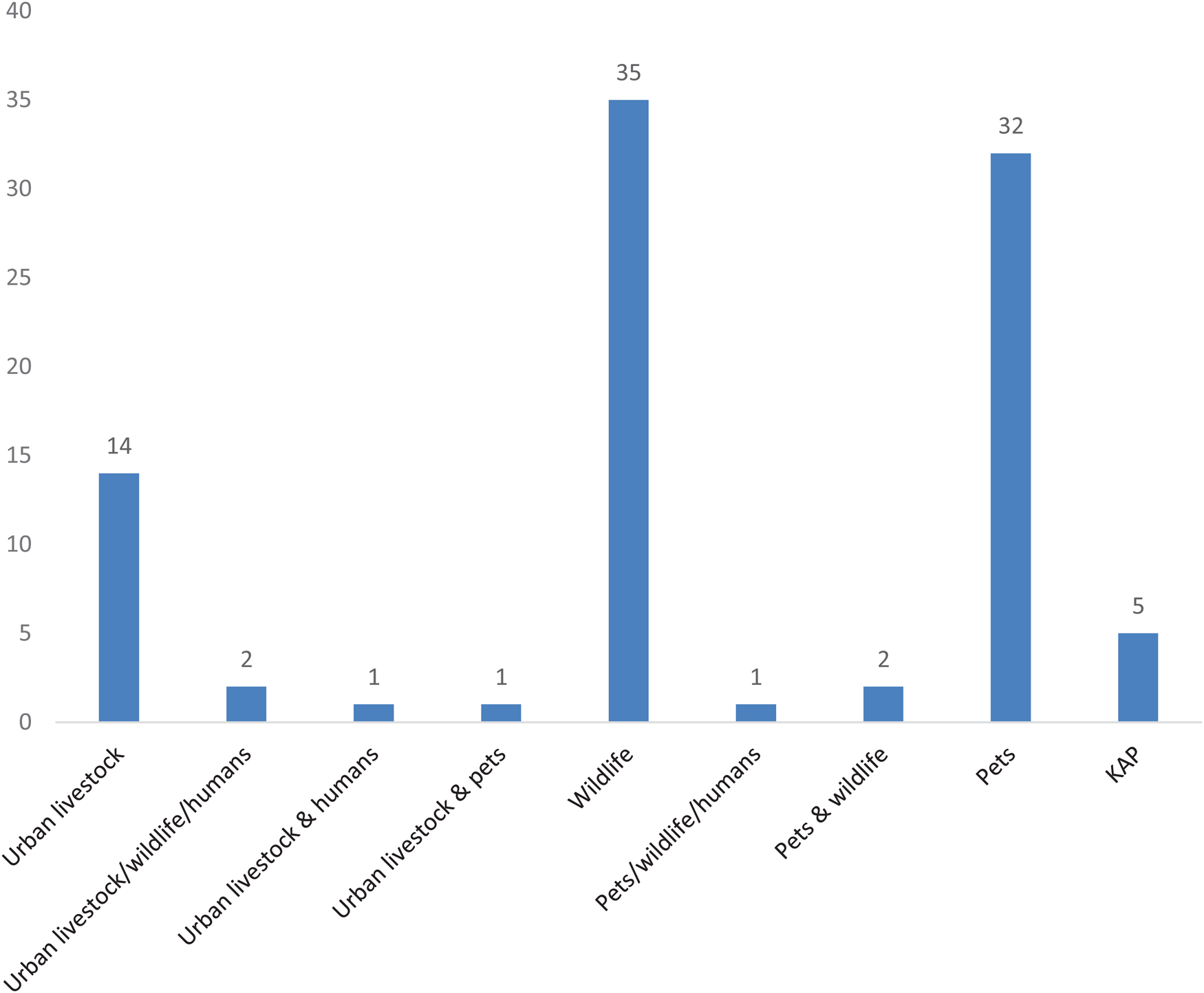
Research had been conducted in all permanently inhabited continents (Fig. 2), with 32% of the papers on studies conducted in South America, 18% in Europe, 16% in North America, 14% in Africa and 6% in South and Southeast Asia, each. Because research purely on human populations were excluded, the remaining papers could be classified as (1) research on domestic animals, 53 papers, including research on livestock and pets, feral and stray domestic animals, (2) research on wild animals, 40 papers, and (3) 5 papers focusing only on knowledge, attitudes and practices (Fig. 3). There was a trend of increasing numbers of papers with time (Fig. 4).

Fig. 2. Number of papers on zoonoses in urban animals in different countries that were included in a systematic review, as well as the proportion of studies conducted in the different continents.

Fig. 3. Number of papers on different types of urban animals in a systematic review on zoonoses in urban animals. Knowledge, attitude, and practice (KAP) studies were broken into a separate category.

Fig. 4. Year of publication of the included papers on zoonoses in urban animals. The search included papers until November 2013.
Research was conducted either in urban, peri-urban or both urban and rural. In addition, one study was defined as suburban, and two were described as semi-urban; for the purpose of the summary, these were judged to be peri-urban studies. In five papers, a description of the study site could not be found that enabled classifying into either category. In 68 papers (72%) there was no definition of urban and peri-urban, or any description of the sampling locations that could facilitate comparisons with other studies (Fig. 5).

Fig. 5. Number of papers based on the classification of study area, and if the paper contained a definition or a description of how the area was classified.
A purposive sampling methodology was stated in five papers, six papers stated a probabilistic sampling without explaining how, and 12 papers described the way a probabilistic sampling had been conducted. The most common way (28 out of 39 studies) of investigating wild animals was through opportunistic traps or road kills. For the majority of papers, sampling was either not probabilistic, or it could not be concluded from the paper.
Urban zoonotic pathogens
Most studies focused on only one pathogen. However, 27 studies included multiple pathogens, especially studies on gastrointestinal helminths (14 papers). In total, 55 studies included parasites, 30 bacterial diseases, 18 viral, and one fungal. The most frequently studied pathogens are listed in Table 2.
Table 2. Number of papers within each pathogen group and the host species and countries of the studies
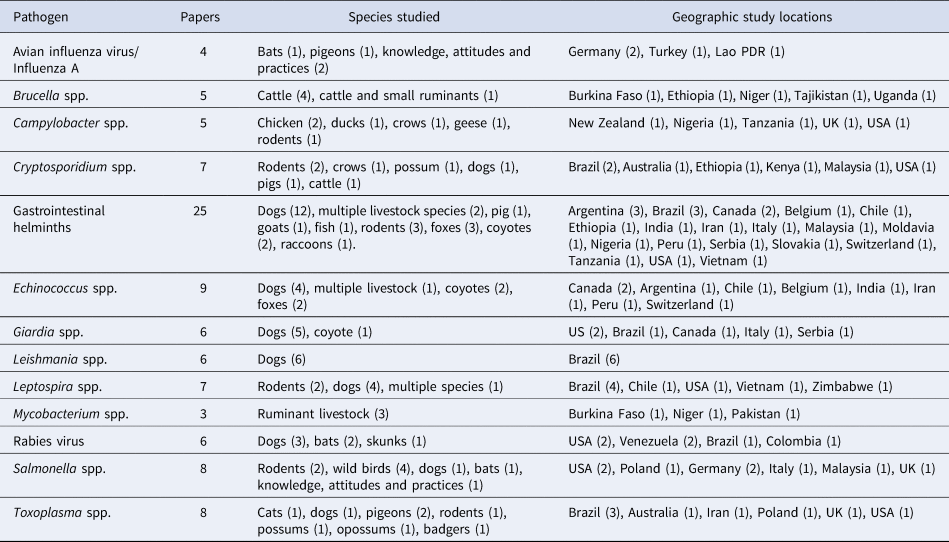
Echinococcus is included in the group gastrointestinal helminths, but also reported on a separate row.
Viruses
Avian influenza virus. Half of the papers on avian influenza or influenza A focused on screening for the virus, and the other half were on knowledge, attitudes and practices. Both surveys screening for influenza were from Germany and did not find the virus in neither 364 pigeons (Kohls et al., Reference Kohls, Lüschow, Lierz and Hafez2011) nor 486 bats (Mühldorfer et al., Reference Mühldorfer, Speck, Kurth, Lesnik, Freuling, Müller, Kramer-Schadt and Wibbelt2011). The other two papers reported on knowledge, attitudes, and practices among households in Turkey and Laos (Barennes et al., Reference Barennes, Harimanana, Lorvongseng, Ongkhammy, Chu, Ly, Kerkhove, Holl, Froehlich, Vong, Puthavathana, Sangsiriwut, Korkusol, Pooruk, Auewarakul, Pittayawanganon, Sutdan, Kitphati, Sawanpanyalert, Phommasack, Bounlu, Ungchusak, Webster, Guan, Poon, Krauss, Webby, Govorkovai, Peiris, Barennes, Martinez-Aussel, Vongphrachanh, Strobel, Takeuchi, Lau, Kim, Tsui, Griffiths, Zwart, Veldhuijzen, Elam, Aro, Abraham, Bishop, Richardus, Brug, Mcleod, Morgan, Prakash, Hinrichs, Peltz, Galit, Ventura-Gabay and Bar-Dayan2010; Bakiray Küçükkaya et al., Reference Bakiray Küçükkaya, Erbaydar, Stöhr, Edirne, Avci, Dagkara, Aslan, Oner, Bay, Arslan, Sarikaya, Erbaydar, Sur, Tasdemir, Cekin, Olsen, Laosiritaworn, Pattanasin, Capua, Mutinelli, Poza, Oyewo, Raufu, Ogunlana, Oyedele, Koch, Elbers, Kruk, Cevizci and Erginoz2011), with both studies emphasizing that more awareness was warranted in both urban and rural areas.
Rabies virus. All six studies on rabies were conducted in the Americas, half on wildlife (two on bats) and half on dogs. In Texas, US, trends of increasing rabies-positive skunks and bats were seen in urban areas (Oertli et al., Reference Oertli, Wilson, Hunt, Sidwa and Rohde2009; Mayes et al., Reference Mayes, Wilson, Oertli, Hunt and Rohde2013). Studies in Maracaibo, Venezuela, found more than 1000 cases of canine rabies in the city, and an association with dogs having access to the streets and the social status of the family (Prieto et al., Reference Prieto, Garcia, Sanchez, Medina and de Vale2006; Fuentes et al., Reference Fuentes, Panunzio, Larreal, Leal, Villarroel, Parral, Velasco and Prieto2008). Paez et al. (Reference Páez, Rey, Agudelo, Dulce, Parra, Díaz-Granados, Heredia and Polo2009) studied control of urban rabies outbreaks in Santa Maria, Colombia, and found it was important with urgent vaccination programs during outbreaks, but in spite of this less than 50% of dogs had protective titers after vaccination.
Other viruses. The other publications about viruses included reoviruses, picobirnaviruses, arenaviruses, hantaviruses, and the vector-borne West Nile virus.
Bacteria
Brucella spp. Studies on Brucella spp. were all focused on seroprevalence in ruminants (mainly cattle) in Asia and Africa, although in one proceeding the methodology was unclear and it was unknown how many animals originated from urban areas. Apart from one study in Tajikistan (Jackson et al., Reference Jackson, Ward, Kennard, Amirbekov, Stack, Amanfu, El-Idrissi and Otto2007) which included small ruminants, the other four studies were conducted in Africa on cattle only. The highest prevalence reported at household level, both rural and urban, was in Tajikistan with up to 17% among households with both goats and sheep, and it was concluded that the situation was out of control, also in urban areas (Jackson et al., Reference Jackson, Ward, Kennard, Amirbekov, Stack, Amanfu, El-Idrissi and Otto2007). This was the largest study that sampled more than 13,000 livestock, but only 620 cattle and 176 small ruminants were said to have come from urban areas, with no definition of this being provided. Conurbations in Ethiopia had a herd prevalence of 8.6% (209 farms sampled), similar to the adjusted herd prevalence of 6.5% (177 farms sampled) in Kampala, Uganda (Makita et al., Reference Makita, Fèvre, Waiswa, Eisler, Thrusfield and Welburn2011; Asmare et al., Reference Asmare, Sibhat, Molla, Ayelet, Shiferaw, Martin, Skjerve and Godfroid2013). Increasing age, larger herds, free grazing of cattle, and purchasing animals were identified as risk factors (Jackson et al., Reference Jackson, Ward, Kennard, Amirbekov, Stack, Amanfu, El-Idrissi and Otto2007; Makita et al., Reference Makita, Fèvre, Waiswa, Eisler, Thrusfield and Welburn2011; Asmare et al., Reference Asmare, Sibhat, Molla, Ayelet, Shiferaw, Martin, Skjerve and Godfroid2013). In a cross-sectional survey among ruminants in Niamey, Niger, it was found that cattle had higher seroprevalence than small ruminants, and the highest prevalence was found in rural areas, with lower in peri-urban and lowest in urban (21.3, 5.1 and 3.3%, respectively) (Boukary et al., Reference Boukary, Saegerman, Rigouts, Matthys, Berkvens and Thys2010).
Campylobacter spp. Campylobacter spp. were studied in birds (wild birds or poultry) in four out of five papers. Positivity rates were frequently high, with 57% of backyard chicken flocks having C. jejuni in Canterbury, New Zealand (Anderson et al., Reference Anderson, Horn and Gilpin2012). Studies in Morogoro, Tanzania, showed around 70% of both chicken (532 sampled) and crows (22 sampled) being positive, similar to 63% of 192 sampled ducks in Makurdi, Nigeria (Mdegela et al., Reference Mdegela, Nonga, Ngowi and Kazwala2006; Akwuobu and Ofukwu, Reference Akwuobu and Ofukwu2010). However, the prevalence seems to be able to vary locally, and one study in Canada geese in Greensboro, US, showed an average rate of 16%, while in one residential area, it was as high as 80% (Rutledge et al., Reference Rutledge, Siletzky, Gu, Degernes, Moorman, DePerno and Kathariou2013). One proceeding studied rodents, but it was not possible to extract results from this.
Leptospira spp. Four of the seven studies on leptospirosis were conducted on dogs, three studies included rodents, and one of these included multiple species. Around 16% of dogs in Harare, Zimbabwe, and Curitiba, Brazil, were seropositive (Bier et al., Reference Bier, Martins-Bede, Morikawa, Ullmann, Kikuti, Langoni, Canever, Biondo and Molento2012; Dhliwayo et al., Reference Dhliwayo, Matope, Marabini, Dutlow and Pfukenyi2012), while other cities in Brazil had between 7% seropositive in Ilheus, and 48% in Porto Alegre (Oliveira Lavinsky et al., Reference Oliveira Lavinsky, Said, Strenzel and Langoni2012; de Oliveira et al., Reference Oliveira Lavinsky, Said, Strenzel and Langoni2010), although sampling in the latter was targeted to high-risk dogs. Urban rodents also contribute to the risk of exposure. In Baltimore, US, 65% of rats were seropositive, while 15–32% of rodents in Vietnamese cities were seropositive (Easterbrook et al., Reference Easterbrook, Kaplan, Vanasco, Reeves, Purcell, Kosoy, Glass, Watson and Klein2007; Koma et al., Reference Koma, Yoshimatsu, Yasuda, Li, Amada, Shimizu, Isozumi, Mai, Hoa, Nguyen, Yamashiro, Hasabe and Arikawa2013).
Mycobacterium spp. One of the three studies on Mycobacterium spp. included abattoir diagnosis of cattle in Niamey, Niger, where 0.19% of carcasses had visible tuberculosis lesions (Boukary et al., Reference Boukary, Saegerman, Rigouts, Matthys, Berkvens and Thys2010). The other two studies used intradermal skin tests for diagnosis, and in peri-urban Faisalabad, Pakistan, 2.5% of buffaloes were found to be positive reactors (Arshad et al., Reference Arshad, Ifrahim, Ashraf, Rehman and Khan2012), while 6.1% of cattle in peri-urban/urban Ouagadougou, Burkina Faso, were positive (Boussini et al., Reference Boussini, Traore, Tamboura, Bessin, Boly and Ouedraogo2012).
Salmonella. In total, eight papers included studies about Salmonella spp., but one of these papers focused on assessing knowledge, attitudes, and practices. Four papers studied the prevalence of Salmonella spp in wild animals, recording positive rates of up to 11% in pigeons in Wroclaw, Poland (Piasecki, Reference Piasecki2006). One study in dog and pigeon excretions in Italy failed to detect Salmonella spp.
Other bacteria. There were two publications each on Bartonella spp. and Yersinia spp., as well as one study focused on antimicrobial-resistant bacteria (Guenther et al., Reference Guenther, Bethe, Fruth, Semmler, Ulrich, Wieler and Ewers2012).
Parasites
Cryptosporidium spp. Seven papers reported studies on cryptosporidiosis, four in wild animals, one in dogs, and two in livestock. A study on cattle in Nairobi, Kenya, found a higher prevalence in the dry compared to the wet season, with an individual prevalence being as high as 15% and herd prevalence 29% in the dry season (Kange'the et al., Reference Kange'the, McDermott, Grace, Mbae, Mulinge, Monda, Nyongesa, Ambia and Njehu2012). The same study also found Cryptosporidium spp. in humans from households with dairy cattle, as well as in their neighbors (4 and 5%, respectively). Similar to the results on Giardia, Wang et al. (Reference Wang, Ruch-Gallie, Scorza, Lin and Lappin2012) found only Cryptosporidium spp. (3/129 tested) in dogs using dog parks. In wild animals, positive rates of 35 and 52% were reported in mice and rats, respectively, in Umuarama, Brazil (Kozerski et al., Reference Kozerski, Caldato, de A Ceranto, von Söhsten, Messa and da Silva2012; Silva et al., Reference Silva, Richtzenhain, Barros, Gomes, Silva, Kozerski, de Araújo Ceranto, Keid and Soares2013). In Sydney, Australia, 133 possums were caught in traps and 11% were positive (Hill et al., Reference Hill, Deane and Power2008).
Gastrointestinal helminthes, with focus on Echinococcus spp. In total, 25 papers reported on gastrointestinal helminthes in urban animals, of which nine specifically reported on echinococcosis/hydatidosis. Nine papers included wildlife and 12 dogs. Three papers stated that random sampling was used, and two of these explained the methodology. In some papers sampling was not directly from animals but from animal feces. All studies did find zoonotic parasites in urban animals, but there was a wide range of parasites discovered and varying positive rates. In red foxes in Geneva, Switzerland, it was found that the proportion positive for echinococcosis was lower in urban areas (31%, compared to 46% overall) (Fischer et al., Reference Fischer, Reperant, Weber, Hegglin and Deplazes2005). Similar high levels of echinococcosis (25–30%) was found in cities in Argentina and Canada (Catalano et al., Reference Catalano, Lejeune, Liccioli, Verocai, Gesy, Jenkins, Kutz, Fuentealba, Duignan and Massolo2012; Liccioli et al., Reference Liccioli, Catalano, Kutz, Lejeune, Verocai, Duignan, Fuentealba, Hart, Ruckstuhl and Massolo2012; Casas et al., Reference Casas, Costas Otero, Cespedes, Sosa and Santillan2013). In contrast, no positive sample for E. multilocularis was found in 160 road-killed foxes in Brussels, Belgium (Brochier et al., Reference Brochier, De Blander, Hanosset, Berkvens, Losson and Saegerman2007), and in a study on E. granulosus in dogs in Chile, it was found that urban dogs had a higher positive rate (11.7%, compared to 7.2% overall) (Acosta-Jamett et al., Reference Acosta-Jamett, Cleaveland, Bronsvoort, Cunningham, Bradshaw and Craig2010).
Other parasitic species frequently studied include Toxocara canis and Toxascaris leonina, which are common dog parasites; however, occasional studies also look at urban livestock. In Mwanza city, Tanzania, higher infection rates with strongyles and coccidia in goats were found in the urban areas (Mhoma et al., Reference Mhoma, Kanyari and Kagira2011), and 95% infection rate with Ascaris suum was found in urban pigs in Ethiopia (Zewdneh et al., Reference Zewdneh, Ekwal, Tsegabirhan, Yohannes and Kidane2013). While dogs were more frequently found infected when they lived on the streets and in low-income areas, compared to house dogs and more affluent areas of the city (Martin and Demonte, Reference Martin and Demonte2008; Nikolić et al., Reference Nikolić, Dimitrijević, Katić-Radivojević, Klun, Bobić and Djurković-Djaković2008; Antolova et al., Reference Antolova, Reiterova, Miterpakova, Stanko and Dubinsky2004), zoonotic parasites were also found in dogs that were not let onto the streets at all in Pinhais, Brazil (Martins et al., Reference Martins, Barros, Bier, Marinho, Figueiredo, Hoffmann, Molento and Biondo2012).
Giardia spp. Out of the six papers reporting on Giardia spp., five were on dogs and one on coyote, and no study reported a probabilistic sampling. A Bayesian model for dogs in Pisa, Italy, estimated 29% of dogs were infected (Papini et al., Reference Papini, Carreras, Marangi, Mancianti and Giangaspero2013), while 19.8% of coyote carcasses in Calgary, Canada, and 17.3% of dogs in Botucatu, Brazil were positive (Liccioli et al., Reference Liccioli, Catalano, Kutz, Lejeune, Verocai, Duignan, Fuentealba, Hart, Ruckstuhl and Massolo2012; Silva et al., Reference Silva, Monobe, Lopes and Araujo2012). A study from the US found only 5 dogs positive out of 129, and all dogs that were positive were using dog parks, identified as the main risk factor (Wang et al., Reference Wang, Ruch-Gallie, Scorza, Lin and Lappin2012).
Leishmania spp. All the six studies included on Leishmania spp. had been conducted on dogs in Brazil, two with purposive sampling and four with unknown sampling methodology. Different detection methods yielded different estimates in urban prevalence, with a serological study in Cuiabá having the highest proportion positive of 27% in urban dogs (Mestre et al., Reference Mestre, Ribeiro, Miyazaki, Rodrigues, Almeida, Sousa and Missawa2011), but the paper contained no information about sampling methodology. In comparison, a study in Dias D’Ávila found only 2.5% positive dogs in the city and 29.9% positive in rural areas, and concluded that leishmaniasis was significantly more common in the countryside (de Oliveira et al., Reference Oliveira, Messick, Biondo, Santos, Stedile, Dalmolin, Guimaraes, Mohamed, Riediger and González2010).
Toxoplasma: Of the eight papers reporting on Toxoplasma gondii, two were on pets (dogs and stray cats), and the rest on wildlife, and only one paper reported on random selection, but without explaining methodology. Among the dogs sampled in Botucato, Brazil, 33.1% had antibodies against Toxoplasma (Langoni et al., Reference Langoni, Modolo, Pezerico, Silva, Castro, da Silva and Padovani2006), whereas only 5% of pigeons in the same country had it (de Lima et al., Reference de Lima, Langoni, da Silva, Pezerico, de Castro, da Silva, Araujo and Araújo2011). Contrastingly, 75.6% of pigeons were seropositive in Wroclaw, Poland, (Piasecki, Reference Piasecki2006).
Other parasites. Other parasites studied included the vector-borne Trypanosoma cruzi and Babesia spp., miscellaneous protozoan parasites (Blastocystis, Neospora), and macroparasites (Trichinella, Tunga penetrans, Paragonium), bacterial (two publications each on Bartonella and Yersinia), viruses (reoviruses, picobirnaviruses, arenaviruses, hantaviruses). One study focused on antimicrobial-resistant bacteria (Guenther et al., Reference Guenther, Bethe, Fruth, Semmler, Ulrich, Wieler and Ewers2012).
Discussion
This review used a number of search strings to screen databases to identify papers published on the topics of zoonotic infections in animals in urban areas. While research solely on human disease, prevalence or incidence was excluded, papers were included on peridomestic wildlife and domestic animals, including pets and livestock, and it was found that most studies were about pets and urban wildlife, while urban livestock was studied more seldom. This may be due to the fact that the importance of urban livestock keeping often has been underestimated when it comes to both their contribution to food security and nutrition as well as to pathogen transmission and disease emergence. However, there are also still prevailing perceptions that livestock keeping is mainly a rural livelihood. Also, it may be due to difficulties in researching an animal husbandry practice that is informal or illegal in many countries, and when authorities are not willing to admit the extent of such practices (Grace et al., Reference Grace, Lindahl, Correa, Kakkar, de Zeeuw and Drechsel2015). The keeping of urban livestock combined with increased human population densities may facilitate spread of diseases, and urbanization is considered one of the drivers behind disease emergence due to the extensive interface and the high number of susceptible and infectious individuals that may meet and interact (Lindahl and Grace, Reference Lindahl and Grace2015).
It is evident in the papers reviewed that when zoonotic pathogens are looked for, they are often detected in urban areas, and most studies conclude that zoonoses are a threat to human inhabitants. One may, however, suspect that there is a publication bias against studies looking for zoonotic pathogens in urban environments and not finding them, as negative results are less likely to get published (Dickersin, Reference Dickersin1990; Dirnagl and Lauritzen, Reference Dirnagl and Lauritzen2010; Koricheva et al., Reference Koricheva, Gurevitch and Mengersen2013). One rare exception included in this review is the report by Kohls et al. (Reference Kohls, Lüschow, Lierz and Hafez2011) showing negative results for influenza. However, this same paper also reported on the presence of paramyxovirus, which may have facilitated its publication.
Many low-income countries with large and rapidly expanding cities were not represented in this screening, or were represented only by very few publications. It is remarkable that only 5 papers each came from the low- and middle-income countries in South and Southeast Asia, regions with dense populations in multiple mega-cities, with more than 10 million people. Similarly, countries such as Russia, China, and South Africa were not represented, possibly because our search strings did not detect them. However, searching a database like PubMed with the search terms zoonotic or zoonoses, urban and Russia, also yielded few results. There may be a tendency to publish in national journals in some countries, and thus the results are not available for the international research community which could explain why our search did not retrieve the articles. However, it may also be an indication of that the topic of urban animals and zoonoses has long had a very low priority. Considering that sub-Saharan Africa is foreseen to be responsible for a large part of the population growth in the future, with many cities with high growth rate and often extensive informal settlements (Gerland et al., Reference Gerland, Raftery, Sevčíková, Li, Gu, Spoorenberg, Alkema, Fosdick, Chunn, Lalic, Bay, Buettner, Heilig and Wilmoth2014; Bloom, Reference Bloom2011), it is worrisome that not more than 13 papers were found relating to zoonoses in African cities. Scarcity on published papers on zoonoses in Africa has however been noted before, and may have multiple reasons. For example, difficulties for scientists to get research published in journals accessible online, or low priority among research donors (Alonso et al., Reference Alonso, Lindahl, Roesel, Traore, Yobouet, Ndour, Carron and Grace2016).
The most frequently studied pathogens were gastrointestinal helminths, followed by the diarrheal pathogens Salmonella, Giardia, and Campylobacter. The reasons for selection of these pathogens may be the focus on certain host species, or more pragmatic ones, such as the relative ease to collect fecal samples or a straightforward diagnostic methodology. However, in OIE's annual report on human cases of zoonosis (OIE 2017), brucellosis and leptospirosis, which are sparsely found in this review, were the diseases reported from most countries in 2015. On the other hand, with respect to the number of human cases, the diarrheal diseases salmonellosis and campylobacteriosis seems to be the most frequent. Notably, also the large Global Burden of Disease Study 2015, points out diarrheal diseases as the infectious disease contributing to most disability-adjusted life-years (DALYs) (GBD 2015 DALYs and HALE Collaborators, 2016). Among the major zoonoses, the WHO deals with (WHO 2017), food-borne zoonoses and leptospirosis are also found in this review. In contrast, other WHO-prioritized zoonoses like anthrax and prion diseases are not found at all in the review. It is interesting to note that for some of the food-borne pathogens, the majority of the studies found in this review were conducted in high-income countries, while the burden of these diseases is proportionally much higher in low-income countries (Engels and Savioli, Reference Engels and Savioli2006). In summary, the literature found here on urban zoonoses deals with several of the pathogens that may be regarded as the most important zoonotic pathogens globally. However, at the same time, some of the important zoonotic pathogens are just sparsely studied or not studied at all in urban animals, or are not studied in countries where the burden is high.
This review has several limitations, and the narrow scope of only including papers written in English and only search in international databases, likely made some countries under-represented here. While we used some very general search terms, such as, ‘Urban AND (‘Animal Diseases’ OR ‘Human Diseases’) AND Health AND Cities’, we did not include the names of specific diseases, nor all hosting animal species, which may have contributed to less consideration of some relevant studies. However, the main purpose of this review was to get an overview of where most of the peer-reviewed research has been conducted and where the research focus has been, and we believe that this was still achieved. One interesting finding was that, in spite of many search terms being aimed specifically at picking up studies on urban livestock, this topic was very much under-represented, which further indicates a need for covering that knowledge gap.
In conclusion, this review points out three areas where science-based knowledge is limited. First, with respect to the traditional research focus on zoonoses in livestock in rural areas, we may be at a point where we need to shift focus towards the crowded urban settings where we may see hot-spots for disease emergence in the future. Second, it is worrisome that so few studies are from the rapidly expanding cities in low- and middle-income countries, where urban livestock keeping is far more prominent than in high-income countries. Third, there are arguments to consider other zoonotic pathogens in future studies on zoonoses in urban animals. Overall, there are significant research gaps that should be filled in order to mitigate risks of emergence of zoonoses from urban animals.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1466252319000100.
Acknowledgements
The authors would like to acknowledge Ms Kristin Follis Bergman for help searching publications. This work was funded by a grant from SLU Global and the Swedish International Agricultural Network Initiative.
Financial support
This work was funded by a grant from SLU Global and the Swedish International Agricultural Network Initiative.
Conflict of interest
None.