Introduction
Staphylococcus aureus small colony variants (SCVs) are naturally occurring populations that were first described about 100 years ago, often as ‘G’ forms or ‘dwarf’ colonies of many bacterial species including S. aureus (Swingle, Reference Swingle1935; Wise and Spink, Reference Wise and Spink1954; Goudie and Goudie, Reference Goudie and Goudie1955; Proctor et al., Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006; von Eiff et al., Reference von Eiff, Peters and Becker2006). In early reports SCVs were suggested as gonidial mutants or ‘G’ forms that developed within specialized mother cells under unfavorable conditions and had exceedingly small size, possibly representing a primitive phase of the bacterial life cycle (Swingle, Reference Swingle1935; Wise and Spink, Reference Wise and Spink1954). Other studies reported the isolation of ‘dwarf’ colonies from animals and humans following antibiotic treatment (Goudie and Goudie, Reference Goudie and Goudie1955; Sompolinsky et al., Reference Sompolinsky, Cohen and Ziv1974).
S. aureus SCVs have been extensively investigated in human medicine because of their association with persistent and relapsing infections (Proctor et al., Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006), but have been mainly overlooked in veterinary medicine. However, in one study almost 40 years ago a dwarf-colony variant of S. aureus was linked to chronic bovine mastitis in an Israeli herd (Sompolinsky et al., Reference Sompolinsky, Cohen and Ziv1974). The authors suggested that the frequent use of antibiotics for the treatment of bovine mastitis may have selective advantages for staphylococci with metabolic disorders but their biological significance was not fully understood.
In the mid-1990s, the clinical importance of S. aureus SCVs that are defective in electron transport gained considerable attention, and SCVs were linked to persistent and relapsing human infections (Proctor et al., Reference Proctor, van, Kristjansson, Maslow and Arbeit1995). Subsequently, clinical SCVs were frequently isolated from humans with persistent and relapsing infection including septicemic arthritis, osteomyelitis, unmanageable wound infections or cystic fibrosis following antimicrobial therapy with gentamicin or trimethoprim–sulfamethoxazole (von Eiff et al., Reference von Eiff, Heilmann, Proctor, Woltz, Peters and Gotz1997; Abele-Horn et al., Reference Abele-Horn, Schupfner, Emmerling, Waldner and Goring2000; Sadowska et al., Reference Sadowska, Bonar, von Eiff, Proctor, Chmiela, Rudnicka and Rozalska2002; Kahl et al., Reference Kahl, Duebbers, Lubritz, Haeberle, Koch, Ritzerfeld, Reilly, Harms, Proctor, Herrmann and Peters2003; Lattar et al., Reference Lattar, Tuchscherr, Caccuri, Centron, Becker, Alonso, Barberis, Miranda, Buzzola, von and Sordelli2009). Naturally occurring SCVs have been selected by the intracellular environment of cultured endothelial cells (Vesga et al., Reference Vesga, Groeschel, Otten, Brar, Vann and Proctor1996) as well as following in vitro exposure to certain concentrations of antibiotics, especially aminoglycosides, that eliminate or inhibit the growth of the parental phenotype and permit the SCV phenotype to become detectable (Wise and Spink, Reference Wise and Spink1954; Musher et al., Reference Musher, Baughn, Templeton and Minuth1977; Miller et al., Reference Miller, Edberg, Mandel, Behar and Steigbigel1980; Balwit et al., Reference Balwit, van, Vann and Proctor1994; Massey et al., Reference Massey, Buckling and Peacock2001; Sadowska et al., Reference Sadowska, Bonar, von Eiff, Proctor, Chmiela, Rudnicka and Rozalska2002; Schaaff et al., Reference Schaaff, Bierbaum, Baumert, Bartmann and Sahl2003).
More recently, Atalla et al. (Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008) reported the isolation of naturally occurring bovine SCVs from dairy cows with a history of chronic intramammary S. aureus infection and suggested possible association between persistent bovine mastitis and the formation of SCVs. Colonies with the appearance of SCV were detected in initial cultures of quarter milk samples of 1 of 11 cows, but these colonies reverted to the typical large-colony phenotype upon subculture. Following in vitro enrichment in the presence of sub-inhibitory concentrations of gentamicin (1 μg ml−1), S. aureus of the SCV phenotype were recovered from six of the cows. These results are likely due to the fact that naturally occurring SCVs are often found in mixed population and are easily outgrown by bacteria of the parent phenotype; the findings are consistent with previous observations on human SCVs by Balwit et al. (Reference Balwit, van, Vann and Proctor1994) and Sadowska et al. (Reference Sadowska, Bonar, von Eiff, Proctor, Chmiela, Rudnicka and Rozalska2002).
Phenotypic properties of S. aureus SCVs
S. aureus SCVs exhibit atypical morphological and biochemical properties relative to the parental phenotype that seem to be associated with disruption in the electron transport system or inability to synthesize thymidine (Gilligan et al., Reference Gilligan, Gage, Welch, Muszynski and Wait1987; Balwit et al., Reference Balwit, van, Vann and Proctor1994; McNamara and Proctor, Reference McNamara and Proctor2000; Proctor et al., Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006; von Eiff et al., Reference von Eiff, Peters and Becker2006; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). Typically, SCVs have a slow growth rate and require up to 48 h to form visible colonies on the solid agar medium supplemented with 5% rabbit or sheep blood (Fig. 1). They form tiny colonies that are about one-tenth the size of the parent strain, are non-pigmented, non-hemolytic or have a greatly reduced zone of hemolysis (Proctor and Peters, Reference Proctor and Peters1998; Kahl et al., Reference Kahl, Duebbers, Lubritz, Haeberle, Koch, Ritzerfeld, Reilly, Harms, Proctor, Herrmann and Peters2003; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). Reduced α-hemolysin production by S. aureus SCVs has been suggested to be responsible for reduced virulence in a nematode model of infection and their high capacity for intracellular persistence within non-professional phagocytes compared with their wild-type parental strains (Bates et al., Reference Bates, von, McNamara, Peters, Yeaman, Bayer and Proctor2003; Jonsson et al., Reference Jonsson, von, Proctor, Peters, Ryden and Tarkowski2003; Brouillette et al., Reference Brouillette, Martinez, Boyll, Allen and Malouin2004; Proctor et al., Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006; Sifri et al., Reference Sifri, Baresch-Bernal, Calderwood and von Eiff2006). While SCVs utilize glucose and fructose, they fail to ferment mannitol or other sugars (Becker et al., Reference Becker, Bierbaum, von, Engelmann, Gotz, Hacker, Hecker, Peters, Rosenstein and Ziebuhr2007; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008).

Fig. 1. Morphology of small-colony variant (SCV) Heba3231 on Columbia blood agar plate compared to its parent phenotype. The bovine SCV Heba3231 formed tiny non-hemolytic colonies that are typical for the SCV compared to its parent strain 3231.
SCVs have reduced coagulase production and require more than 18 h of incubation to be coagulase positive (Proctor et al., Reference Proctor, van, Kristjansson, Maslow and Arbeit1995; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). Unlike other secreted proteins, the exoprotein coagulase is expressed during the exponential growth phase by almost all S. aureus strains including bovine isolates (Lebeau et al., Reference Lebeau, Vandenesch, Greenland, Novick and Etienne1994). The expression of this protein is controlled by the accessory gene regulator (agr) locus and accordingly its expression is inhibited during the post-exponential phase. However, a previous study demonstrated that S. aureus strains that are agr-deficient continuously expressed an intermediate level of coagulase during the growth cycle (Lebeau et al., Reference Lebeau, Vandenesch, Greenland, Novick and Etienne1994). This observation could explain the delayed coagulase activity of S. aureus strains with the SCV phenotype. Coagulase-deficient mutants were not altered in their in vitro adherence to fibrinogen and in their infectivity in the rat model of endocarditis (Moreillon et al., Reference Moreillon, Entenza, Francioli, McDevitt, Foster, Francois and Vaudaux1995). The role of coagulase in the pathogenesis of bovine mastitis has never been established (Phonimdaeng et al., Reference Phonimdaeng, O'Reilly, Nowlan, Bramley and Foster1990; Baddour et al., Reference Baddour, Tayidi, Walker, McDevitt and Foster1994; Sutra and Poutrel, Reference Sutra and Poutrel1994; Moreillon et al., Reference Moreillon, Entenza, Francioli, McDevitt, Foster, Francois and Vaudaux1995; Kerro et al., Reference Kerro, van Dijk and Nederbragt2002).
Growth curve characteristics of S. aureus SCVs
Growth curve analysis of SCVs shows an extended lag phase (Kahl et al., Reference Kahl, Belling, Becker, Chatterjee, Wardecki, Hilgert, Cheung, Peters and Herrmann2005; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008) and a slower generation time compared with the parental strain (Wise and Spink, Reference Wise and Spink1954; Proctor et al., Reference Proctor, Kahl, von, Vaudaux, Lew and Peters1998). For example, the bovine SCV Heba3231 grew slowly in brain heart infusion (BHI) broth containing 1 μg ml−1 gentamicin and reached a plateau at a low cell density compared with its parent strain 3231 and the prototype strain Newbould 305 (Fig. 2). The rate of growth of the SCV was approximately one-ninth that of the parent (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008).

Fig. 2. Growth curve of SCV Heba3231 in brain heart infusion (BHI) broth with gentamicin in comparison with its parent strain and the prototypic strain Newbould 305.
A common feature of naturally occurring and in vitro-induced SCV S. aureus is the tendency to revert to the parental phenotype after cessation of antibiotic exposure (Swingle, Reference Swingle1935; Wise and Spink, Reference Wise and Spink1954; Massey et al., Reference Massey, Buckling and Peacock2001; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). Reversion also occurs spontaneously on transfer from stock culture or after prolonged incubation in a culture medium, most often as 1–2 large colonies among numerous small colonies (Swingle, Reference Swingle1935). While the bovine SCV Heba3231 was stable on solid media, the colonies reverted to the parent phenotype in antibiotic-free BHI broth (Fig. 3) and the reversion was cell density dependent, occurring at >20×106 colony-forming unit (CFU) ml−1.

Fig. 3. Reversion of S. aureus SCV Heba3231 to the typical phenotype on Columbia blood agar plate when cultured in an antibiotic-free medium.
Phenotypic switching between an SCV and a wild-type strain may be explained by a number of mechanisms, but the exact mechanism is not known. Regulatory mechanisms likely play a role in SCV selection (Proctor et al., Reference Proctor, Balwit and Vesga1994; Hoffman et al., Reference Hoffman, Deziel, D'Argenio, Lepine, Emerson, McNamara, Gibson, Ramsey and Miller2006; Mitchell et al., Reference Mitchell, Lamontagne, Brouillette, Grondin, Talbot, Grandbois and Malouin2008), but mutational changes in genes with putative functions in hemin or menadione biosynthesis were reported, especially in gentamicin-induced SCVs (Schaaff et al., Reference Schaaff, Bierbaum, Baumert, Bartmann and Sahl2003) or in SCVs from patients receiving systemic antibiotic therapy (Lannergård et al., Reference Lannergård, von, Sander, Cordes, Seggewiss, Peters, Proctor, Becker and Hughes2008). Because SCVs are unstable in most in vitro systems and the genetic changes in clinical isolates are undefined, constructed SCV mutants have been generated by disrupting the hemB and/or menD genes in S. aureus (von Eiff et al., Reference von Eiff, Heilmann, Proctor, Woltz, Peters and Gotz1997; Bates et al., Reference Bates, von, McNamara, Peters, Yeaman, Bayer and Proctor2003; Brouillette et al., Reference Brouillette, Martinez, Boyll, Allen and Malouin2004). These genetically stable SCV mutants mimic the typical phenotypic features of clinical SCVs and have been successfully employed for phenotypic, transcriptomic and proteomic characterizations of SCVs and for the assessment of their pathogenic potential (Bates et al., Reference Bates, von, McNamara, Peters, Yeaman, Bayer and Proctor2003; Jonsson et al., Reference Jonsson, von, Proctor, Peters, Ryden and Tarkowski2003; Brouillette et al., Reference Brouillette, Martinez, Boyll, Allen and Malouin2004; Senn et al., Reference Senn, Bischoff, von Eiff and Berger-Bachi2005; Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006; Seggewiss et al., Reference Seggewiss, Becker, Kotte, Eisenacher, Yazdi, Fischer, McNamara, Al, Proctor, Peters, Heinemann and von Eiff2006; Sifri et al., Reference Sifri, Baresch-Bernal, Calderwood and von Eiff2006; von Eiff et al., Reference von Eiff, Peters and Becker2006; Besier et al., Reference Besier, Ludwig, Ohlsen, Brade and Wichelhaus2007; Chatterjee et al., Reference Chatterjee, Kriegeskorte, Fischer, Deiwick, Theimann, Proctor, Peters, Herrmann and Kahl2008; Lannergård et al., Reference Lannergård, von, Sander, Cordes, Seggewiss, Peters, Proctor, Becker and Hughes2008).
Auxotrophism in S. aureus SCVs
The phenotypic characteristics of S. aureus SCVs are mostly related to alterations in hemin and/or menadione biosynthesis required for the electron transport system or to thymidine deficiency, but SCVs with other metabolic disorders have been reported (Acar et al., Reference Acar, Goldstein and Lagrange1978; Proctor et al., Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006; Seggewiss et al., Reference Seggewiss, Becker, Kotte, Eisenacher, Yazdi, Fischer, McNamara, Al, Proctor, Peters, Heinemann and von Eiff2006; Besier et al., Reference Besier, Ludwig, Ohlsen, Brade and Wichelhaus2007; Kohler et al., Reference Kohler, von, Liebeke, McNamara, Lalk, Proctor, Hecker and Engelmann2008). Hemin and menadione are required for the biosynthesis of cytochromes and menaquinone to complete the electron transport chain and generate large quantities of ATP. The production of ATP is required for biosynthesis of cell-wall teichoic acid, rapid growth and formation of large colonies, carotenoid biosynthesis, protein biosynthesis and increased electrochemical gradient to positively charged antibiotics including aminoglycosides and cationic peptides (Proctor et al., 1998, Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006). Thymidine is required for the synthesis of electron transport chain components, yet the mechanisms involved in thymidine-dependent SCV are unlikely to be related to the electron transport defect (Kahl et al., Reference Kahl, Duebbers, Lubritz, Haeberle, Koch, Ritzerfeld, Reilly, Harms, Proctor, Herrmann and Peters2003). Thymidine-dependent SCVs emerged after long-term therapy of cystic fibrosis patients with trimethoprim sulphamethoxazole (STX) that interferes with a coenzyme required for downstream DNA synthesis (Proctor et al., Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006). The survival of the STX-resistant SCV is dependent on exogenous thymidine that is abundant in airway secretions. In addition to the typical pinpoint colony morphology, thymidine-dependent SCVs often exhibit a ‘fried-egg’ appearance with translucent edges surrounding an elevated pigmented center (Kahl et al., Reference Kahl, Duebbers, Lubritz, Haeberle, Koch, Ritzerfeld, Reilly, Harms, Proctor, Herrmann and Peters2003).
Investigation of auxotrophism showed that supplementation of growth medium with hemin, menadione or thymidine restores the size of colonies (Wise and Spink, Reference Wise and Spink1955; Balwit et al., Reference Balwit, van, Vann and Proctor1994; Kahl et al., Reference Kahl, Herrmann, Everding, Koch, Becker, Harms, Proctor and Peters1998; Brouillette et al., Reference Brouillette, Martinez, Boyll, Allen and Malouin2004; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). The bovine SCV strain Heba3231 failed to grow on chemically defined medium supplemented with 1, 5 or 10 μg ml−1 hemin, menadione and/or thymidine suggesting that the bovine SCV may have unknown metabolic defects and require additional nutrients to support its growth (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). While the bovine SCV failed to grow on Muller Hinton (MH) agar plates, it formed minute colonies in 48 h on chocolate MH or when MH was supplemented with 5% sheep blood. Similarly, supplementation of MH with 1 μg ml−1 hemin or menadione enhanced SCV growth in 18 or 48 h. However, it failed to grow on MH supplemented with thymidine (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008).
Antimicrobial susceptibility of S. aureus SCVs
In humans, S. aureus is problematic in healthcare and community settings and is featured as a ‘superbug’ for its increasing antimicrobial resistance (Foster, Reference Foster2004; Lindsay and Holden, Reference Lindsay and Holden2006). Antimicrobial therapy of clinical or subclinical S. aureus mastitis is generally not successful. Most antibiotic treatments result in short-term clinical cure and persistent or relapsing infection of the treated quarters (Barkema et al., Reference Barkema, Schukken and Zadoks2006). The inability of antimicrobial drugs to clear most S. aureus infections including bovine mastitis, coupled with the increasing rise of multidrug-resistant strains in humans, has led to extensive antimicrobial resistance studies involving bovine S. aureus isolates (Erskine et al., Reference Erskine, Cullor, Schaellibaum, Yancey and Zecconi2004; Barkema et al., Reference Barkema, Schukken and Zadoks2006).
There is no evidence to support a widespread increase in penicillin resistance of bovine S. aureus isolates over the past 35 years (Erskine et al., Reference Erskine, Cullor, Schaellibaum, Yancey and Zecconi2004; Call et al., Reference Call, Davis and Sawant2008). In fact, recent studies show a decrease in penicillin resistance in several countries during the past 10 years (Werckenthin et al., Reference Werckenthin, Cardoso, Martel and Schwarz2001; Erskine et al., Reference Erskine, Walker, Bolin, Bartlett and White2002; Vintov et al., Reference Vintov, Aarestrup, Zinn and Olsen2003; Bennedsgaard et al., Reference Bennedsgaard, Thamsborg, Aarestrup, Enevoldsen, Vaarst and Christoffersen2006; Hendriksen et al., Reference Hendriksen, Mevius, Schroeter, Teale, Meunier, Butaye, Franco, Utinane, Amado, Moreno, Greko, Stark, Berghold, Myllyniemi, Wasyl, Sunde and Aarestrup2008). Penicillin-resistant S. aureus seem to be a herd problem often related to inappropriate use of penicillin (Bennedsgaard et al., Reference Bennedsgaard, Thamsborg, Aarestrup, Enevoldsen, Vaarst and Christoffersen2006). While methicillin-resistant S. aureus (MRSA) has been a stumbling block for human antimicrobial therapy in community and healthcare settings, methicillin resistance seems uncommon among bovine isolates (Moon et al., Reference Moon, Lee, Kang, Lee, Joo, Park, Kim and Koo2007; Monecke et al., Reference Monecke, Kuhnert, Hotzel, Slickers and Ehricht2007; Morgan, Reference Morgan2008). Transmission of MRSA between cows and attendants has recently been reported in one province in Canada (Patrick Boerlin, Department of Pathobiology, Ontario Veterinary College, personal communication), Belgium (Vanderhaeghen et al., Reference Vanderhaeghen, Cerpentier, Adriaensen, Vicca, Hermans and Butaye2010), Hungary (Juhász-Kaszanyitzky et al., Reference Juhász-Kaszanyitzky, Janosi, Somogyi, Dan, van der Graaf-van Bloois, van Duijkeren and Wagenaar2007) and Korea (Lee, Reference Lee2003). Therapeutic success of bovine S. aureus mastitis is dependent on pathogen, cow and treatment-related factors, and SCVs may be of considerable importance in internalization of bovine mastitis-associated S. aureus strains within cells of the mammary gland, making them inaccessible to β-lactam antibiotics (Barkema et al., Reference Barkema, Schukken and Zadoks2006). Furthermore, SCVs have novel mechanisms for antibiotic resistance and appear to be selected by exposure to certain antimicrobials (Proctor et al., Reference Kahl, Herrmann, Everding, Koch, Becker, Harms, Proctor and Peters1998).
Clinical SCVs are often more resistant to antimicrobial compounds especially to aminoglycosides compared to wild-type parent strains (Proctor et al., Reference Kahl, Herrmann, Everding, Koch, Becker, Harms, Proctor and Peters1998; Seifert et al., Reference Seifert, von Eiff and Fatkenheuer1999; von Eiff et al., Reference von Eiff, Proctor and Peters2000; Brouillette et al., Reference Brouillette, Martinez, Boyll, Allen and Malouin2004; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). An interruption of the electron transport chain affects the electrochemical gradient across the bacterial cell membrane to positively charged antibiotics including aminoglycosides and cationic peptides. The minimum inhibitory concentration (MIC) of gentamicin for the bovine SCV Heba3231 was at least 16 times that of its parent strain and the prototype strain Newbould 305, but there was no difference in MICs of other antimicrobials (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). Reduced susceptibility to gentamicin is typical of a respiratory deficient mutant due to alteration in the electromembrane potential. However, clinical isolates are often found in mixed populations of SCV and the parent, and even when SCVs constitute a high percentage of colonies, they are easily overgrown in overnight broth culture, presenting a challenge for antimicrobial testing (von Eiff et al., Reference von Eiff, Peters and Becker2006). Interestingly, while the bovine prototype strain Newbould 305 and its isogenic hemB mutant were susceptible to the beta-lactam antibiotic cephapirin in in vitro assays, the hemB mutant was over 100 times more persistent in the mouse mammary gland than was Newbould 305 during antimicrobial therapy with cephapirin (Brouillette et al., Reference Brouillette, Martinez, Boyll, Allen and Malouin2004).
Pathogenicity of S. aureus SCVs
Pathogenicity of S. aureus SCVs of human or bovine origin has been evaluated in a number of infection models including a mouse model of arthritis (Jonsson et al., Reference Jonsson, von, Proctor, Peters, Ryden and Tarkowski2003), a rabbit model of endocarditis (Bates et al., Reference Bates, von, McNamara, Peters, Yeaman, Bayer and Proctor2003), a nematode Caenorhabditis elegans model of infection (Sifri et al., Reference Sifri, Baresch-Bernal, Calderwood and von Eiff2006), a mouse model of mastitis (Brouillette et al., Reference Brouillette, Martinez, Boyll, Allen and Malouin2004) and in dairy cows (Atalla et al., Reference Atalla, Gyles, Wilkie, Leslie and Mallard2009). Studies have reported that SCVs varied from being highly virulent, equally virulent or less virulent compared to their wild-type strains. In the mouse model of arthritis, the hemB mutant was more highly virulent than the wild-type strains and its severity was likely related to excessive production of destructive proteases (Jonsson et al., Reference Jonsson, von, Proctor, Peters, Ryden and Tarkowski2003). In the rabbit model of endocarditis, a hemB mutant was as virulent as its wild-type parent strain as determined by bacterial dissemination, and as sensitive to oxacillin therapy, suggesting possible reversion of the hemB mutant to the parental phenotype due to the availability of hemin in the embolic infarcts; a menD mutant strain showed less colonization and more resistance to oxacillin (Bates et al., Reference Bates, von, McNamara, Peters, Yeaman, Bayer and Proctor2003).
In an early study, rabbits injected intravenously with the parent strains most often became emaciated and died, whereas those receiving the SCV appeared healthy and lacked any macroscopic lesions, suggesting reduced virulence of the SCV strains (Swingle, Reference Swingle1935). In C. elegans, although the hemB mutant successfully colonized the nematode gut, it was less virulent than the wild-type strain (Sifri et al., Reference Sifri, Baresch-Bernal, Calderwood and von Eiff2006), mostly associated with reduced production of α-toxin and other virulence determinants. Although the bovine Newbould hemB mutant has reduced ability to colonize the mouse mammary gland, it was greater than 100 times more persistent than the parent strain Newbould 305 (Brouillette et al., Reference Brouillette, Martinez, Boyll, Allen and Malouin2004). Additionally, intramammary challenge of dairy cows with the SCV Heba3231 or the Newbould hemB strain resulted in mastitis that was mild compared with that induced by their wild-type parent strains, based on systemic and localized signs (Atalla et al., Reference Atalla, Gyles, Wilkie, Leslie and Mallard2009). The pathogenicity of SCVs appears to rely on their ability to survive intracellularly compared to toxin-producing wild-type strains (von Eiff et al., Reference von Eiff, Heilmann, Proctor, Woltz, Peters and Gotz1997; Kahl et al., Reference Kahl, Herrmann, Everding, Koch, Becker, Harms, Proctor and Peters1998; von Eiff et al., Reference von Eiff, Proctor and Peters2000; Vaudaux et al., Reference Vaudaux, Francois, Bisognano, Kelley, Lew, Schrenzel, Proctor, McNamara, Peters and von Eiff2002; Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006; Sendi and Proctor, Reference Sendi and Proctor2009). The intracellular environment provides protection from host defenses and antibiotics and allows for long-term persistence in the host.
Prevalence of S. aureus SCVs
In view of the aforementioned unusual phenotypic characteristics, S. aureus SCVs present a challenge to clinicians and clinical microbiologists and are easily overlooked by diagnostic laboratories (Proctor et al., Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006; von Eiff, Reference von Eiff2008). The estimated frequency of occurrence of human S. aureus SCVs varies between 1 and 30% of clinical samples (Proctor et al., Reference Proctor, von Eiff, Kahl, Becker, McNamara, Herrmann and Peters2006). S. aureus SCV was found in ~29% of patients with osteomyelitis (von Eiff et al., Reference von Eiff, Heilmann, Proctor, Woltz, Peters and Gotz1997), 17–46% of patients with cystic fibrosis who were chronically colonized with S. aureus (Kahl et al., Reference Kahl, Duebbers, Lubritz, Haeberle, Koch, Ritzerfeld, Reilly, Harms, Proctor, Herrmann and Peters2003; Besier et al., Reference Besier, Ludwig, Ohlsen, Brade and Wichelhaus2007), and in about 1% of isolates in a general microbiology laboratory (Acar et al., Reference Acar, Goldstein and Lagrange1978). Naturally occurring bovine SCV was detected in three out of six (50%) S. aureus- positive milk samples from dairy cows with a history of chronic mastitis (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). Considering that SCVs grow slowly and are easily overgrown by their parents and readily revert to the typical parental phenotype, there is pressing need for improved isolation techniques that enrich for the SCV population. This will allow for further studies to determine the prevalence of S. aureus SCV in dairy cows.
Transcriptomics, clonal characteristics of S. aureus SCVs
DNA microarrays were used to compare the transcriptome of S. aureus strains with normal phenotype to their artificially created SCV mutants (Seggewiss et al., Reference Seggewiss, Becker, Kotte, Eisenacher, Yazdi, Fischer, McNamara, Al, Proctor, Peters, Heinemann and von Eiff2006; Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006; Kohler et al., Reference Kohler, von, Liebeke, McNamara, Lalk, Proctor, Hecker and Engelmann2008) or naturally occurring SCVs (Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). A S. aureus-specific DNA microarray was used to determine the heterogeneity of gene expression associated with the bovine SCV Heba3231 phenotype (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). The DNA array contained 460 genes including virulence, secretion, general stress response, regulatory systems, iron transport, antibiotic resistance and general biosynthesis genes (amplified from DNA of S. aureus strains N315, MRSA COL and Mu50). Genes encoding for the effector molecule of the agr, α-toxin and coagulase were down-regulated in SCV Heba3231, whereas genes encoding for fermentative and arginine–deiminase pathways, capsular biosynthesis and those associated with up-regulation of the alternative factor sigB were up-regulated (Table 1). Of note, genes involved in iron metabolism were down-regulated in SCV Heba3231.
Table 1. Expression of some marker genes in naturally occurring bovine small colony variant (SCV) Heba3231 compared to the genetically constructed Newbould hemB mutant

A ⩾2-fold increase or decrease was considered significant.
a A decrease is indicated by minus sign.
b Atalla et al. (Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008).
While naturally occurring SCVs of bovine and human origins share common features with genetically defined SCVs that explain the SCV phenotypic characteristics, they had a unique transcriptional signature (Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). For example, genes that are positively controlled by the alternative factor sigB, such as those encoding for capsular biosynthesis and shock proteins, were up-regulated in SCV Heba3231 (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008) and clinical human SCVs isolated from cystic fibrosis patients (Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006).
Increased activity of the alternative factor sigB is known to up-regulate the transcription of several surface adhesins and is associated with adherence, invasion and persistence within host cells (Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008; Mitchell et al., Reference Mitchell, Lamontagne, Brouillette, Grondin, Talbot, Grandbois and Malouin2008). Naturally occurring SCVs with high sigB activity were successful persisters within host cells compared to Newbould hemB and a sigB mutant that was shown to be a poor persister (Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006) as well as compared to their wild-type parental strains (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008, Reference Atalla, Gyles and Mallard2010a).
To determine genome relatedness between SCV Heba3231 and its parent strain 3231, genomic compositions were studied by comparative genomic hybridization using a DNA microarray containing 460 genes representing sequences of S. aureus strains N315, MRSA COL and Mu50 (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008). Results were also compared to those for the Newbould hemB mutant and its parent strain, the prototype Newbould 305 (Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006) to identify genetic differences between bovine isolates that may influence host–pathogen interaction. Clonal characterization confirmed that wild-type strain 3231 and the SCV Heba3231 are of the same clone but were different from the prototype strain Newbould 305 and its isogenic hemB mutant (Table 2).
Table 2. Genome relatedness between naturally occurring bovine SCV (Heba3231) and its parent strain 3231 compared to the genetically constructed Newbould hemB mutant and its parent strain the prototype Newbould 305 as determined by comparative genomic hybridization
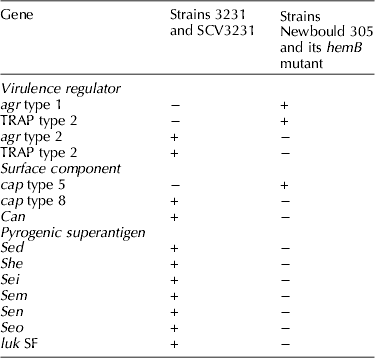
Important differences included the presence of a set of pyrogenic superantigens such as staphylococcal enterotoxin D (SED) in strains 3231 and SCV Heba3231 that appear to play a role in immune response modulation. In our recent study (Atalla et al., Reference Atalla, Gyles and Mallard2010a, Reference Atalla, Wilkie, Gyles, Leslie, Mutharia and Mallardb), differences in antibody-mediated immune response (AMIR) and cell-mediated immune response (CMIR) were clearly evident in the response to SCV Heba3231 and its parent strain 3231 compared with the hemB mutant and its parent strain Newbould 305, following intramammary infection (IMI) of cattle. Predominance of IgG2 antibody in sera and induction of CMIR to the SCV Heba3231 were noted and are typical of a type 1-biased immune response. These findings correlate with the ability of the bovine SCV to adapt to an intracellular lifestyle for better adaptation to the bovine mammary gland and for long-term persistence. Similar to the SCV Heba3231, the parent strain 3231 stimulated both AMIR and CMIR. However, polarization of the immune response toward type 1 or type 2 was not evident. In fact, involvement of both types 1 and 2 in domestic species including cattle is the usual outcome in response to most pathogens (Estes and Brown, Reference Estes and Brown2002; Crawley et al., Reference Crawley, Mallard and Wilkie2005).
On the other hand, both the hemB strain and its parent Newbould 305 stimulated production of specific antibody responses predominated by IgG1 and failed to induce DTH, which is typical for a type 2-biased immune response. The differences in immune response seem to be related to differences in virulence attributes of the S. aureus strains involving mostly exoproteins such as SED and staphylococcal enterotoxin-like gene sen that are expressed by SCV Heba3231 and 3231 strains but not the other strains. Specifically, SED has been reported to mediate a type 1 biased immune response characterized by predominance of IgG2 antibody in secreted milk during experimental IMI of dairy cattle (Tollersrud et al., Reference Tollersrud, Kampen and Kenny2006).
Intracellular survival of S. aureus SCVs
Traditionally, S. aureus has been viewed as an extracellular pathogen. However, there are several reports of its ability to invade and survive intracellularly within professional phagocytes including neutrophils and monocyte-derived macrophages (Gresham et al., Reference Gresham, Lowrance, TCaver, Wilson, Cheung and Lindberg2000; Voyich et al., Reference Voyich, Braughton, Sturdevant, Whitney, Said-Salim, Porcella, Long, Dorward, Gardner, Kreiswirth, Musser and DeLeo2005), as well as non-professional phagocytic cells such as cultured bovine aortic endothelial cells (BAEC) (Vann and Proctor, Reference Vann and Proctor1987; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008) and a bovine mammary epithelial cell line (MAC-T) (Almeida et al., Reference Almeida, Matthews, Cifrian, Guidry and Oliver1996; Bayles et al., Reference Bayles, Wesson, Liou, Fox, Bohach and Trumble1998; Atalla et al., Reference Atalla, Gyles and Mallard2010a, Reference Atalla, Wilkie, Gyles, Leslie, Mutharia and Mallardb). Unlike facultative intracellular pathogens that survive inside infected phagocytes and mammalian cells for long periods of times, internalized S. aureus often reside for a short time, escape into the cytosol and induce apoptosis in in vitro infection models (Bayles et al., Reference Bayles, Wesson, Liou, Fox, Bohach and Trumble1998; Menzies and Kourteva, Reference Menzies and Kourteva1998; Murai et al., Reference Murai, Sakurada, Seki, Shinji, Hirota and Masuda1999; Kahl et al., Reference Kahl, Goulian, van, Herrmann, Simon, Kaplan, Peter and Cheung2000; Wesson et al., Reference Wesson, Deringer, Liou, Bayles, Bohach and Trumble2000; Smagur et al., Reference Smagur, Guzik, Magiera, Bzowska, Gruca, Thogersen, Enghild and Potempa2009).
Compared to wild-type S. aureus strains, clinical SCVs of human and bovine origin were shown to invade and persist longer inside non-phagocytic cells in culture with minimal deleterious effects compared to wild-type strains (Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006; Schröder et al., Reference Schröder, Kland, Peschel, von Eiff and Aepfelbacher2006; Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008, Reference Atalla, Gyles and Mallard2010a, Reference Atalla, Wilkie, Gyles, Leslie, Mutharia and Mallardb). Monolayer MAC-T cells with internalized SCV Heba3231 following exposure at multiplicity of infection (MOI) 100 appeared healthy with minimal loss of integrity of the cell monolayer; viable CFU were detected in cell lysates 96 h after infection. By contrast and in line with previous observations (Bayles et al., Reference Bayles, Wesson, Liou, Fox, Bohach and Trumble1998; Menzies and Kourteva, Reference Menzies and Kourteva1998) cells infected with either the parent strain 3231 or Newbould 305 underwent progressive loss of viability at 3.8 h to complete detachment of cell monolayers by 24 h after infection. There was a marked reduction in viable bacteria in cell lysates at 3.8 h and complete absence at 24 h. The minimal tissue damage caused by the SCV Heba3231 is likely related to its diminished α-toxin production as determined by phenotypic and transcriptional analyses (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008), whereas the considerable tissue damage caused by the wild-type strains with the continuous loss of viable bacteria was likely due to expression of the membrane damaging α-toxin and subsequent elimination by lysostaphin of bacteria released into the extracellular fluid.
Role of S. aureus SCVs in pathogenesis
The pathogenesis of S. aureus infections involves several stages including entry into the host, rapid replication, avoidance or subversion of innate defenses, attachment and colonization, tissue invasion and tropism and post-invasion events (Frost et al., Reference Frost, Wanasinghe and Woolcock1977; Sutra and Poutrel, Reference Sutra and Poutrel1994; Alexander and Hudson, Reference Alexander and Hudson2001; Kerro et al., Reference Kerro, van Dijk and Nederbragt2002; Garzoni and Kelley, Reference Garzoni and Kelley2009; Sinha and Fraunholz, Reference Sinha and Fraunholz2010). The outcome of the interaction between the host and a microbe is influenced by virulence that is a function of certain factors produced by the microbe (Casadevall, Reference Casadevall2008). S. aureus is equipped with several virulence factors that promote colonization, infection (caused by attachment, growth of invading bacteria and subversion of host defenses) and overt disease (often as a result of damaging toxins or induced host response).
In the case of bovine mastitis, access of S. aureus into the mammary gland often occurs as a consequence of disruption of the teat or mucosal barriers. Breaching of the protective physical barriers allows S. aureus to gain access to the sterile environment of the mammary gland either through progressive colonization of the teat canal and/or transfer with milk into the teat sinus at the end of milking (Sutra and Poutrel, Reference Sutra and Poutrel1994). While little is known about the clinical importance of S. aureus SCVs in spontaneous bovine mastitis, intramammary challenge of dairy cows through the teat canal with ~5000 CFU of SCV Heba3231 per gland-induced localized signs of mastitis in 3/5 cows and virtual absence of systemic response during the 5-days post-challenge (Atalla et al., Reference Atalla, Gyles, Wilkie, Leslie and Mallard2009). Bacterial shedding in milk from SCV Heba3231 challenged quarters was generally low and first detected at day 4 post-challenge (Atalla et al., Reference Atalla, Gyles, Wilkie, Leslie and Mallard2009). While bacteria were not detected in all challenged quarters at any of the sampling points, SCVs were recovered from the milk of three cows during the first 5 days, and at days 21 and 36 post-challenge, and of all five cows at day 14 post-challenge. Somatic cell scores were significantly higher (P<0.5) during the first week and up to day 36 post-challenge compared to day 0 before challenge (Atalla et al., Reference Atalla, Gyles, Wilkie, Leslie and Mallard2009).
Upon breaching the compromised anatomical barriers, S. aureus invades the bovine MAC-T by receptor-mediated endocytosis involving adhesion to host cells, signal transduction and cytoskeletal rearrangement and subsequent bacterial uptake (Almeida et al., Reference Almeida, Matthews, Cifrian, Guidry and Oliver1996; Bayles et al., Reference Bayles, Wesson, Liou, Fox, Bohach and Trumble1998; Dziewanowska et al., Reference Dziewanowska, Patti, Deobald, Bayles, Trumble and Bohach1999; Lammers et al., Reference Lammers, Nuijten, Kruijt, Stockhofe-Zurwieden, Vecht, Smith and van Zijderveld1999; Sinha et al., Reference Sinha, Francois, Nusse, Foti, Hartford, Vaudaux, Foster, Lew, Herrmann and Krause1999, Reference Dziewanowska, Carson, Patti, Deobald, Bayles and Bohach2000; Alexander and Hudson, Reference Alexander and Hudson2001; Menzies, Reference Menzies2003; Garzoni and Kelley, Reference Garzoni and Kelley2009). The intimate adherence of S. aureus to the MAC-T is the first and most crucial event for teat colonization and development of IMI (Frost, Reference Frost1975; Frost et al., Reference Frost, Wanasinghe and Woolcock1977; Wanasinghe, Reference Wanasinghe1981; Gudding et al., Reference Gudding, McDonald and Cheville1984; Opdebeeck et al., Reference Opdebeeck, Frost and O'Boyle1988; Sordillo et al., Reference Sordillo, Doymaz and Oliver1989; Cifrian et al., Reference Cifrian, Guidry, O'Brien, Nickerson and Marquardt1994; Hensen et al., Reference Hensen, Pavicic, Lohuis and Poutrel2000; Kerro et al., Reference Kerro, van Dijk and Nederbragt2002). The high capacity for adhesion of S. aureus to epithelial cells was linked to the high prevalence of chronic mastitis in dairy herds with relapsing acute episodes compared to the sporadic infections associated with coliforms (Frost, Reference Frost1975; Frost et al., Reference Frost, Wanasinghe and Woolcock1977).
S. aureus adhesion is mediated by the expression of several surface adhesion molecules that bind directly to host cells or through bridging ligands such as fibronectin (Fn), fibrinogen and collagen (Clarke and Foster, Reference Clarke and Foster2006). Binding of S. aureus to epithelial cells often occurs through Fn that acts as a bridging molecule between Fn-binding protein (FnBP) and host cells α5β1-integrins (Lammers et al., Reference Lammers, Nuijten, Kruijt, Stockhofe-Zurwieden, Vecht, Smith and van Zijderveld1999; Dziewanowska et al., Reference Dziewanowska, Carson, Patti, Deobald, Bayles and Bohach2000). In addition, FnBPs can bind to both heat shock protein 60 (HSP60) expressed on the cell surface and Fn linked to α5β1-integrins, or may bind directly to HSP60 independent of β1-integrins (Dziewanowska et al., Reference Dziewanowska, Carson, Patti, Deobald, Bayles and Bohach2000). The interaction between S. aureus and α5β1-integrins leads to integrin clustering that triggers a signaling cascade across the cell membrane and activation of several protein tyrosine kinases (PTK). The signal transduction leads to cytoskeletal rearrangement through polymerization of actin microfilaments and bacterial uptake in a membrane-bound vacuole via a zipper-like mechanism (Bayles et al., Reference Bayles, Wesson, Liou, Fox, Bohach and Trumble1998; Dziewanowska et al., Reference Dziewanowska, Patti, Deobald, Bayles, Trumble and Bohach1999; Sinha and Herrmann, Reference Sinha and Herrmann2005). S. aureus internalization occurs in a time-dose-dependent manner (Sinha and Herrmann, Reference Sinha and Herrmann2005).
As with the wild-type parents SCV adhesion and internalization by host cells appear to be mediated by Fn–FnBP interaction (Vaudaux et al., Reference Vaudaux, Francois, Bisognano, Kelley, Lew, Schrenzel, Proctor, McNamara, Peters and von Eiff2002; Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006; Sendi and Proctor, Reference Sendi and Proctor2009). In SCVs, surface adhesins such as FnBP and fibrinogen-binding proteins are over-expressed under the influence of increased sigB activity and down-regulation of the agr locus compared to parent strains; the enhanced expression promotes efficient adhesion and internalization by host cells (Vaudaux et al., Reference Vaudaux, Francois, Bisognano, Kelley, Lew, Schrenzel, Proctor, McNamara, Peters and von Eiff2002; Moisan et al., Reference Moisan, Brouillette, Jacob, Langlois-Begin, Michaud and Malouin2006). Noteworthy, Fn is not expressed on the apical surface of epithelial cells in the bovine mammary gland, but on the surface of myoepithelial cells located beneath the luminal epithelial cells (Lammers et al., Reference Lammers, Nuijten, Kruijt, Stockhofe-Zurwieden, Vecht, Smith and van Zijderveld1999). Accordingly, Fn may not be accessible for early tissue adhesion and establishment of IMI, instead it may play a role in the spread of infection. In addition to the role of FnBPs in the pathogenesis of S. aureus infections, the involvement of other surface proteins such as Eap that compensate for the loss of FnBPs and wall teichoic acid have been reported in successful S. aureus internalization within human eukaryotic cells (Hussain et al., Reference Hussain, Haggar, Peters, Chhatwal, Herrmann, Flock and Sinha2008; Weidenmaier and Peschel, Reference Weidenmaier and Peschel2008).
Unlike entry into cells which can be a microbe or host-active process, the intracellular survival strategy is determined largely by the microbe (Casadevall, Reference Casadevall2008). Once inside the host cell, microbial pathogens use different strategies to avoid the lethal phagolysosomal environment through preventing lysosomal fusion or acidification (e.g. Salmonella spp. and Mycobacterium tuberculosis), surviving within the harsh lysosome environment (e.g. Coxiella burnetii) or by escaping from their endocytic vesicles into the cytoplasm (e.g. Listeria monocytogenes) (Gruenheid and Finlay, Reference Gruenheid and Finlay2003; Luzio et al., Reference Luzio, Pryor and Bright2007). Despite being recognized to survive well within non-phagocytic cells (Garzoni and Kelley, Reference Garzoni and Kelley2009; Sinha and Fraunholz, Reference Sinha and Fraunholz2010), trafficking pathway studies present conflicting results regarding the fate of S. aureus enclosed within vacuoles. The different bacterial fates are likely related to strain variation and variations in their gene expression, the type of in vitro infection model, MOI and the time points at which the cells are examined (Lowy et al., Reference Lowy, Fant, Higgins, Ogawa and Hatcher1988; Almeida et al., Reference Almeida, Matthews, Cifrian, Guidry and Oliver1996; Bayles et al., Reference Bayles, Wesson, Liou, Fox, Bohach and Trumble1998; Kahl et al., Reference Kahl, Goulian, van, Herrmann, Simon, Kaplan, Peter and Cheung2000; Atalla et al., Reference Atalla, Gyles and Mallard2010a, Reference Atalla, Wilkie, Gyles, Leslie, Mutharia and Mallardb).
Recent ultrastructural analysis of MAC-T cells infected with the SCV Heba3231 and its parent strain (MOI 100) provided an insight into intracellular aspects of pathogenesis (Atalla et al., Reference Atalla, Gyles and Mallard2010a, Reference Atalla, Wilkie, Gyles, Leslie, Mutharia and Mallardb). Both SCV Heba3231 and its parent strain adhered, and were internalized within MAC-T cells following the same sequence of events including adherence of bacteria to the epithelial cell surface, formation of pseudopod-like structures around the adherent bacteria, and engulfment of bacteria within endocytic vesicles (Fig. 4a and c). Nevertheless, differences were seen in their intracellular fates. While SCV Heba3231 remained localized within the endocytic membrane at 24 h of incubation (Fig. 4b), the wild-type strain 3231 escaped from the vacuoles into the host cytosol at 3.5 h and induced complete destruction of the epithelial cells by 24 h (Fig. 4C4 and 6), similar to previous observations (Bayles et al., Reference Bayles, Wesson, Liou, Fox, Bohach and Trumble1998; Schröder et al., Reference Schröder, Kland, Peschel, von Eiff and Aepfelbacher2006). In agreement with other studies (Bayles et al., Reference Bayles, Wesson, Liou, Fox, Bohach and Trumble1998; Schröder et al., Reference Schröder, Kland, Peschel, von Eiff and Aepfelbacher2006), the highly toxigenic parent strain 3231 likely escaped from the endocytic vacuole into the cytoplasm due to the release of α-toxin and proteases; in the cytoplasm, the organisms replicate rapidly causing cell death, contributing to spread of infection and development of acute mastitis.

Fig. 4. (a) Transmission electron micrographs (TEM) of bovine mammary epithelial cells (MAC-T) infected with SCV Heba3231 (MOI 100), demonstrating the proposed sequence of events of SCV uptake: (1) SCV in close proximity with epithelial cell surface, (2) adherence of SCV to the epithelial cell surface, (3) elongation of epithelial cell surface around adherent SCV, (4) formation of pseudopod-like structure surrounding SCV and (5 and 6) one and two SCVs enclosed within a vacuole at 1 and 3.5 h. Scale bars: 1 μm. (b) TEM of MAC-T cells infected with SCV Heba3231 (MOI 100) at 24 h showing: (1) epithelial cell packed with large vacuoles each containing 5–10 bacteria, and (2) epithelial cell occupied by large spacious vacuole that incorporates small vacuoles and contains few bacteria. Bars=5 μm. (c) TEM of MAC-T cells infected with 3231 strain (MOI 100) demonstrating the proposed sequence of events of 3231 uptake: (1) adherence of 3231 to epithelial cell surface and formation of elongated appendages, (2) formation of pseudopod-like structure surrounding 3231, (3) single coccus of 3231 strain within large vacuole, (4) cocci of 3231 strain partially surrounded by a degraded vacuolar membrane – some appear to be dividing and are entirely free in the cytoplasm at 3.5 h, (5) membrane-bound vacuole (phagosome) in contact with electron dense lysosome, (6) cocci of 3231 strain released from damaged MAC-T cells at 24 h. Scale bars=1 μm.
Survival of S. aureus SCV within non-phagocytic cells is a successful strategy for persistence within the host as the intracellular compartment shields the SCV from immune defenses and antimicrobial therapy. In view of the SCV Heba3231 transcriptome and up-regulation of genes involved in alternative respiration (Atalla et al., Reference Atalla, Gyles, Jacob, Moisan, Malouin and Mallard2008), it is conceivable that internalized SCV Heba3231 use the arginine–deiminase pathway to produce ATP and the released ammonia to counteract the lethal acidic environment of the phagolysosome, contributing to SCV survival and persistence (Atalla et al., Reference Atalla, Gyles and Mallard2010a, Reference Atalla, Wilkie, Gyles, Leslie, Mutharia and Mallardb).
Conclusion
Persistent S. aureus infection has been related to the extraordinary versatility of this pathogen and the development of diverse strategies to overcome the host immune system and antimicrobial therapy. The ability of strains of S. aureus to form slow growing subpopulations called SCVs is a unique feature that may sustain intracellular persistence and modulate innate and adaptive defense mechanisms. While S. aureus SCVs have been extensively studied in human medicine and often linked to persistent and recurrent human infections, they are neglected and often overlooked in veterinary medicine.
Among the common features of S. aureus SCV are the up-regulation of genes for surface molecules that allow for intimate adherence to and internalization by host cells, and down-regulation of genes for secreted toxins and enzymes to facilitate persistence for the lifetime of the infected host cells. Internalization and persistence of the bovine SCV within MAC-T cells has major implications. First, it highlights the potential role of bovine MAC-T in persistent infection. Second, it explains the inability of antimicrobial therapy to clear IMI and of currently available vaccines to provide protection against S. aureus mastitis. Third, it indicates the need for effective CMIR and AMIR to control persistent strains, especially those that generate SCVs. Lastly, it explains the frequent failure to detect S. aureus in milk from cows with subclinical mastitis.








