Introduction
Composition of the dietary-derived lipids, specifically carbon chain length, distribution of fatty acids in triacylglycerols and degree of saturation of fat, affect lipid metabolism in piglets (Gu and Li, Reference Gu and Li2003). Medium-chain fatty acids (MCFAs) are saturated 6–12 carbon fatty acids, which occur naturally as medium-chain triglycerides (MCTs) in milk fat and various feed materials, especially coconut, palm oils and Cuphea seed oils (Graham, Reference Graham1989; Dierick et al., Reference Dierick, Decuypere, Degeyter and Dierick2003; Marten et al., Reference Marten, Pfeuffer and Schrezenmeir2006). During palm or coconut oil refining, MCT oils are produced as by-products from hydrolyzed triglycerides by re-esterification of glycerol and fatty acid distillates enriched by fractional distillation or the free fatty acids are used without further processing (Nandi et al., Reference Nandi, Gangopadhyay and Ghosh2005). Both MCFA and MCTs have specific nutritional and metabolic effects, including rapid digestion, passive absorption and obligatory oxidation, making them particularly interesting for the nutrition of young animals (Odle, Reference Odle1997). Antimicrobial effects of MCTs and MCFAs have been described as well as a protective effect on the intestinal microarchitecture based on studies in pigs (Dierick et al., Reference Dierick, Decuypere, Degeyter and Dierick2003). MCFAs have also been suggested to have immune-modulating effects (Wang et al., Reference Wang, Wu, Simonavicius, Tian and Ling2006), but evidence from the pig is lacking (Buchheit, Reference Buchheit2009).
The weaner piglet is an ideal target for exploiting the nutritional and physiological effects of MCFA and MCTs in the post-weaning period. In piglets, the changes associated with the critical post-weaning phase often result in reduced feed intake, causing energy deficiency, modifications in the intestinal morphology, reduced absorptive and barrier functions, impaired immune reactivity and altered composition of the intestinal microbiota (Pluske et al., Reference Pluske, Hampson and Williams1997; Konstantinov and Smidt, Reference Konstantinov, Smidt, Mengheri, Britti and Finamore2006; Lallès et al., Reference Lallès, Bosi, Smidt and Stokes2007). The gradual decrease in maternal immunoglobulins from the sow's colostrum (Rooke and Bland, Reference Rooke and Bland2002), the not yet fully developed active immune system of piglets (Scharek and Tedin, Reference Scharek and Tedin2007; Butler et al., Reference Butler, Lager, Splichal, Francis, Kacskovics, Sinkora, Wertz, Sun, Zhao, Brown, Dewald, Dierks, Muyldermans, Lunney, McCray, Rogers, Welsh, Navarro, Klobasa, Habe and Ramsoondar2009) and the continuous contact with new microbes from the environment are factors associated with a high risk of gastrointestinal disease, which can cause diarrheal disorders and considerable piglet losses (Lalles et al., Reference Lalles, Bosi, Smidt and Stokes2007).
The administration of MCFAs appears to provide a promising approach to reduce complications associated with the post-weaning phase in piglets. The modes of action, however, are not fully understood. This review article is focused on the mechanisms of action and the nutritional and physiological efficacy of MCFAs that are of particular interest in piglet nutrition and may serve as an alternative, non-antibiotic approach to improve performance and health.
Chemical structure and occurrence of MCFAs
MCFAs are saturated and unbranched monocarboxylic acids. This group consists of caproic acid (C6:0, hexanoic acid), caprylic acid (C8:0, octanoic acid) and capric acid (C10:0, decanoic acid). Lauric acid (dodecanoic acid) with 12 carbon atoms is also often classed with the group of MCFAs (Bach and Babayan, Reference Bach and Babayan1982). All naturally occurring fatty acids are composed of C2-units (acetyl-CoA) and thereby have an even number of carbon atoms.
Due to a shorter hydrocarbon chain length compared to long-chain fatty acids, MCFAs have a low melting point and a comparatively high solubility in water. At a neutral pH, the MCFAs are mostly dissociated (ionized) (Bach and Babayan, Reference Bach and Babayan1982). The standard chemical properties of caproic, caprylic, capric and lauric acid are summarized in Table 1.
Table 1. Chemical properties of caproic, caprylic, capric and lauric acids

pK a, negative logarithm of the acid dissociation constant.
References: HSDB (2011), Hsiao and Siebert (Reference Hsiao and Siebert1999).
For young animals, the milk of their mothers is a crucial source of MCFAs, occurring in varying concentrations depending on the species. High concentrations of MCFAs are found in the milk of the mouse, rat, rabbit, goat, horse and elephant. Cow and sheep milk and human breast milk contain only small amounts of MCFAs. Merely trace amounts can be detected in the milk of the sow, the camel and the guinea pig (Witter and Rook, Reference Witter and Rook1970; Decuypere and Dierick, Reference Decuypere and Dierick2003). It is not fully understood as to why MCFA concentrations differ among species; however, species-specific discrepancies in the activities of tissue-specific thioesterases appear to be responsible for the varying efficacies in synthesis (Libertini and Smith, Reference Libertini and Smith1978; Rudolph et al., Reference Rudolph, Neville and Anderson2007). MCFAs occur naturally as parts of triglycerides in various vegetable fats/oils, particularly, coconut and palm. Typically, MCFA content in coconut oil is high; of the oil fraction 3.4–15% is composed of caprylic acid (C8:0), 3.2–15% of capric acid (C10:0) and 41–56% of lauric acid (C12:0). High contents of caprylic (2.4–6.2%), capric (2.6–7.0%) and lauric acid (41–55%) can also be found in palm kernel oil (Young, Reference Young1983). Cuphea seeds (family of loosestrife) have a broad species-dependent diversity in MCFA. Oil from Cuphea seeds is a source with extraordinarily high content of MCFA, showing a remarkable diversity in composition between species (Graham et al., Reference Graham, Hirsinger and Röbbelen1981). Cuphea lanceolata and Cuphea ignea oils containing over 80% capric acid and only small amounts of caprylic acid have been used in studies as sources of MCTs in piglets (Dierick et al., Reference Dierick, Decuypere, Degeyter and Dierick2003).
Digestion, absorption and enterocyte metabolism of MCFAs
As a result of their chemical and physical properties, MCTs differ significantly from long-chained triglycerides (LCTs) with regard to digestion, absorption and metabolism. Due to the relative insolubility of LCT and long chain fatty acid (LCFA), bile salts play a significant role in the emulsification of LCT, permitting efficient hydrolysis by pancreatic lipases and the formation of micelles containing LCFA and monoglyceride digestion products. Micelles deliver these products to the brush border membrane permitting passive diffusion into the enterocyte. Re-esterification of LCFA and monoglycerides to triglycerides takes place within the enterocytes. The re-formed triglycerides reach the lymph, the thoracic duct and finally the bloodstream via water-soluble lipoproteins (chylomicrons).
The hydrolysis of MCTs occurs rapidly in comparison with that of LCTs and due to higher water solubility, without the necessity for emulsion with bile. Lingual and gastric lipases are initially active against MCT in the stomach generating significant MCFAs and monoglycerides prior to release into the duodenum. With the addition of pancreatic lipases in the duodenum, MCFAs are made rapidly available for absorption in the upper small intestine (Ramirez et al., Reference Ramirez, Amate and Gil2001). Most of the MCFAs are absorbed by passive diffusion in their free form, but absorption as acylester was also demonstrated (Carvajal et al., Reference Carvajal, Nakayama, Kishi, Sato, Ikeda, Sugano and Imaizumi2000). Within the enterocyte, MCFAs have a low affinity for fatty acid binding protein and are therefore largely not re-esterified but diffuse in to portal blood and associate with albumin for transport directly to the liver (Bloch, Reference Bloch1974; Guillot et al., Reference Guillot, Vaugelade, Lemarchal and Rerat1993, Reference Guillot, Lemarchal and Dhorne1994). Only a small portion of the MCFAs is taken up by the chylomicrons (Greenberger and Skillmann, Reference Greenberger and Skillmann1969; Bach and Babayan, Reference Bach and Babayan1982).
Hydrolysis of MCT may not be necessary for absorption to occur. In the presence of a digestive disorder such as pancreatic insufficiency, intact MCTs are partially taken up by the enterocytes and cleaved hydrolytically within the cells (Playoust and Isselbacher, Reference Playoust and Isselbacher1964; Valdivieso, Reference Valdivieso1972). Evidence for this differentiated absorptive mechanism was found in experiments using fistulated pigs, as a biphasic concentration curve was observed in the portal blood subsequent to repeated infusions of MCTs into the duodenum. An initial increase in concentration was observed 15 min after the infusion was administered, probably indicating the direct absorption of MCFAs. The second peak was detected after 75–95 min, indicating a slower process, for instance, direct absorption of MCTs by enterocytes and subsequent hydrolysis in the enterocyte (Guillot et al., Reference Guillot, Vaugelade, Lemarchal and Rerat1993, Reference Guillot, Lemarchal and Dhorne1994).
Intermediary metabolism of MCFAs
It is clear that the majority of MCFAs are efficiently used for energy production by mitochondrial β-oxidation after portal transport to the liver (Wojtczak and Schönfeld, Reference Wojtczak and Schönfeld1993; Turner et al., Reference Turner, Hariharan, Tidang, Frangioudakis, Beale, Wright, Zeng, Leslie, Li, Kraegen, Cooney and Ye2009). Most of the absorbed MCFAs are bound to serum albumin with a high affinity and capacity during direct transport to the liver in portal blood (Ashbrook et al., Reference Ashbrook, Spectro, Fletcher, Ashbrook, Spectro and Fletcher1972; Kenyon and Hamilton, Reference Kenyon and Hamilton1994). Once in the liver, the MCFAs can pass through the mitochondrial membrane independently of the carnitine palmitoyltransferase (Sidossis et al., Reference Sidossis, Stuart, Shulman, Lopaschuk and Wolfe1996; Rasmussen et al., Reference Rasmussen, Holmback, Volpi, Morio-Liondore, Paddon-Jones and Wolfe2002). Before oxidation, the MCFAs are activated by the medium-chain octanoyl-CoA synthetase, stimulated by carnitine in piglets (van Kempen and Odle, Reference Van Kempen and Odle1993). Since MCFA freely enter hepatocyte mitochondria compared to LCFA, they are thought to be more prone to ketone body synthesis (Odle, Reference Odle1997) resulting in increased plasma ketones that may serve as energy substrate for peripheral tissues. Swine are able to activate MCFAs to CoA thioester in colonocytes using the MCFA:CoA ligase, and to utilize them in their intestinal epithelium for energy production (Vessey, Reference Vessey2001). Due to the low concentrations in the digesta arising from fermentation, the contribution of MCFA to the energy supply of the distal epithelium is probably of minor significance compared to butyrate, the main energy yielding substrate. Depending on substrate availability, intramitochondrial β-oxidation results in energy production and represents the main metabolic pathway of the MCFAs (Bach and Babayan, Reference Bach and Babayan1982; Odle, Reference Odle1997). A small portion of absorbed MCFA is stored in adipose tissue (Sarda et al., Reference Sarda, Lepage, Roy and Chessex1987) or is elongated to LCFAs and serves to resynthesize triglycerides (Hill et al., Reference Hill, Peters, Swift, Yang, Sharp, Abumrad and Greene1990; Carnielli et al., Reference Carnielli, Rossi, Badon, Gregori, Verlato, Orzali and Zacchello1996).
Studies on energetics in newborn piglets clearly demonstrated a superior energetic exploitation of the MCFAs in comparison with the LCFAs. Compared with values obtained subsequent to the feeding of LCTs, the plasma concentrations for 3-hydroxybutyric acid increased more significantly after the application of MCT (Odle et al., Reference Odle, Benevenga and Crenshaw1989). In 1-day-old piglets, the plasma concentration of hydrobutyric acid thereby is negatively correlated with the chain length. Odd chain MCFA (C7–C9) were utilized more efficiently by hepatocytes than C8 and especially C10, probably due to the effects on propionyl-CoA metabolism (Odle et al., Reference Odle, Benevenga and Crenshaw1991b). Results from in vitro studies using isolated hepatocytes support these findings, indicating that the metabolism is dependent on chain length, as shorter fatty acids were metabolized to CO2 as well as other degradation products such as ketone bodies 40% more rapidly compared with longer-chain fatty acids (Odle et al., Reference Odle, Benevenga and Crenshaw1991a).
MCFAs as energy sources in piglets
MCFAs represent immediately available sources of energy that can be supplemented to the diets of neonates and young animals to improve their energy supply. In patients, MCFAs are administered in the treatment of diseases such as lipid absorption disorders, malabsorption syndromes, pancreatic insufficiency, disorders of the gall bladder, gastroenteritis, diabetes mellitus and as a source of energy in premature babies (Borum, Reference Borum1992; Heird et al., Reference Heird, Jensen, Gomez, Heird, Jensen and Gomez1992). A high postnatal capacity to oxidize fatty acids was identified in many species including newborn and young piglets (van Kempen and Odle, Reference van Kempen and Odle1993; Odle et al., Reference Odle, Lin, Wieland and Kempen1994; Heo et al., Reference Heo, Lin, Han and Odle2002). These energy-providing properties of MCTs are of high interest for the nutrition of young pigs.
The feeding of MCTs to sows in the late gestation phase in comparison with LCTs increased the survival rate of neonatal piglets (Newcomb et al., Reference Newcomb, Harmon, Nelssen, Thulin and Allee1991; Azain, Reference Azain1993; Jean and Chiang, Reference Jean and Chiang1999). In one study, supplementation of gestating sows with diets containing 10% MCTs (weight basis) did not result in changes in the birth weights or the number of live piglets per sow but did increase the survival rate of the piglets after 3 days of age and beyond the weaning phase. Improvement in survival rate was greatest in piglets with a birth weight of less than 900 g (Azain, Reference Azain1993). Further, increased blood glucose levels on the day of birth indicated improved energy status of the piglets, possibly accounting for the increased neonatal survival rate.
Given the metabolism of MCFA by liver and limited inclusion in chylomicrons, supplementation of lactating sows with MCT would be unlikely to markedly impact on milk MCFA content as a means of supplementing suckling pigs. Accordingly, MCT-supplemented diets only led to minor changes in the milk fat composition, indicating that no connection could be established between the MCFA content in the milk of the sows and the increased survival rate of the piglets (Azain, Reference Azain1993). The direct application of MCTs to suckling piglets resulted in an improved energy balance in neonatal animals; however, the survival rate of the piglets may not be improved (Odle, Reference Odle1997).
In weaned pigs, the combined dietary supplementation of MCTs with different lipases resulted in an increase in the daily live weight gain (Dierick et al., Reference Dierick, Decuypere, Molly, Beek and Vanderbeke2002). An examination of the effects of various sources of fat (MCT, soybean oil and animal fat) on weight gain, feed intake and feed conversion demonstrated that the dietary inclusion of 5% MCTs (weight basis) within the first 14 days post-weaning provided the greatest increase in weight gain and a better feed conversion of the piglets (Dove, Reference Dove1993). In contrast, the formulation of diets with free MCFAs in this context often led to a reduction in feed intake (Odle et al., Reference Odle, Benevenga and Crenshaw1991a, Reference Odle, Benevenga and Crenshawb; Decuypere and Dierick, Reference Decuypere and Dierick2003). The intense, goatish smell of the non-esterified free fatty acids and the low tolerance to changes in taste may be factors that contribute to decreased acceptance (Decuypere and Dierick, Reference Decuypere and Dierick2003). In an experiment with 21-day-old piglets 8% lipids from a medium-chain free fatty acid mixture (60% C 8:0, 40% C 10:0) were compared with beef tallow. Although this level of MCFA must have resulted in an extremely strong odor, compared to a beef tallow group, feed intake was reduced by only 4% while growth rate was 6.3% better (Cera et al., Reference Cera, Mahan and Reinhart1989). Furthermore, the MCFAs can induce the secretion of cholecystokinin and possibly other intestinal hormones, which influence the feeling of satiety and therefore feed intake (Mabayo et al., Reference Mabayo, Furuse, Yang and Okumura1992). However, more recent studies attribute only a minor influence of the MCFAs on the secretion of cholecystokinin (Symersky et al., Reference Symersky, Vu, Frolich, Biemond and Masclee2002).
Influence of MCFAs on the intestinal morphology and physiology and on the gut-associated immune system
The transitional reduction of intestinal integrity in the piglet post-weaning is associated with decreased digestive capacity, mainly due to shorter villi in the small intestine and reduced digestive enzyme activity (Miller et al., Reference Miller, James, Smith and Bourne1986; van Dijk et al., Reference Van Dijk, Niewold, Nabuurs, Hees, Bot, Stockhofe-Zurwieden, Ubbink-Blanksma and Beynen2002; Montagne et al., Reference Montagne, Boudry, Favier, Huerou-Luron, Lalles and Seve2007). The morphological and physiological changes are reflected in a decline in growth rates, an increased susceptibility to enteric diseases and immunosuppression (Bailey et al., Reference Bailey, Clarke, Wilson, Williams and Stokes1992; Lalles et al., Reference Lalles, Bosi, Smidt and Stokes2007). MCFAs can be utilized directly by the enterocytes for energy production and thereby help to support the integrity of the intestinal tissue in post-weaning piglets (Guillot et al., Reference Guillot, Vaugelade, Lemarchal and Rerat1993). Feeding of MCTs to rats positively influenced the intestinal morphology, resulting in an augmentation of mucosa, a higher phospholipid/protein ratio in jejunal mucosal microvillus lipids, longer intestinal villi and shorter crypts, and increased activity of membrane-bound enzymes (Takase and Goda, Reference Takase and Goda1990). Similar results were achieved using MCFAs in swine; a significant increase in the length of the villi in the small intestine combined with a lower crypt depth and a lower number of intraepithelial lymphocytes (IELs) were observed (Dierick et al., Reference Dierick, Decuypere, Degeyter and Dierick2003). Villus length and crypt depth are often used as indicators for the evaluation of the mucosal turnover. A reduced number of IELs could reflect a lower apoptosis rate and be associated with the increase in villus length and the decrease in crypt depth or altered immune surveillance. However, little is known about the influence of MCFAs on gut-associated and systemic immune reactions and the results do not allow a concise conclusion on the direction of immunomodulatory mechanisms.
MCFAs can act as ligands for the orphan receptor GPR84, a signaling protein highly expressed in immune cells. The activation of GPR84 in monocytes and macrophages enhanced lipopolysaccharide (LPS)-stimulated interleukin (IL)-12 p40 production, indicating a mechanism linking free fatty acids with immune reactions (Wang et al., Reference Wang, Wu, Simonavicius, Tian and Ling2006). Although MCFAs are readily absorbed in the upper intestine, colonic cells were used to study the impact of MCFA on ILs. Capric acid enhanced IL-8 production in human Caco-2 cells (Tanaka et al., Reference Tanaka, Saitoh, Tabata, Matsuse, Kojima, Sugi, Nakagawa, Kayazawa, Teranishi, Uchida, Hirata and Katsu2001), while caprylic acid and MCT suppressed IL-8 secretion in Caco-2 cells after 24 h preincubation by inhibition of the IL-8 promoter (Hoshimoto et al., Reference Hoshimoto, Suzuki, Katsuno, Nakajima and Saito2002). Rats fed MCTs through a feeding tube showed a significant increase in the expression of IL-6, followed by the secretion of immunoglobulin A (IgA) after the injection of bacterial LPS (Kono et al., Reference Kono, Fujii, Asakawa, Maki, Amemiya, Hirai, Matsuda and Yamamoto2004). In the same study, the LPS-induced expression of proinflammatory cytokines and chemokines (tumor necrosis factor-α (TNF-α), IL-18, macrophage inflammatory protein-2 and monocyte chemoattractant protein-1) was significantly lowered by MCTs and the expression of the immune modulating and anti-inflammatory cytokine IL-10 in the ileum and Peyer's patches was significantly greater in the MCT group. The expression of interferon-γ (IFN-γ) was also blunted by MCTs. The secretion of IgA and the modulation of the cytokine release subsequent to the LPS injection were suggested to mediate positive effects on the intestinal health of the animals (Kono et al., Reference Kono, Fujii, Asakawa, Maki, Amemiya, Hirai, Matsuda and Yamamoto2004).
Dendritic cells isolated from the thoracic duct lymph showed similar phagocytic activity independently whether long-chain (arachidonic or oleic acid) or caprylic acid was added to an ex vivo culture, but the long-chain fatty acids suppressed Major Histocompatibility Complex (MHC) class II molecule expression. This might be considered as an indicator of better antigen presentation ability and immune response of the gut-associated lymphatic tissue when MCFAs are fed (Tsuzuki et al., Reference Tsuzuki, Miyazaki, Matsuzaki, Okada, Hokari, Kawaguchi, Nagao, Itoh and Miura2006). Murine-stimulated IELs had reduced IFN-γ production when exposed to long-chain fatty acids, while caprylic acid did not cause changes in IFN-γ production (Hara et al., Reference Hara, Miura, Komoto, Inamura, Koseki, Watanabe, Hokari, Tsuzuki, Ogino, Nagata, Hachimura, Kaminogawa and Ishii2003). In piglets, a mixture of MCFA (caprylic and capric acid in a mixture at 0.15% in the diet) did not affect the phagocytic activity of isolated blood-neutrophils or the relative proportions of CD4−CD8+, CD4+CD8− and CD4+CD8+ lymphocytes and MHC−CD21+, MHC+CD21− and MHC+CD21+ cells in the blood or the mesenteric lymph nodes. Ileal tissue samples from piglets fed MCFA did not display changes in mRNA expression of makrophage inflammatory protein (MIP1-β), IL-1β, IFN-γ, TNF-α and monocyte chemoattractant protein (MCP-1) compared to control animals (Buchheit, Reference Buchheit2009).
Influence of MCFAs on the intestinal microbiota
Due to their antibacterial effects, MCFAs were initially used in the preservation of feed, specifically in silage (Woolford, Reference Woolford1975) and foods (Freese et al., Reference Freese, Sheu and Galliers1973). Many in vitro studies have evidenced that MCFAs and their monoglycerides are able to inactivate pathogenic bacteria, viruses and parasites. MCFA were mainly considered to be anionic surfactants, which, as a result of this property, have antibacterial effects (Mroz et al., Reference Mroz, Koopmans, Bannink, Partanen, Krasucki, Overland, Radcliffe, Mosenthin, Zentek and Zebrowska2006). Membrane destabilization by the incorporation of MCFAs into the bacterial cell wall and cytoplasmic membrane, as well as the inhibition of bacterial lipases, which are necessary for the colonization of the skin and the intestinal mucosa, may be the cardinal mechanisms (Isaacs et al., Reference Isaacs, Litov and Thormar1995; Bergsson et al., Reference Bergsson, Arnfinnsson, Karlsson, Steingrímsson and Thormar1998, Reference Bergsson, Steingrímsson and Thormar2002). Changes in the elementary bodies, the infectious particles, were detected by electron microscopy following the treatment of Chlamydia trachomatis with monocapring; however, the host cell was not impaired (Bergsson et al., Reference Bergsson, Arnfinnsson, Karlsson, Steingrímsson and Thormar1998). Besides the direct lytic effects of MCFA, the activation of bacterial autolytic enzymes might also play a role in the activity against pathogens (Tsuchido et al., Reference Tsuchido, Hiraoka, Takano and Shibasaki1985).
Alternatively, the uptake of undissociated fatty acids into the bacterial cell appears to have cytotoxic effects. The MCFAs dissociate into protons and anions in the basic cytoplasm of the cell, decreasing the pH. Cytoplasmic enzymes are inactivated as a result, leading to the death of the bacterial cell (Freese et al., Reference Freese, Sheu and Galliers1973; Hsiao and Siebert, Reference Hsiao and Siebert1999). In vitro studies showed a negative correlation between increasing pH values and the efficacy of the MCFAs, indicating that the antibacterial effects depend on the degree of dissociation of the fatty acids. The undissociated form generally has stronger effects. The pH of the surrounding environment is therefore of considerable importance and appears to significantly influence the efficacy of the MCFAs (Hsiao and Siebert, Reference Hsiao and Siebert1999; Sun et al., Reference Sun, O'Connor and Roberton2002). It can be assumed that the antibacterial effect of the MCFAs in the gastrointestinal tract is limited to the stomach and the proximal small intestine (duodenum), as MCFAs are rapidly absorbed and predominantly found in the dissociated form at neutral pH. The undissociated form is found at pH between 3 and 6 (Dierick et al., Reference Dierick, Decuypere, Molly, Beek and Vanderbeke2002). Therefore, it can be speculated that targeted acidification of the stomach, for instance, by using diets with low buffering capacity and including organic acids may improve the antibacterial effects of MCFAs.
In vitro studies have demonstrated that MCFAs and their monoglycerides inactivate bacteria, viruses and parasites. The results of these studies are shown in Tables 2–4 for caprylic and capric acid.
Table 2. Overview of the effects of caprylic and capric acids on selected Gram-positive bacteria in in vitro experiments
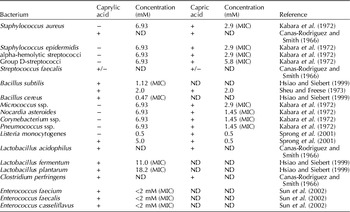
+, Inhibition; −, no inhibition; +/−, marginal inhibition; ND, no data; MIC, minimal inhibitory concentration; mM, millimoles per liter.
Table 3. Overview of the effects of caprylic and capric acids on selected Gram-negative bacteria in in vitro experiments

+, Inhibition; −, no inhibition; +/−, marginal inhibition; ND, no data; MIC, minimal inhibitory concentration.
Table 4. Overview of the effects of caprylic and capric acids on viruses, Candida albicans and Giardia lamblia in in vitro experiments

a Except for G. lamblia, where the data are expressed as μM for the LD50.
b Except for respiratory syncytial virus, where the data are expressed as M and for G. lamblia, where the data are expressed as μM for the LD50.
+, Inhibition; −, no inhibition; +/−, marginal inhibition; ND, no data available; mM, millimoles per liter; μM, micromoles per liter; LD50, median lethal dose.
The results summarized in Tables 2–4 are partly contradictory. In most studies, caprylic and capric acid were effective against a number of Gram-positive bacteria species. However, efficacy of these acids against Gram-negative bacteria was observed only rarely and inconsistently. In addition, many studies found lauric acid (C12:0) to have a pronounced antibacterial effect, which in some cases surpassed the effects of the other free fatty acids (Canas-Rodriguez and Smith, Reference Canas-Rodriguez and Smith1966; Sun et al., Reference Sun, O'Connor and Roberton2002). Lauric acid is especially effective against Gram-positive bacteria. The monoglycerides of capric and lauric acid seemed considerably more effective against bacteria than were their free fatty acids in a number of studies (Kabara et al., Reference Kabara, Swieczkowski, Conley and Truant1972; Bergsson et al., Reference Bergsson, Arnfinnsson, Karlsson, Steingrímsson and Thormar1998, Reference Bergsson, Steingrimsson and Thormar1999, Reference Bergsson, Steingrímsson and Thormar2002; Petschow et al., Reference Petschow, Batema, Talbott and Ford1998; Sprong et al., Reference Sprong, Hulstein and Van der Meer1999). Results for relevant pathogenic bacteria such as Escherichia coli and Salmonella Enteritidis, the former being specifically important for weaned piglets, are highly inconsistent, which is most likely the result of the use of different concentrations, culture media, strains of bacteria and pH values. For example, in one study, caprylic and capric acids had no effect on Gram-negative bacteria at a concentration of 6.93 mM (Kabara et al., Reference Kabara, Swieczkowski, Conley and Truant1972). However, others attained a significant reduction of E. coli and Salmonella Enteritidis using capric acid at a concentration of 0.5 mM (Sprong et al., Reference Sprong, Hulstein and Van der Meer2001). In contrast, the minimum inhibitory concentration of caprylic acid for E. coli was reported as 12 mM (1.73 g/l) (Hsiao and Siebert, Reference Hsiao and Siebert1999). Caprylic acid was effective at inhibiting Salmonella Enteritidis only at higher concentrations (10 mM) (van Immerseel et al., Reference Van Immerseel, Buck, Boyen, Bohez, Pasmans, Volf, Sevcik, Rychlik, Haesebrouck and Ducatelle2004).
In combination, caprylic and capric acids have synergistic effects. Under simulated pig gastric conditions a total concentration of 0.35 g MCFAs (C8:0+C10:0) per 100 g medium at a pH of 5 resulted in a decline of the bacterial flora by 1-log; the proportions of the individual fatty acids were not crucial for the efficacy (Dierick et al., Reference Dierick, Decuypere, Molly, Beek and Vanderbeke2002). The inhibition of Salmonella Typhimurium and coliforms by 15 mM caprylate was also demonstrated using a porcine continuous culture system, simulating the porcine caecum; bifidobacteria and streptococci were less affected (Messens et al., Reference Messens, Goris, Dierick, Herman and Heyndrickx2010).
MCFA activities described in vitro were also characterized in feeding studies in milk-fed animals. During the search for antimicrobial factors in milk, it was found that rabbit milk fat, having about 40% MCFAs (Maertens, Reference Maertens1998), neutralized bacteria isolated from the stomach, with caprylic and capric acids showing the highest activity. No effect could be observed in the digesta of the small intestine (Canas-Rodriguez and Smith, Reference Canas-Rodriguez and Smith1966). Milk fat of the rabbit showed a higher antibacterial effect against Staphylococcus aureus, Candida albicans, Lactobacillus acidophilus and Clostridium perfringens, in comparison with E. coli and Streptococcus faecalis.
Other in vitro studies confirmed the antibacterial activities using MCTs in combination with lipases in a test model involving weaned piglets. A 10-fold reduction of the total anaerobic bacteria plate count, and viable counts for lactobacilli and E. coli was achieved in the digesta of the stomach and duodenum by MCFAs and microbial lipase (Dierick et al., Reference Dierick, Decuypere, Molly, Beek and Vanderbeke2002). Besides the effects on the luminal bacteria, it seems interesting to consider the impact on bacterial adherence. Sodium caproate was found to prevent the adhesion to and subsequent invasion of the ileal mucosa in rats by Salmonella Typhimurium (Cox et al., Reference Cox, Rawlinson, Baird, Bzik and Brayden2008). Furthermore, this study demonstrated a bactericidal effect against Salmonella Typhimurium, depending on the concentration of the MCFA. After the simultaneous oral application of capric acid and Vibrio cholerae to mice, the pathogen could not be detected in the ileum or the caecum, suggesting a potential prophylactic use against intestinal infections (Petschow et al., Reference Petschow, Batema, Talbott and Ford1998). Two reports suggest that Campylobacter infection can be controlled in poultry by feeding caprylic acid beginning at 1 day of age (Solis de Los Santos et al., Reference Solis De Los Santos, Donoghue, Venkitanarayanan, Dirain, Reyes-Herrera, Blore and Donoghue2008, Reference Solis De Los Santos, Hume, Venkitanarayanan, Donoghue, Hanning, Slavik, Aguiar, Metcalf, Reyes-Herrera, Blore and Donoghue2010).
Effects on performance of piglets
The potential use of MCFAs as rapidly available and easily metabolizable sources of energy has been examined in the feeding of pigs in which the MCFAs were usually administered as intact MCTs in the feed. Performance of artificially reared suckling piglets was reduced using a diet containing 24% but not 13.5% MCTs (Newport et al., Reference Newport, Storry and Tuckley1979). The results of studies using weaned piglets have been inconsistent. No differences occurred with regard to feed intake, feed efficiency and weight gain when comparing a diet with 10% MCTs versus diets containing the same amount of tallow, pig fat or corn oil (Allee et al., Reference Allee, Romsos, Leveille and Baker1972). In contrast, significantly higher (6–8% in average) growth rates were found for the MCTs in a study comparing rations with three different copper levels and 5% lipid from MCT with soya oil or animal fats (Dove, Reference Dove1993). Also, a 10% increase in the daily weight gain was found in piglets fed 2.5% MCTs selectively processed from coconut oil (enriched for capric acid) compared to soybean oil (Dierick et al., Reference Dierick, Decuypere, Molly, Beek and Vanderbeke2002). The growth response was observed for MCTs whether or not it was supplemented with lipase, although addition of lipase resulted in more dramatic effects on gastric and duodenal bacterial populations.
Although the evidence for the favorable energetic attributes of MCT is strong, many authors consider that the mechanisms resulting in improved piglet performance are associated with the antibacterial effects of the MCFAs in the intestinal lumen, specifically against pathogenic strains (Decuypere and Dierick, Reference Decuypere and Dierick2003). Others have associated performance effects with the direct and indirect influence of MCFA on epithelial function (villus length, crypt depth) in the upper small intestine. An increased absorptive surface could facilitate increased uptake and a more efficient utilization of nutrients for growth.
Toxicity of MCFAs
Many studies have investigated potential oral, parenteral and dermal toxicity using laboratory animals and humans; however, the findings have unanimously indicated a low toxicity. Diets containing MCTs comprising up to 15% of the calories or up to 50% of the total fat are considered to have a low toxicity or to be non-toxic (Traul et al., Reference Traul, Driedger, Ingle and Nakhasi2000). In neonatal piglets, these effects may be ketogenic or narcotic (Lin et al., Reference Lin, Chiang and Lee1995). To date, no indications have been found for an allergenic potential. However, the application of higher doses led to weak irritation of the mucosa of the eyes and the skin (Traul et al., Reference Traul, Driedger, Ingle and Nakhasi2000). Stagnation in growth and morphological changes were observed in mammalian cell cultures (HeLa, human fibroblasts and murine neuroblastoma cells) treated with millimolar concentrations of C6:0 and C10:0 fatty acids, which may be the result of structural alterations of the cell membrane (Sheu et al., Reference Sheu, Salomon, Simmons, Sreevalsan and Freese1975). The results of the in vitro studies are important for the characterization of potential toxic effects. However, it must be noted that the results of in vitro models cannot be readily extrapolated to in vivo situations. The cytotoxic effects that have been mentioned may not be of consequence in the living organism as possible negative side effects may be neutralized by the complex interactions of physiological factors such as digesta, mucins and serum.
Conclusion
In conclusion, MCFAs and MCTs can be used in piglet nutrition to improve performance parameters including piglet survival, feed intake and feed-to-gain ratio. Inclusion levels of MCTs up to 15% are possible, whereas dietary addition of free MCFA is limited due to their negative sensory effects. Inclusion levels of 8% were described in diets for young pigs. There is good evidence indicating that MCFAs offer a highly available and efficient energy source as compared to LCT associated with their passive absorption, portal transport to liver and efficient oxidation. The contribution of the antimicrobial and immunomodulatory properties of MCFAs to the observed performance responses are less clear and may warrant further characterization.






