Pestivirus infections in cattle are of major concern worldwide as they lead to significant economic losses for both dairy and beef producers. Pestiviruses compose a genus within the family Flaviviridae. Within this genus are four recognized species, bovine viral diarrhea virus types 1 and 2 (BVDV-1 and BVDV-2), classical swine fever virus (CSFV) and border disease virus (BDV) (Simmonds, Reference Simmonds2011). In addition to the recognized species, four additional Pestivirus species have been proposed. These putative species include Giraffe virus, Pronghorn virus, Bungowannah virus, and HoBi-like viruses (Liu et al., Reference Liu, Xia, Wahlberg, Belak and Baule2009b; Bauermann et al., Reference Bauermann, Ridpath, Weiblen and Flores2013). Although the first three cited putative pestivirus species are geographically restricted, the distribution of HoBi-like viruses has reached a worldwide scale (Vilcek et al., Reference Vilcek, Ridpath, Van Campen, Cavender and Warg2005; Cortez et al., Reference Cortez, Heinemann, De Castro, Soares, Pinto, Alfieri, Flores, Leite and Richtzenhain2006; Kirkland et al., Reference Kirkland, Frost, Finlaison, King, Ridpath and Gu2007; Liu et al., Reference Liu, Kampa, Belak and Baule2009a; Bianchi et al., Reference Bianchi, Martins, Weiblen and Flores2011; Decaro et al., Reference Decaro, Lucente, Mari, Cirone, Cordioli, Camero, Sciarretta, Losurdo, Lorusso and Buonavoglia2011; Mishra et al., Reference Mishra, Rajukumar, Pateriya, Kumar, Dubey, Behera, Verma, Bhardwaj, Kulkarni, Vijaykrishna and Reddy2014).
Viruses of this group are also referred as BVDV-3 or atypical pestiviruses, and have been isolated on multiple occasions from South American and Asian cattle herds (Kampa et al., Reference Kampa, Alenius, Emanuelson, Chanlun and Aiumlamai2009; Liu et al., Reference Liu, Kampa, Belak and Baule2009a; Bauermann et al., Reference Bauermann, Ridpath, Weiblen and Flores2013; Haider et al., Reference Haider, Rahman, Khan, Mikolon, Gurley, Osmani, Shanta, Paul, Macfarlane-Berry, Islam, Desmond, Epstein, Daszak, Azim, Luby, Zeidner and Rahman2014; Mishra et al., Reference Mishra, Rajukumar, Pateriya, Kumar, Dubey, Behera, Verma, Bhardwaj, Kulkarni, Vijaykrishna and Reddy2014; Weber et al., Reference Weber, Mosena, Simoes, Almeida, Pessoa, Budaszewski, Silva, Ridpath, Riet-Correa, Driemeier and Canal2014). After the identification of HoBi-like viruses in Brazilian fetal bovine serum (FBS) (Schirrmeier et al., Reference Schirrmeier, Strebelow, Depner, Hoffmann and Beer2004), the virus was described, by report chronology, in Thailand (Kampa et al., Reference Kampa, Alenius, Emanuelson, Chanlun and Aiumlamai2009; Liu et al., Reference Liu, Kampa, Belak and Baule2009a), Italy (Decaro et al., Reference Decaro, Lucente, Mari, Cirone, Cordioli, Camero, Sciarretta, Losurdo, Lorusso and Buonavoglia2011), Bangladesh (Haider et al., Reference Haider, Rahman, Khan, Mikolon, Gurley, Osmani, Shanta, Paul, Macfarlane-Berry, Islam, Desmond, Epstein, Daszak, Azim, Luby, Zeidner and Rahman2014), and India (Mishra et al., Reference Mishra, Rajukumar, Pateriya, Kumar, Dubey, Behera, Verma, Bhardwaj, Kulkarni, Vijaykrishna and Reddy2014) (Fig. 1). So far, no identification of infected calves in Australia has been reported, although identification of HoBi-like virus in FBS lots labeled as originating and processed in that country has been described (Xia et al., Reference Xia, Larska, Giammarioli, De Mia, Cardeti, Zhou, Alenius, Belak and Liu2013). Similarly, two lots of FBS labeled as originating in North American but processed in Europe were positive for HoBi-like viruses (Xia et al., Reference Xia, Vijayaraghavan, Belak and Liu2011). A follow-up investigation sampling only FBS lots both originating and processed in North America found no evidence of HoBi-like viruses (Bauermann et al., Reference Bauermann, Flores, Falkenberg, Weiblen and Ridpath2014a).
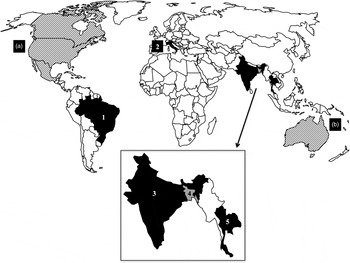
Fig. 1. HoBi-like virus distribution. HoBi-like viruses were detected in FBS lots claimed to originate in North America (a) and Australia (b). Cattle and buffalo infection were verified in Brazil (1) dating back to the late 1990s. The clinical presentation in cattle included respiratory, enteric, reproductive, and hemorrhagic syndromes. In Italy (2) similar clinical presentations were verified in cattle in 2007, 2010–2011. In India (3), Bangladesh (4), and Thailand (5), there have been several identifications of HoBi-like viruses. HoBi-like viruses were detected both in herds with no recent history of disease and herds that undergo respiratory or reproductive disease. No clear correlation of HoBi-like virus in any outbreaks was reported in Asia. The published data might suggest that this group of virus is the ‘typical’ bovine pestivirus in the region. World map available at www.freeworldmaps.net
The first isolation of HoBi-like virus dates to late in the 1990s, and from a water buffalo sample originating in Brazil (Claudio Wageck Canal, personal communication 2011). Initial characterization efforts were limited and it was only in subsequent phylogenetic analysis conducted a decade later that it was determined to be a HoBi-like virus (Stalder et al., Reference Stalder, Meier, Pfaffen, Wageck-Canal, Rufenacht, Schaller, Bachofen, Marti, Vogt and Peterhans2005). Aborted fetal tissues samples, originating in the South East region of Brazil and dated 2002 and 2004, were also reported as positive for HoBi-like viruses (Cortez et al., Reference Cortez, Heinemann, De Castro, Soares, Pinto, Alfieri, Flores, Leite and Richtzenhain2006). Additional HoBi-like virus was isolated from buffy coat samples, dated 2011, originating from cattle herds from the South region of Brazil. Another HoBi-like isolate was found in a commercial bovine semen sample (Bianchi et al., Reference Bianchi, Martins, Weiblen and Flores2011; Bauermann et al., Reference Bauermann, Ridpath, Weiblen and Flores2013). Sample submission for viral isolation occurred following several descriptions of blind newborn calves in herds using semen from that animal. Samples from the same lot of semen were submitted to a wide series of diagnostic assays and no other agent besides the HoBi-like virus was detected (Bianchi et al., Reference Bianchi, Martins, Weiblen and Flores2011; Bauermann et al., Reference Bauermann, Ridpath, Weiblen and Flores2013).
In 2014 an interesting case was described in a Brown Swiss cattle herd in North Eastern part of Brazil (Weber et al., Reference Weber, Mosena, Simoes, Almeida, Pessoa, Budaszewski, Silva, Ridpath, Riet-Correa, Driemeier and Canal2014). This herd experienced above normal rates of return to estrus and abortions, and following birth, about 10% of the calves died within the first year. Four calves were found as positive for HoBi-like virus by reverse transcription polymerase chain reaction (RT–PCR). During necropsy, among other symptoms, one of these calves presented erosions in the lips and hard palate, ulceration on the coronary band, and corneal opacity. The second calf had multiple erosions in the hard palate and cheeks. Ulcerations were also verified in the tongue and in the abomasum. Linear erosions were verified in the esophagus (Weber et al., Reference Weber, Mosena, Simoes, Almeida, Pessoa, Budaszewski, Silva, Ridpath, Riet-Correa, Driemeier and Canal2014). This case resembles the BVDV presentation known as mucosal disease (MD) and is one of the first descriptions of an MD-like syndrome associated with HoBi-like virus infection.
The identification of HoBi-like viruses in multiple regions across Brazil over a time span from the 1990s to the present, along with evidence that a significant portion of Brazilian FBS lots are contaminated with HoBi-like viruses, suggests that this viral group is likely endemic in Brazil.
In Asia, the history of HoBi-like viruses started in an epidemiological study performed between 2002 and 2004, in Thailand. The study focused on determining the prevalence of animals infected with bovine herpesvirus type 1 (BoHV-1) and BVDV, or harboring serum neutralizing antibodies against these agents. The authors detected the specific seroconversion to HoBi-like virus in 4 of 186 tested herds. While seroconversion was reported, no signs of related disease could be verified. One serum sample was identified by antigen capture ELISA (ACE) as positive for HoBi-like virus (Kampa et al., Reference Kampa, Alenius, Emanuelson, Chanlun and Aiumlamai2009).
In 2014, two reports described the presence of HoBi-like viruses in Asian cattle herds (Haider et al., Reference Haider, Rahman, Khan, Mikolon, Gurley, Osmani, Shanta, Paul, Macfarlane-Berry, Islam, Desmond, Epstein, Daszak, Azim, Luby, Zeidner and Rahman2014; Mishra et al., Reference Mishra, Rajukumar, Pateriya, Kumar, Dubey, Behera, Verma, Bhardwaj, Kulkarni, Vijaykrishna and Reddy2014). The first was in Bangladesh, in a study that searched for pestivirus in samples from animals admitted to three governmental veterinary hospitals in that country. Admitted animals presented at least one of the following symptoms: fever, diarrhea, and/or respiratory distress. Samples were tested by BVDV ACE and 16 positive animals were identified. From ACE positive samples, 14 were processed for RT–PCR and sequencing of the 5'UTR region. Phylogenetic analyses of the three sequences, successfully retrieved, revealed that all the three belonged to HoBi-like virus group. Interestingly, these samples were genetically distinct from sequences of HoBi-like viruses identified up to that moment and were tentatively grouped in a novel subgenotype (Haider et al., Reference Haider, Rahman, Khan, Mikolon, Gurley, Osmani, Shanta, Paul, Macfarlane-Berry, Islam, Desmond, Epstein, Daszak, Azim, Luby, Zeidner and Rahman2014) (Fig. 2). Also in 2014, researchers reported the presence of HoBi-like viruses in India. In that study, 1049 blood samples were collected from 21 dairy farms in eight states. The sampled animals were apparently healthy or had a history of diarrhea, respiratory, and/or reproductive disease. By RT–PCR, 20 samples were detected as positive for pestivirus. Phylogenetic analysis of sequences generated from the amplicon revealed that one belonged to the BVDV-1 species while the other 19 samples were classified as HoBi-like virus. Sequences from these 19 samples were grouped in two different clusters, and both groups are genetically divergent from known HoBi-like sequences. HoBi-like virus was identified across three states in western, central, and northern India (Mishra et al., Reference Mishra, Rajukumar, Pateriya, Kumar, Dubey, Behera, Verma, Bhardwaj, Kulkarni, Vijaykrishna and Reddy2014). These findings suggest that the ‘atypical’ HoBi-like pestivirus may be the ‘typical’ pestivirus circulating in Indian cattle herds and likely in nearby regions.

Fig. 2. Bovine pestiviruses phylogenetic tree. Phylogenetic comparison of the putative HoBi-like virus species, with BVDV 1 and 2 species. Bungowannah virus was used as outgroup. Gray insert details the genetic divergence within the HoBi-like isolates and the country of sample origin. The phylogenetic tree is based on the comparison of the viral 5′UTR sequences. The phylogenetic relationship was determined using the Neighbor-Joining method (Saitou and Nei, Reference Saitou and Nei1987). The evolutionary distances were computed using the Kimura 2-parameter method (Kimura, Reference Kimura1980) and are in the units of the number of base substitutions per site. Phylogenetic analyses were conducted using MEGA6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013).
In Europe, HoBi-like virus activity seems restricted to the Southern Italian region of Basilicata, where in 2010 HoBi-like viruses were identified associated with an outbreak of respiratory disease and cases of reproductive losses (Decaro et al., Reference Decaro, Lucente, Mari, Cirone, Cordioli, Camero, Sciarretta, Losurdo, Lorusso and Buonavoglia2011, Reference Decaro, Lucente, Mari, Sciarretta, Pinto, Buonavoglia, Martella and Buonavoglia2012a). The outbreak of respiratory disease affected 6-month-old calves, and clinical presentation included fever, respiratory distress, and leukopenia. While two animals died during the outbreak, the other 24 affected animals recovered within 2 weeks with supportive treatment. At post-mortem of the two dead animals, the presence of tracheitis and bronchopneumonia involving the apical lung lobes was noted. Lung samples from these calves were submitted to quantitative RT–PCR (qRT–PCR) and HoBi-like virus was detected in both animals. The virus was also detected in nasal swabs from six other calves (Decaro et al., Reference Decaro, Lucente, Mari, Cirone, Cordioli, Camero, Sciarretta, Losurdo, Lorusso and Buonavoglia2011). Reproductive disorders were reported in the same herd, in June of the following year. Reproductive disease presentation included abortions in eight cows between the fourth and sixth month of gestation. HoBi-like viruses were isolated from two of the aborted fetuses (Decaro et al., Reference Decaro, Lucente, Mari, Sciarretta, Pinto, Buonavoglia, Martella and Buonavoglia2012a). HoBi-like infection of pregnant animals in that herd also resulted in the generation and birth of one persistent infected (PI) calf, which was monitored for 17 months. A cytopathic HoBi-like virus was isolated from this animal one month before its death. At post-mortem necrosis of the oral mucosa, hemorrhagic thacheitis, pneumonia, and enteritis were noted (Decaro et al., Reference Decaro, Lanave, Lucente, Mari, Varello, Losurdo, Larocca, Bozzetta, Cavaliere, Martella and Buonavoglia2014). The lesions observed were similar to those reported for BVDV MD.
While the first outbreak of disease associated with HoBi-like virus infection in Italy was observed in 2010, subsequent analysis of archived samples revealed that bovine nasal swabs collected in 2007 were identified as HoBi-like virus positive. These nasal swabs were collected from a herd located in the Basilicata region. These samples were submitted to a diagnostic laboratory because a drastic drop in milk production, cough, and nasal discharge had been observed in the herd. No animal introduction onto the farm was reported, but artificial insemination was routinely performed, and the farm is located a few kilometers from a water buffalo farm (Decaro et al., Reference Decaro, Mari, Lucente, Sciarretta, Elia, Ridpath and Buonavoglia2013). The proximity to the water buffalo farm might be relevant. While HoBi-like viruses might have been introduced to the herd by the use of contaminated FBS in the artificial insemination procedures, it is hypothesized that HoBi-like viruses in cattle result from a spillover from water buffalos (Bauermann et al., Reference Bauermann, Ridpath, Weiblen and Flores2013). Interestingly, the two regions (South America and Asia) where HoBi-like viruses are most frequently reported are regions with significant populations of water buffalo.
Phylogenetic analysis reveals that HoBi-like viruses isolated in Italy group with viruses isolated from samples originating in South America. In contrast, HoBi-like viruses originating in Thailand, India, and Bangladesh appear to belong to different subgenotypes. The divergence of HoBi-like viruses into different subgenotypes that correlate with geographic regions suggests that these viruses have been circulating among domestic and/or wild ruminant populations for a significant time before initial viral identifications (Bauermann et al., Reference Bauermann, Ridpath, Weiblen and Flores2013; Haider et al., Reference Haider, Rahman, Khan, Mikolon, Gurley, Osmani, Shanta, Paul, Macfarlane-Berry, Islam, Desmond, Epstein, Daszak, Azim, Luby, Zeidner and Rahman2014; Mishra et al., Reference Mishra, Rajukumar, Pateriya, Kumar, Dubey, Behera, Verma, Bhardwaj, Kulkarni, Vijaykrishna and Reddy2014).
As described, some of the outbreaks related to HoBi-like viruses are associated with moderate to severe clinical presentation. However, inoculation of susceptible calves with HoBi-like virus, under experimental conditions, did not reproduce the severity of respiratory disease as described in Italy. Under experimental conditions the majority of inoculated animals presented elevated body temperature, slight decrease in white blood cells, especially between day post infection (dpi) 3–9. Seroconversion was observed in all animals, as well as detection of virus in nasal and/or buffy coat for several days (Schirrmeier et al., Reference Schirrmeier, Strebelow, Depner, Hoffmann and Beer2004; Decaro et al., Reference Decaro, Mari, Lucente, Sciarretta, Moreno, Armenise, Losurdo, Camero, Lorusso, Cordioli and Buonavoglia2012b; Ridpath et al., Reference Ridpath, Falkenberg, Bauermann, VanderLey, Do, Flores, Rodman and Neill2013). Clinical presentation observed following experimental exposure of calves using the strain isolated included moderate conjunctivitis, watery-mucoid nasal and ocular discharge, and cough. Depletion in the lymphocyte counts between dpi 2 and 5, returning to normal level at dpi 14. A decrease in platelet counts was detected at dpi 7. Other hematological parameters remained within normal range (Larska et al., Reference Larska, Polak, Riitho, Strong, Belak, Alenius, Uttenthal and Liu2012).
Experimental infection of heifers within the first trimester of gestation demonstrated the ability of these viruses to infect the fetus and establish persistent infection (Bauermann et al., Reference Bauermann, Falkenberg, Vander Ley, Decaro, Brodersen, Harmon, Hessman, Flores and Ridpath2014b). During that study, one previously tested pregnant heifer was diagnosed as non-pregnant about 25 days after viral inoculation, but no sign of abortion was noted. One heifer aborted at the eighth month of gestation. The recovered fetus had no visible malformation and size was consistent with the gestation period. This fetus tested positive for HoBi-like virus by RT–PCR in the abdominal fluid, and by immunohistochemistry (IHC) and ACE on ear skin tissue. The remaining six heifers gave birth to six calves harboring HoBi-like virus at day of birth. Two died shortly after birth and four remaining calves were confirmed as PIs following consecutive positive tests during the first month of age (Bauermann et al., Reference Bauermann, Falkenberg, Vander Ley, Decaro, Brodersen, Harmon, Hessman, Flores and Ridpath2014b).
While all isolations of HoBi-like virus from field samples have been from either water buffalo or cattle, there is experimental evidence that others species could also be infected by these viruses. The inoculation of the Italian isolate (Italy-1/10-1) in 5-month-old sheep resulted in moderate to abundant nasal discharge, but no increase in body temperature. Decreases in lymphocyte counts (equal or lower than 60% from baselines) were verified between dpi 5 and 10. Seroconversion peaked at dpi 21. Viral RNA was intermittently detected between dpi 5 and 21 in nasal secretion and/or buffy coat of sheep (Decaro et al., Reference Decaro, Mari, Lucente, Sciarretta, Moreno, Armenise, Losurdo, Camero, Lorusso, Cordioli and Buonavoglia2012b). Using the same viral strain, inoculation of 2-month-old piglets resulted in no visible clinical sign or hematological change but animals seroconverted (Decaro et al., Reference Decaro, Mari, Lucente, Sciarretta, Moreno, Armenise, Losurdo, Camero, Lorusso, Cordioli and Buonavoglia2012b). Similar results were obtained when inoculating pigs with the strain D32/00 (Schirrmeier et al., Reference Schirrmeier, Strebelow, Depner, Hoffmann and Beer2004). Studies involving the inoculation of HoBi-like viruses in species other than cattle are limited. Results so far demonstrate that besides cattle, sheep could replicate and transmit HoBi-like viruses. It is not known if there is a change in the virus, associated with adaptation to replication in sheep or if HoBi-like viruses can establish PI in sheep.
Studies involving the detection of HoBi-like PIs demonstrated that similar to BVDV PIs, the concentration of viral protein in skin sections, especially ear skin, is significant. IHC staining of ear tissue of HoBi-like PIs using the monoclonal antibody (mAb) 15C5 proved to be highly efficient to detect HoBi-like PI animals (Bauermann et al., Reference Bauermann, Falkenberg, Vander Ley, Decaro, Brodersen, Harmon, Hessman, Flores and Ridpath2014b; Weber et al., Reference Weber, Mosena, Simoes, Almeida, Pessoa, Budaszewski, Silva, Ridpath, Riet-Correa, Driemeier and Canal2014). However, by this IHC method it is not possible to differentiate between BVDV and HoBi-like PIs . The lack of specific mAbs for HoBi-like viruses hinders the design of differential tests. Similarly ACE based on the Erns protein (IDEXX BVDV PI X2 Test) has a high detection rate for HoBi-like PIs samples under experimental conditions (Bauermann et al., Reference Bauermann, Falkenberg, Vander Ley, Decaro, Brodersen, Harmon, Hessman, Flores and Ridpath2014b) but cannot be used to differentiate HoBi-like virus and BVDV infections. When comparing samples collected weekly from day of birth to 3 weeks of age from HoBi-like virus PI calves, ACE positive signals increased significantly with the age of the calf. The addition of a sample digestion step before the ACE procedure increased the sensitivity of the test. In contrast, whether samples are submitted to digestion or not, the use of ACE test based on the NS3 protein yielded no positive result. Therefore, the combination of Erns and NS3 ELISA could be an option for differentiation of HoBi-like and BVDV PIs (Bauermann et al., Reference Bauermann, Falkenberg, Vander Ley, Decaro, Brodersen, Harmon, Hessman, Flores and Ridpath2014b).
The commercial BVDV RT–PCR kits (VetMAX®-Gold BVDV and VIROTYPE® BVDV) were shown to have a high rate of detection of HoBi-like virus in tissues from HoBi-like PIs, especially in ear skin sections (Bauermann et al., Reference Bauermann, Falkenberg, Vander Ley, Decaro, Brodersen, Harmon, Hessman, Flores and Ridpath2014b). There are no RT–PCR or qRT–PCR commercial tests currently available to specifically detect HoBi-like viruses although assays for use in research have been published (Liu et al., Reference Liu, Xia, Belak and Baule2008; Bauermann et al., Reference Bauermann, Flores, Falkenberg, Weiblen and Ridpath2014a). While these assays were successfully used to detect HoBi-like viruses in laboratory generated samples, testing of tissues from HoBi-like PIs resulted in some false negatives. Therefore, further refinements in the assays are required for increased sensitivity, as well as the test of greater number of isolates.
Genetic and antigenic similarities between BVDV and HoBi-like strains is high enough that assays designed to detect BVDV will detect HoBi-like viruses; though the sensitivity may be reduced (Bauermann et al., Reference Bauermann, Flores and Ridpath2012, Reference Bauermann, Falkenberg, Vander Ley, Decaro, Brodersen, Harmon, Hessman, Flores and Ridpath2014b; Decaro et al., Reference Decaro, Mari, Lucente, Sciarretta, Moreno, Armenise, Losurdo, Camero, Lorusso, Cordioli and Buonavoglia2012b; Larska et al., Reference Larska, Polak, Riitho, Strong, Belak, Alenius, Uttenthal and Liu2012). However, testing to date has been based on HoBi-like viruses from South America and Europe and recent publications have indicated that the genetic and antigenic diversity among HoBi-like viruses is greater than previously thought (Haider et al., Reference Haider, Rahman, Khan, Mikolon, Gurley, Osmani, Shanta, Paul, Macfarlane-Berry, Islam, Desmond, Epstein, Daszak, Azim, Luby, Zeidner and Rahman2014; Mishra et al., Reference Mishra, Rajukumar, Pateriya, Kumar, Dubey, Behera, Verma, Bhardwaj, Kulkarni, Vijaykrishna and Reddy2014). The degree of divergence exhibited by HoBi-like viruses isolated in Bangladesh and India is a matter of great concern. For instance, the panpestivirus pair of primers 324–326, which is widely used for bovine pestivirus detection and successfully detects HoBi-like viruses from Brazil, Thailand, and Italy, yielded no positive results in any of the 19 HoBi-like samples tested in India.
The scarcity of information on the prevalence of HoBi-like viruses hinders efforts to estimate the real impact of these viruses on cattle populations. The paucity of information on the economic impact of HoBi-like viruses undermines the development of control programs, including the implementation of systematic diagnostic efforts and international trade regulations for live animals, animal products, and biologicals. The adoption of testing guidelines seems particularly important for the trade of FBS, as South American countries are major suppliers. It has been theorized that HoBi-like viruses were introduced into Europe via the use of contaminated FBS originating in South America. Currently, required testing for commercial FBS does not include testing for HoBi-like viruses. BVDV infections result in significant economic loss around the world. Because the clinical presentations associated with HoBi-like virus infection are similar to those seen following infection with BVDV, it is highly probably that introduction of HoBi-like viruses into new regions of the world would result in significant economic losses. Therefore, major beef and dairy cattle producers would benefit from a complete preparedness to prevent viral introduction and/or dissemination using the specific diagnostic and control approaches.




