Introduction
Escherichia coli is one of the most widely studied organisms in the microbial world. Non-pathogenic strains of E. coli are part of the normal flora of the gastrointestinal tract and for many years have been used in the laboratory as a host organism for recombinant DNA techniques; pathogenic strains have been implicated in causing a wide range of intestinal and extra-intestinal diseases in humans and animals (Kaper and Nataro, Reference Kaper and Nataro2004). The species E. coli, belonging to the family Enterobacteriaceae, is a Gram-negative flagellated rod-shaped bacterium. There is enormous diversity within the species, which is differentiated into subgroups based on many different physiological, morphological and antigenic characteristics.
Initially, E. coli isolates were differentiated from one another by biotypes that were based on fermentation or non-fermentation of a range of carbohydrate substrates. It was soon realized that this method was not optimal for distinguishing strains in epidemiological investigations as it was limited in its discriminatory power. Therefore, immunogenicity of the bacterial surface structures was considered as a potentially more effective method. The combination of three principal surface antigens (O antigens that are a part of the lipopolysaccharide (LPS), the flagellar H antigens and the K capsular antigens (O:K:H)) was developed as a method for subtyping E. coli strains. However, only a few laboratories had the ability to type the K antigen, and therefore, serotyping based on the O and H antigens became the ‘gold standard’ for E. coli. The responsibility for maintaining the reagents for an international typing scheme and for aspects of research in this field rests with the WHO International Escherichia and Klebsiella Centre in Copenhagen, but scientists all over the world contribute to new knowledge in this area.
Further refinement by combining serotypes with biotypes based on phage types, virulence markers, phylogenetic profile and genotypic characteristics has now been adopted for distinguishing E. coli strains. Within a serogroup several subgroups may be identified by the presence of several H antigens associated with the O antigen. The O:H combination is referred to as a serotype. Within a serotype there may be numerous subtypes that are often identified by a variety of gene-based methods.
In the 1940s, Kaufmann (Reference Kaufmann1943, Reference Kaufmann1944, Reference Kaufmann1947) attempted to classify E. coli by serological methods, and by 1945, he successfully classified E. coli into 20 O groups on the basis of the antigenic scheme he developed by using boiled cultures of E. coli for O antisera production. The antisera generated against the O groups were tested with boiled cultures of unknown strains for agglutination reactions for identification of the 20 O groups. Five additional O groups were subsequently added (Knipschildt, Reference Knipschildt1945). Ørskov et al. (Reference Ørskov, Ørskov, Jann and Jann1977) presented a comprehensive serotyping scheme comprising 164 O groups, which has been the basis for O classification for taxonomic and epidemiological studies and for distinguishing strains during outbreaks and for surveillance purposes. Later, Ørskov et al. (Reference Ørskov, Ørskov and Rowe1984) added 6 O groups, O165 through O170, based in part on a selection from 8 O groups that had previously been called OX1 to OX8 by Ewing et al. (Reference Ewing, Tatum, Davis and Reavis1956) ; OX1 was designated as O170, OX2 as O169, OX8 as O166 and OX5 as O168. OX4 and OX6 were found to be similar to O146 and O171, respectively (Ewing, Reference Ewing, Edwards and Ewing1972; Scheutz et al., Reference Scheutz, Cheasty, Woodward and Smith2004), and OX3 and OX7 were never established as serotypes until 2004 when Scheutz et al. (Reference Scheutz, Cheasty, Woodward and Smith2004) designated OX3 and OX7 as O174 and O175. The nucleotide sequence of the OX6 O-antigen gene cluster has been found to be similar to that of O45 (Fratamico et al., unpublished data). Ørskov et al. (Reference Ørskov, Wachsmuth, Taylor, Echeverria, Rowe and Sakazaki1991) reported two more serogroups, O172 and O173, and Scheutz et al. (Reference Scheutz, Cheasty, Woodward and Smith2004) added O176 to O181. Currently there are 174 O groups, identified as O1–O181 O groups except for O groups O31, O47, O67, O72, O93, O94 and O122 which were determined to be no longer valid O groups.
It should be emphasized that the international typing scheme is limited to the typing of E. coli which appear to be important, primarily for their involvement or potential involvement in disease. There are large numbers of strains of E. coli that are designated as ‘untypeable’ because they do not react with antisera in the established typing scheme. These untypeable strains include pathogenic ones (Duda et al., Reference Duda, Lindner, Brade, Leimbach, Brzuszkiewicz, Dobrindt and Holst2011). Some of these strains may be assigned to an O serogroup, based on genetic methods (DebRoy et al., Reference DebRoy, Roberts, Davis and Bumbaugh2010).
LPS
The cell envelope of Gram-negative bacteria is composed of the outer and inner membranes that are separated by the periplasm. The outer membrane is composed of phospholipids, LPSs, membrane proteins and lipoproteins (Bos et al., Reference Bos, Robert and Tommassen2007). LPS, also known as endotoxin, consists of three components: the hydrophobic membrane anchor lipid A region, which is associated with the toxicity of the LPS and is well conserved among Gram-negative bacteria (Raetz and Whitfield, Reference Raetz and Whitfield2002), the distal O-antigen polysaccharide region that is exposed to the surface and the core polysaccharide region that connects the two.
The lipid A moiety consists of a β-1,6-linked d-glucosamine disaccharide carrying ester- and amide-linked 3-hydroxy fatty acids at the 2, 3, 2′ and 3′ positions and phosphate groups at the 1, 4′ positions. The lipid A biosynthetic pathway has been characterized (Raetz and Whitfield, Reference Raetz and Whitfield2002; Wang and Quinn, Reference Wang and Quinn2010). The first step in the biosynthesis of LPS in E. coli is catalyzed by UDP-N-acetylglucosamine (UDP-GlcNAc) acyltransferase (LpxA) leading to the reversible transfer of the R-3-hydroxyacyl chain from R-3-hydroxyacyl acyl carrier protein to the glucosamine 3-OH group of UDP-GlcNAc. A number of enzymes sequentially convert this structure into disaccharide-1-P, Kdo2-lipid A, core-lipid A and eventually LPS (Wang and Quinn, Reference Wang and Quinn2010). The lipid A structure is more conserved than that of the outer core oligosaccharide region, which shows more variability among bacterial species. There are five types of core in E. coli, named R1–R4 and K-12. The core oligosaccharides are sequentially assembled on lipid A at the cytoplasmic surface of the inner membrane. This involves a number of glycotransferases that are membrane bound and use nucleotide sugars as donors. Core oligosaccharides can be divided into two regions, inner and outer core. The inner oligosaccharide component of the core connects to lipid A and the outer component connects to the O antigen repeating units (Raetz et al. Reference Raetz, Reynolds, Trent and Bishop2007).
LPS is responsible for stimulation of the innate immune system. The response from the host depends on the particular structure of lipid A, which is the bioactive component of LPS and is responsible for the toxic effect of Gram-negative bacterial infections (Galanos et al., Reference Galanos, Luderitz, Rietschel, Westphal, Brade, Brade, Freudenberg, Schade, Imoto, Yoshimura, Kusumoto and Shiba1985). LPS is recognized by the Toll-like receptor 4 (TLR4) present on the surface of monocytes, macrophages, neutrophils and dendritic cells, cells of the innate immune system (Poltorak et al., Reference Poltorak, He, Smirnova, Liu, Van Huffel and Du1998; Akira et al., Reference Akira, Uematsu and Takeuchi2006). The lipid A of E. coli, composed of two phosphate groups and six acyl chains containing 12 or 14 carbons, is a powerful activator of the innate immune system (Golenbock et al., Reference Golenbock, Hampton, Qureshi, Takayama and Raetz1991).
The O antigens are thermostable and are found in all smooth bacteria within the Enterobacteriaceae, which means that the bacteria retain their immunogenicity, agglutinating and agglutinin-binding capacity at boiling temperature. This property has been exploited for serological identification of O antigens where the bacteria are boiled to react with antisera for agglutination. Bacteria that have mutated and lost the O antigen specificity are referred to as ‘rough’ forms and cannot be serotyped by agglutination reactions as they have lost the ability to synthesize the O antigens. Interestingly, although LPS contributes to virulence, rough strains of E. coli have been recognized as highly virulent. For example, O rough:H7 Shiga toxin-producing E. coli (STEC) implicated in bloody diarrhea have been shown to be O157:H7 organisms that failed to express their O antigen, because of an insertion in one of the genes in the O-antigen gene cluster (Rump et al., Reference Rump, Feng, Fischer and Monday2010a, Reference Rump, Beutin, Fischer and Fengb).
Biosynthesis of the O antigens
The genes in the E. coli O-antigen gene clusters encode for enzymes responsible for O antigen biosynthesis. There are three major gene classes: nucleotide sugar synthesis genes, sugar transferase genes and O unit processing genes. These genes are responsible for carrying out three distinct processes that are involved in the synthesis and translocation of the O antigen (Bronner et al., Reference Bronner, Clarke and Whitfield1994; Reeves et al., Reference Reeves, Hobbs, Valvano, Skurnik, Whitfield, Coplin, Kido, Klena, Maskell, Raetz and Rick1996; Keenleyside and Whitfield, Reference Keenleyside and Whitfield1996; Daniels et al., Reference Daniels, Vindurampulle and Morona1998; Linton and Higgins, Reference Linton and Higgins1998; Samuel and Reeves, Reference Samuel and Reeves2003; Liu et al., Reference Liu, Knirel, Feng, Perepelov, Senchenkova, Wang, Reeves and Wang2008; Wang et al., Reference Wang, Wang and Reeves2010). Nucleotide sugar synthesis genes encode for proteins that are responsible for sugar synthesis specific to certain O groups. Enzymes of the second group, the glycosyl transferase proteins, transfer various precursor sugars for the specific linkages in the O antigen to form oligosaccharides on the carrier lipid undecaprenyl phosphate (UndP), situated in the inner membrane facing the cytoplasm (Reeves, Reference Reeves1994). The O antigen processing proteins, the Wzx (O antigen flippase) and Wzy (O antigen polymerase), are involved in translocation of the O units across the membrane and in polymerization. The O unit is synthesized by sequential transfer of sugars and other constituents to the first sugar, which is then translocated and flipped across the membrane by the protein Wzx to the periplasmic face. These are further polymerized by Wzy (Mulford and Osborn, Reference Mulford and Osborn1983; McGrath and Osborn, Reference McGrath and Osborn1991; Reeves and Wang, Reference Reeves and Wang2002; Liu et al., Reference Liu, Knirel, Feng, Perepelov, Senchenkova, Wang, Reeves and Wang2008). The number of O units in the final O antigen is regulated by Wzz. The Wzx and Wzy proteins are both hydrophobic and have about 12 predicted transmembrane regions, and a long cytoplasmic region is predicted between the transmembrane domains in Wzy. In some of the E. coli O groups such as O8, O9, O9a, O52 and O99, the translocation of the O antigen is carried out by an ABC transporter protein, Wzm, which is responsible for the export, and Wzt, the ATP-binding component.
O antigens are composed of repeat units of oligosaccharides (O unit) comprising sugar residues, which vary considerably and differ in their arrangement and linkage between and within the O units, making the O antigen the most variable region of the bacterial cell. The O antigens are highly immunogenic and variability among the groups provides the basis for serotyping and classification of E. coli. Carbohydrate analysis methods have been valuable in the identification of the structures of numerous O antigens (MacLean et al., Reference MacLean, Webb and Perry2006, Reference MacLean, Liu, Vinogradov and Perry2010; Chafchaouni-Moussaoui et al., Reference Chafchaouni-Moussaoui, Novikov, Bhrada, Perry, Filali-Maltouf and Caroff2011).
O antigens may contribute to virulence of the organism, and certain O groups are associated with high virulence. It has been shown that specific clones within certain serotypes are associated with specific diseases (Ørskov et al., Reference Ørskov, Ørskov, Evans, Sack, Sack and Wadstrom1976; Achtman and Pluschke, Reference Achtman and Pluschke1986). E. coli O18:K1 was shown to cause newborn meningitis and neonatal bacteremia, multiplying in the bloodstream of infected newborn rats; this pathogen was resistant to the bactericidal effects of complement in the absence of specific antibodies. Loss of the O antigen resulted in the strain becoming more sensitive to the bactericidal effects of the classical complement pathway (Pluschke et al., Reference Pluschke, Mayden, Achtman and Levine1983). Differences in virulence among certain serotypes of E. coli were dependent on specific cell surface structures such as O antigens (O1, O7, O16, O18 and O75), capsule and fimbriae (Kusecek et al., Reference Kusecek, Wloch, Mercer, Vaisänen, Pluschke, Korhonen and Achtman1984). Outbreaks of disease due to E. coli O157:H7, causing severe gastrointestinal illness and affecting 47 people, were described for the first time in 1983 (Riley et al., Reference Riley, Remis, Helgerson, McGee, Wells, Davis, Hebert, Olcott, Johnson, Hargrett, Blake and Cohen1983; Remis et al., Reference Remis, MacDonald, Riley, Puhr, Wells, Davis, Blake and Cohen1984). Since then, many reports have established E. coli O157:H7 carrying Shiga toxin genes as a major pathogen that causes hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS) in humans (Krishnan et al., Reference Krishnan, Fitzgerald, Dakin and Behme1987; Neill et al., Reference Neill, Tarr, Clausen, Christie and Hickman1987). STEC strains belonging to specific O serogroups, including O26, O45, O103, O111, O121 and O145, have been linked to outbreaks of disease that are similar to those caused by O157, but occur at a lower frequency and tend to be less severe (Karmali et al., Reference Karmali, Mascarenhas, Shen, Ziebell, Johnson, Reid-Smith, Isaac-Renton, Clark, Rahn and Kaper2003; Gyles, Reference Gyles2007).
There is evidence to suggest that the O antigens in Enterobacteriaceae play an important role in host–pathogen interactions (West et al., Reference West, Sansonetti, Mounier, Exley, Parsot, Guadagnini, Pre´vost, Prochnicka-Chalufour, Delepierre, Tanguy and Tang2005); therefore, it is possible that the presence of specific O antigens may be a factor contributing to disease caused by certain serogroups. In Yersinia serotype O8, pathogenesis was examined by analysis of mutants with no O antigen, LPS with one O unit and LPS with random distribution of the O antigen. It was found that LPS mutants with attenuated O antigens were severely impaired in their ability to adhere and could not colonize in the spleen and liver in contrast to the wild type (Bengoechea et al., Reference Bengoechea, Najdenski and Skurnik2004). Recently, Vigil et al. (Reference Vigil, Alteri and Mobley2011) reported by whole genome screening approaches, including transcriptomic, proteomic, and signature-tagged mutagenesis, that uropathogenic E. coli strains in humans expressed or required genes for capsule, LPS, translational machinery, type 1 fimbriae and iron acquisition systems to cause urinary tract infection (UTI). Among extra-intestinal pathogenic E. coli (ExPEC) strains that cause UTI, strains belonging to serogroups O4 and O6 have been implicated in causing fatal pneumonia in dogs, cats and tigers (Handt et al., Reference Handt, Stoffregen, Prescott, Pouch, Ngai, Anderson, Gatto, DebRoy, Fairbrother, Motzel and Klein2003; Sura et al., Reference Sura, Van Kruiningen, DebRoy, Hinckley, Greenberg, Gordon and French2007; Carvallo et al., Reference Carvallo, DebRoy, Baeza, Hinckley and Gilbert2010).
Horizontal transfer and recombination can convert E. coli from one O-antigen to another. This is well documented in the case of evolution of E. coli O157:H7 whose O antigen was changed from O55 to O157 (Tarr et al., Reference Tarr, Schoening, Yea, Ward, Jelacic and Whittam2000). Horizontal transfer by bacteriophage (phage conversion) is well known to cause changes in LPS in Salmonella.
O antigen detection
Serotyping
Conventional serotyping for O group identification is based on agglutination reactions, which remains one of the most comprehensive and simple methods for testing O groups. Serotyping is carried out in tubes or 96-well plates or on slides using antisera that are generated by immunization of rabbits with different O group reference strains (Ørskov et al., Reference Ørskov, Ørskov, Jann and Jann1977; Ørskov and Ørskov, Reference Ørskov and Ørskov1984). When antisera containing the antibodies for each O group are allowed to react with an unknown O antigen, released by heating the bacteria for 2 h at 100°C, agglutination, or clumping of the bacteria is observed when the O antigen reacts with the specific antiserum. However, if the organism is capsulated or rough and does not carry LPS, the agglutination reaction fails. Sometimes there may be cross reactions with other O groups, resulting in equivocal results.
Slide agglutination is conducted by adding a small amount of culture growing on an agar plate to a droplet of diluted serum on a microscope slide. The slide is rocked for a few seconds, and agglutination is observed by the naked eye. However, the method is not confirmatory and can result in equivocal results. Currently, serotyping is generally performed by observing for agglutination in 96-well plates. The diluted antisera (20 μl) are then dispensed in 96-well microtiter plates, with each well containing antiserum against a different O group. For O serotyping, bacteria are grown overnight to a concentration of 108–109 colony-forming units (CFU)/ml. The cultures are heated for 2 h at 100°C, diluted with formalinized saline to a McFarland density of 2, and 180 μl of bacterial suspension are added to the antisera in the wells and mixed. The 96-well plates are incubated at 50°C overnight, then checked for agglutination reactions. Titers, which indicate the relative strength of the antiserum, are expressed as the reciprocal of the highest dilution that resulted in agglutination. A comparison of the titers of the antisera of known potency against the unknown E. coli isolate and the homologous strain is a rough guide to the relationship of the unknown O antigen to that of the O antigen used to prepare the reference antiserum.
Restriction fragment length polymorphism (RFLP) analysis of O-antigen gene clusters
O-antigen gene clusters are located at position 44–45 min on the chromosome of E. coli, at 200–2000 base pairs (bp) upstream of gnd encoding for 6-phosphogluconate dehydrogenase (Batchelor et al., Reference Batchelor, Alifano, Biffali, Hull and Hull1992; Bastin et al. Reference Bastin, Stevenson, Brown and Reeves1993). There is a highly conserved 39-bp JUMPstart sequence upstream of the O-antigen gene cluster (Hobbs and Reeves, Reference Hobbs and Reeves1994). Using primer sequences complementary to these regions, Wang and Reeves (Reference Wang and Reeves1998) sequenced the E. coli O157 O-antigen gene cluster and determined specific sequences that could be used to identify the O157 serogroup. Subsequently, the genes for many O groups have been sequenced using this strategy. Coimbra et al. (Reference Coimbra, Grimont, Lenormand, Burguiere, Beutin and Grimont2000) used the same strategy and amplified the O-antigen gene clusters of 148 O serogroups and digested the amplification product with the restriction enzyme MboII. Profiles were compared and 147 patterns were obtained. Although the authors claimed that the patterns were unique, 13 RFLP patterns were shared by two or more O groups, making it difficult to substitute this method for serotyping for routine diagnostic testing.
PCR assays for O antigen detection
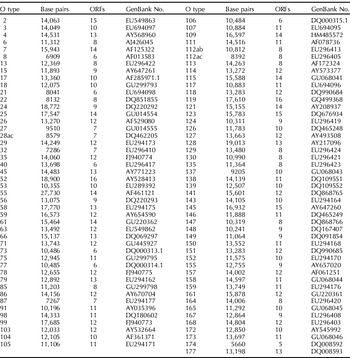
The O-antigen gene clusters for many O groups have now been sequenced (Marolda and Valvano, Reference Marolda and Valvano1993; Bastin and Reeves, Reference Bastin and Reeves1995; Amor and Whitfield, Reference Amor and Whitfield1997; Wang and Reeves, Reference Wang and Reeves1998; Wang L et al., Reference Wang, Curd, Qu and Reeves1998, Reference Wang, Briggs, Rothemund, Fratamico, Luchansky and Reeves2001, Reference Wang, Huskic, Cisterne, Rothemund and Reeves2002, Reference Wang, Perepelov, Feng, Shevelev, Wang, Senchenkova, Han, Li, Shashkov, Knirel, Reeves and Wang2007, Reference Wang, Perepelov, Feng, Knirel, Li and Wang2009, Reference Wang, Wang and Reeves2010, Paton and Paton, Reference Paton and Paton1999; Shepherd et al., Reference Shepherd, Wang and Reeves2000; D'Souza et al., Reference D'Souza, Wang and Reeves2002, Reference D'Souza, Samuel and Reeves2005; Grozdanov et al., Reference Grozdanov, Zahringer, Blum-Oehler, Brade, Henne, Knirel, Schombel, Schulze, Sonnenborn, Gottschalk, Hacker, Rietschel and Dobrindt2002; Perelle, et al., Reference Perelle, Dilasser, Grout and Fach2002; Shao et al., Reference Shao, Li, Jia, Lu and Wang2003; Fratamico et al., Reference Fratamico, Briggs, Needle, Chen and DebRoy2003, Reference Fratamico, DebRoy, Strobaugh and Chen2005, Reference Fratamico, DebRoy and Liu2009a, Reference Fratamico, DebRoy, Miyamoto and Liub, Reference Fratamico, Yan, Liu, DebRoy, Byrne, Monaghan, Fanning and Bolton2010; Feng et al., Reference Feng, Senchenkova, Yang, Shashkov, Tao, Guo, Cheng, Ren, Knirel, Reeves and Wang2004a, Reference Feng, Wang, Tao, Guo, Krause, Beutin and Wangb, Reference Feng, Han, Wang, Bastin and Wang2005a, Reference Feng, Senchenkova, Tao, Shashkov, Liu, Shevelev, Reeves, Xu, Knirel and Wangb, Reference Feng, Perepelov, Zhao, Shevelev, Wang, Senchenkova, Shashkov, Geng, Reeves, Knirel and Wang2007; Guo et al., Reference Guo, Feng, Tao, Zhang and Wang2004, Reference Guo, Kong, Cheng, Wang and Feng2005; Beutin et al., Reference Beutin, Kong, Feng, Wang, Krause, Leomil, Jin and Wang2005a, Reference Beutin, Tao, Feng, Krause, Zimmermann, Gleier, Xia and Wangb, Reference Beutin, Wang, Naumann, Han, Krause, Leomil, Wang and Feng2007; DebRoy et al., Reference DebRoy, Fratamico, Roberts, Davis and Liu2005; Cheng et al., Reference Cheng, Wang, Wang, Wang, Wang and Feng2006, Reference Cheng, Liu, Bastin, Han, Wang and Feng2007; Cunneen and Reeves, Reference Cunneen and Reeves2007; Han et al., Reference Han, Liu, Cao, Beutin, Kruger, Liu, Li, Liu, Feng and Wang2007; Liu Y et al., Reference Liu, DebRoy and Fratamico2007, Reference Liu, Knirel, Feng, Perepelov, Senchenkova, Wang, Reeves and Wang2008; Liu B et al., Reference Liu, Wu, Li, Beutin, Chen, Cao and Wang2010; Ren et al., Reference Ren, Liu, Cheng, Liu, Feng and Wang2008; Perepelov et al., Reference Perepelov, Li, Liu, Senchenkova, Guo, Shevelev, Shashkov, Guo, Feng, Knirel and Wang2009, Reference Perepelov, Li, Liu, Senchenkova, Guo, Shashkov, Feng, Knirel and Wang2011a, Reference Perepelov, Ni, Wang, Shevelev, Senchenkova, Shahskov, Wang and Knirelb; Wang Q et al., 2009, 2010a, b; Li et al., Reference Li, Liu, Chen, Guo, Guo, Liu, Feng and Wang2010a, Reference Li, Perepelov, Wang, Senchenkova, Liu, Shevelev, Guo, Shashkov, Chen, Wang and Knirelb). DNA sequences for about 95 O-antigen gene clusters have been deposited in GenBank so far, and the accession numbers are listed in Table 1. The genetics of the O-antigen gene clusters are directly correlated with the structural variations of the O antigens. Genes in the O-antigen gene clusters for all O groups sequenced are transcribed in one direction (Fig. 1).
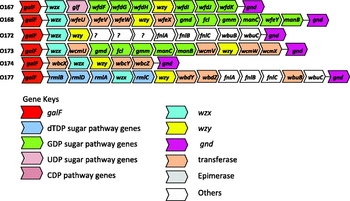
Fig. 1. O antigen gene clusters of all E. coli O antigens from GenBank. The arrows represent the location and direction of translation for putative genes in the clusters. The genes are not represented in scale.
Table 1. Accession numbers in GenBank for all O antigen gene clusters that have been sequenced
Nucleotide sequences of the O-antigen gene clusters for O107 and O117 are homologous (Wang et al., Reference Wang, Perepelov, Feng, Knirel, Li and Wang2009), and these two O groups have been found to cross-react in serotyping assays. More recently, Wanget al. (Reference Wang, Perepelov, Feng, Knirel, Li and Wang2009) have shown these two O-antigen gene clusters, having 98.6% DNA identity, encode genes that result in differences in 1 sugar residue. They identified a nucleotide substitution in 1 gene which resulted in a Glc-transferase in O117 and a Glc-NAc transferase in O107. The O-antigen gene clusters of E. coli O129 and O135 also exhibit 100% homology (Liu Y et al., Reference Liu, Knirel, Feng, Perepelov, Senchenkova, Wang, Reeves and Wang2008), and the DNA sequences of O118 and O151 are alike except for two nucleotides that are substituted in O151 thereby changing two amino acids in the proteins that are transcribed (Liu Y et al., Reference Liu, Knirel, Feng, Perepelov, Senchenkova, Wang, Reeves and Wang2008). O groups with homologous sequences may be merged to avoid confusion.
Plainvert et al. (Reference Plainvert, Bidet, Peigne, Barbe, Médigue, Denamur, Bingen and Bonacorsi2007) identified a meningitis-causing extraintestinal pathogenic E. coli clone that belonged to serogroup O45; however, there were clear differences in the sequence of the O-antigen gene cluster of this clone (S88) with that of the O45:H2 96-3285 strain that had been previously published (DebRoy et al., Reference DebRoy, Fratamico, Roberts, Davis and Liu2005). The putative Wzx of strain S88 showed 23% identity and 42% similarity to the putative Wzx of E. coli 96-3285. It was suggested that multiple recombination events led to the horizontal acquisition of the new O-antigen gene cluster, referred to as the O45S88 antigen, leading to the emergence of a virulent O45:H7 clone.
The O-antigen gene clusters are generally highly heterologous with considerable nucleotide differences between the clusters. However, the predominant genes in the clusters are those involved in nucleotide sugar synthesis, and encoding for synthetases, transferases, epimerases and other enzymes. Although the genes encoding specific proteins may have similarities in functions among the O groups, the DNA sequences may be quite variable. In most of the clusters, two genes, encoding the O antigen flippase (wzx) and the O antigen polymerase (wzy) responsible for O antigen translocation across the membrane and for polymerization, are unique, and therefore have been used as targets in genetic-based methods for serogroup identification (Fig. 2). A list of primer sequences used for detecting the O groups is presented in Table 2. Singleplex, multiplex and real-time PCR assays that are highly sensitive and specific have been developed for the detection and identification of many O groups of E. coli (Bastin and Reeves, Reference Bastin and Reeves1995; Wang et al., Reference Wang, Curd, Qu and Reeves1998, Reference Wang, Briggs, Rothemund, Fratamico, Luchansky and Reeves2001, Reference Wang, Huskic, Cisterne, Rothemund and Reeves2002, Reference Wang, Liu, Kong, Steinruck, Krause, Beutin and Feng2005, Reference Wang, Perepelov, Feng, Knirel, Li and Wang2009, Reference Wang, Ruan, Wei, Hu, Wu, Yu, Feng and Wang2010a, Reference Wang, Wang, Beutin, Cao, Feng and Wangb; Grozdanov et al., Reference Grozdanov, Zahringer, Blum-Oehler, Brade, Henne, Knirel, Schombel, Schulze, Sonnenborn, Gottschalk, Hacker, Rietschel and Dobrindt2002; Perelle et al., Reference Perelle, Dilasser, Grout and Fach2002; Shao et al., Reference Shao, Li, Jia, Lu and Wang2003; Fratamico et al., Reference Fratamico, Briggs, Needle, Chen and DebRoy2003, Reference Fratamico, DebRoy, Strobaugh and Chen2005, Reference Fratamico, DebRoy and Liu2009a, Reference Fratamico, DebRoy, Miyamoto and Liub, Reference Fratamico, Yan, Liu, DebRoy, Byrne, Monaghan, Fanning and Bolton2010, Reference Fratamico, Bagi, Cray, Narang, Yan, Medina and Liu2011; DebRoy et al., Reference DebRoy, Roberts, Kundrat, Davis, Briggs and Fratamico2004, Reference DebRoy, Fratamico, Roberts, Davis and Liu2005, Reference DebRoy, Roberts, Davis and Bumbaugh2010, Reference DebRoy, Roberts, Valadez, Dudley and Cutter2011; Feng et al., Reference Feng, Senchenkova, Yang, Shashkov, Tao, Guo, Cheng, Ren, Knirel, Reeves and Wang2004a, Reference Feng, Wang, Tao, Guo, Krause, Beutin and Wangb, Reference Feng, Han, Wang, Bastin and Wang2005a, Reference Feng, Senchenkova, Tao, Shashkov, Liu, Shevelev, Reeves, Xu, Knirel and Wangb, Reference Feng, Perepelov, Zhao, Shevelev, Wang, Senchenkova, Shashkov, Geng, Reeves, Knirel and Wang2007; Guo et al., Reference Guo, Feng, Tao, Zhang and Wang2004, Reference Guo, Kong, Cheng, Wang and Feng2005; Beutin et al., Reference Beutin, Kong, Feng, Wang, Krause, Leomil, Jin and Wang2005a, Reference Beutin, Tao, Feng, Krause, Zimmermann, Gleier, Xia and Wangb, Reference Beutin, Wang, Naumann, Han, Krause, Leomil, Wang and Feng2007; Cheng et al., Reference Cheng, Liu, Bastin, Han, Wang and Feng2007; Liu Y et al., Reference Liu, DebRoy and Fratamico2007, Reference Liu, Knirel, Feng, Perepelov, Senchenkova, Wang, Reeves and Wang2008; Ren et al., Reference Ren, Liu, Cheng, Liu, Feng and Wang2008; Han et al., Reference Han, Liu, Cao, Beutin, Kruger, Liu, Li, Liu, Feng and Wang2007; Goswami et al., Reference Goswami, Gyles, Friendship, Poppe, Vinogradov and Boerlin2010; Li et al., Reference Li, Liu, Cao, Han, Liu, Liu, Guo, Bastin, Feng and Wang2006, Reference Li, Liu, Chen, Guo, Guo, Liu, Feng and Wang2010a, Reference Li, Perepelov, Wang, Senchenkova, Liu, Shevelev, Guo, Shashkov, Chen, Wang and Knirelb).

Fig. 2. Multiplex polymerase chain reaction (m-PCR) assay for detecting STEC O-groups targeting the wzx gene. Lane 1, molecular weight markers; lane 2, pooled amplified DNA generated from eight STEC O-groups in one m-PCR reaction; lane 3, negative control strain E. coli K12; lane 4, O26 (155 bp); lane 5, O45 (238 bp); lane 6, O103 (321 bp); lane 7, O111 (438 bp); lane 8, O113 (514 bp); lane 9, O121 (628 bp); lane 10, O145 (750 bp); lane 11, O157 (894 bp). Reproduced with permission from DebRoy et al. (Reference DebRoy, Roberts, Valadez, Dudley and Cutter2011).
Table 2. Primer sequences for the detection of O groups by PCR

Detection of E. coli serogroups belonging to different pathotypes
Use of multiplex PCR assays targeting a serogroup-specific region within the E. coli O antigen gene cluster, for example, the wzx or wzy gene, as well as genes encoding for the Shiga toxins or other virulence genes has been reported (Fratamico et al., Reference Fratamico, DebRoy, Strobaugh and Chen2005, Reference Fratamico, DebRoy and Liu2009a, Reference Fratamico, DebRoy, Miyamoto and Liub, Reference Fratamico, Yan, Liu, DebRoy, Byrne, Monaghan, Fanning and Bolton2010). The multiplex assays can be used to simultaneously identify the E. coli serogroup and determine the specific pathotype, such as STEC (Fig. 3) or enteroinvasive E. coli (EIEC). Alternatively, food samples or other types of samples can be subjected first to screening for virulence genes of interest, followed by testing for specific E. coli O groups of interest, targeting genes within the O-antigen gene cluster (Perelle et al., Reference Perelle, Dilasser, Grout and Fach2004; Fratamico et al., Reference Fratamico, Bagi, Cray, Narang, Yan, Medina and Liu2011). Bugarel et al. (Reference Bugarel, Beutin, Martin, Gill and Fach2010) utilized GeneDisc® array (Gene Systems, Bruz, France) technology to identify 12 E. coli O serogroups and 7 H flagellar types, as well as the presence of virulence genes in one platform.

Fig. 3. Multiplex PCR detection of E. coli O22:H1 E14a targeting the wzx and wzy genes in spiked dog feces and ground beef. Lane M, molecular weight markers. Lane 1, PCR products for O22 wzx (458 bp) and O22 wzy (246 bp) from standard reference strain E14a (positive control) and 16S rRNA internal control (99 bp). Lane 2, H2O (negative control). Lane 3, O22 wzx and wzy genes amplified from dog feces spiked with E. coli O22 at 105 CFU/g of dog feces. Lane 4, dog feces spiked with E. coli O22 at 106 CFU/g. Lanes 5–10, multiplex PCR results for detection of O22:H5 95–3322 and O91:H21 96.1516 in ground beef. Lanes 5 and 6, samples inoculated with E. coli O22:H5 at 2 CFU/25 g. Lane 7, sample inoculated with 20 CFU//25 g and all samples subjected to enrichment for 18 h at 42°C (target genes: O22 wzx-458 bp, stx1/stx2-305 bp, O22 wzy-246 bp and 16S rRNA internal control-99 bp). Lanes 8 and 9, samples inoculated with O91:H21 96.1516 at 2 CFU/25 g. Lane 10, sample inoculated with 20 CFU/25 g and subjected to enrichment for 18 h at 42°C (target genes: O91 wzy-277 bp, stx1/stx2-305 bp and 16S rRNA internal control-99 bp). Reproduced with permission from Fratamico et al. (Reference Fratamico, DebRoy and Liu2009a).
Detection of O groups by microarrays
Microarrays have been developed for identification of E. coli O groups (Liu and Fratamico, Reference Liu and Fratamico2006) and for serotyping of enterotoxigenic E. coli (Wang et al., Reference Wang, Wang, Beutin, Cao, Feng and Wang2010b). Model DNA microarrays developed by Liu and Fratamico (Reference Liu and Fratamico2006) employed either oligonucleotides or PCR products spotted onto arrays followed by hybridization with labeled long PCR products representing the entire O-antigen gene cluster of specific E. coli serogroups. This microarray format can be expanded to target all of the E. coli O serogroups, and potentially also identify the H type by targeting genes encoding for the E. coli H flagellar antigens (e.g., fliC), as well (Wang et al., Reference Wang, Rothemund, Curd and Reeves2003). DNA microarrays have been developed for detecting strains involved in bovine diarrhea and septicemia (Liu et al., Reference Liu, Wu, Li, Beutin, Chen, Cao and Wang2010) and for the detection of E. coli causing post-weaning diarrhea and edema disease in pigs (Han et al., Reference Han, Liu, Cao, Beutin, Kruger, Liu, Li, Liu, Feng and Wang2007). Li et al. (Reference Li, Liu, Cao, Han, Liu, Liu, Guo, Bastin, Feng and Wang2006) developed a serotype-specific DNA microarray for identification of serogroups E. coli O55, O111, O114, O128 and O157.
Future directions
Although the DNA sequences of 96 O antigen gene clusters have already been reported, the nucleotide sequences of another 78 O groups are still being pursued. Comparative genomics of the O-antigen gene clusters and significance of genetic aberrations in the clusters will be apparent once all the clusters are sequenced. A better understanding of the genetic loci encoding all O-antigens that confer pathogenicity would lead to a more comprehensive view of the structural patterns and biosynthetic pathways used to build such antigens and of their role in pathogenesis. The genomic sequences will enable researchers to: (a) determine the role and relevance of genes encoding the O antigen that may be implicated as determinants of pathogenicity and disease specificity in humans and animals; (b) evaluate the molecular mechanisms for host specificity associated with different O groups in human and animal infections; (c) elucidate the molecular mechanisms of host adaptation and immune system evasion; (d) identify specific genes and proteins suitable for use in the development of the next generation of diagnostic, therapeutic and immunoprophylactic agents.







