Dental modification was commonly practiced in pre-Hispanic populations throughout Mesoamerica. It first aroused the curiosity of dentists (e.g. Van Rippen 1917; Whittlesey 1935) who were attracted to the skill and technical aspects involved in its execution. Although types have been created for the extreme variation in form and artistry (e.g. Romero 1958, 1970; Rubín de la Borbolla 1940; see Figure 1), it is only recently that attention has been turned to its cultural meaning in a systematic way (e.g. Havill et al. 1997; López Olivares 1997; Tiesler Blos 1999, 2001). There are two types of dental modification: filing and inlay. Dental filing is the alteration of tooth shape involving the creation of notches, grooves, or points. Dental inlay involves drilling holes and inserting various materials therein. The two types of dental modification are not mutually exclusive, although the frequency of their use varies with time. Dental filing seems to have been the earliest type of modification technique to appear among the Maya, originating in the Early Preclassic period (1400–1000 b.c.); dental inlay was introduced in the Middle Preclassic period (900–600 b.c.; Romero 1970). Filing and inlay were both very common during the Late Classic period (a.d. 700–900), but filing seems to have been the most common form of dental modification during the Postclassic period (a.d. 1000–1500; Romero 1970; Tiesler Blos 2001). Diego de Landa's (1941) ethnohistoric account described the practice of dental filing well into the Colonial period, which is corroborated by Lorena Havill and colleagues (1997), who observed it as the only form of dental modification in the colonial population at Tipu, Belize. Both dental filing and inlay are commonly depicted in ceramics and iconography from Mesoamerica (Caceres 1938; Rubín de la Borbolla 1940), the most popular example of this being the Sun God's T-shaped central incisors (Figure 2; Linné 1940). There is no evidence that dental filing or inlay was therapeutic; rather, it is believed to have been done for ritual or aesthetic purposes (Fastlicht 1948; Van Rippen 1917).
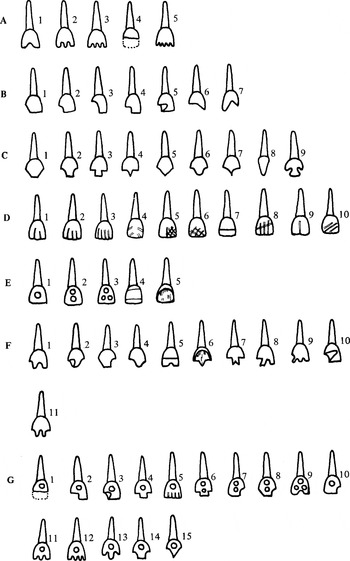
Romero's system of classification for teeth with dental modification (Romero Molina 1986:11, modified from Romero 1970).
Sun God with T-shaped central incisors (Linné 1940:13, from Joyce 1914:Figure 72).
Dental modification has been suggested as a marker of elite status (Becker 1973; Romero 1958; Smith 1972), but the majority of studies present little or no evidence for a relationship between socioeconomic status and dental modification within the ancient Maya (Fastlicht 1962; Havill et al. 1997; Massey and Steele 1997; Saul and Hammond 1973; Saul and Saul 1991; Saul and Saul 1997; Tiesler Blos 2001; Willey et al. 1965). Evidence from other cultural groups in Mesoamerica and South America similarly provides minimal support for a relationship between status and dental modification (Fastlicht and Romero 1951; Romero 1958). For example, the frequency of dental modification in low-status individuals from archaeological sites in Mexico such as Cholula, Tamuin, and Monte Negro was similar to that for elite individuals (Romero 1970). In general, males and females have similar rates of dental filing (Fastlicht 1948, 1962; Havill et al. 1997; Massey and Steele 1997; Romero 1970; Saul and Saul 1997; Smith 1972; but see Tiesler Blos 2001). This corroborates ethnohistorical data that describe dental filing in both males and females (Landa 1941). Dental modification is found primarily in individuals older than 15 (Romero 1970; Tiesler Blos 2001); the near-absence of modification in younger subadults suggests social proscription against members below a certain age.
The purpose of this study was to test further the hypothesis that dental modification is unrelated to sex or socioeconomic status using a Postclassic sample of 61 adults (individuals older than 16 years) from Lamanai, Belize. This study was underpinned by two main assumptions: (1) socioeconomic status can be indicated by burial offerings, grave type, and grave location; and (2) dietary differences can be related to status differences.
Dental Modification
Numerous studies have investigated how dental filing and inlay were created (Fastlicht 1948, 1962; Gwinnett and Gorelick 1979; Havill et al. 1997; Rubín de la Borbolla 1940; Sweet 1963; Van Rippen 1917; Whittlesey 1935). According to Landa (1941), old women performed the filing using stone abraders and water. Dental inlay, however, was not described by the European chroniclers and was less common in later periods. Consequently, there is little documentation of either the inlay specialists or their techniques.
The actual toolkit for dental modification is unknown but may have included flint blades and worked bone (Romero 1958). Most investigators believe that dental modification was performed on living subjects and that dental filing was achieved relatively easily by abrasion and not by chipping (Fastlicht 1948; Havill et al. 1997; Romero 1970; Rubín de la Borbolla 1940). The holes for the dental inlay were probably created by a drill, similar to that used to make holes in beads (Fastlicht 1948; Gwinnett and Gorelick 1979; Romero 1970; Rubín de la Borbolla 1940). This drill may have been a quartzite tube or a hardened bone used in conjunction with water and an abrasive material such as fine sand (Romero 1970). The types of stones used for inlay vary geographically and temporally but include pyrite, jade, turquoise, jadeite, hematite, and obsidian (Sweet 1963). Inlays were secured by either pressure or cement (Rubín de la Borbolla 1940), the composition of which is virtually identical to that of Portland cement, which is used in brick-laying and construction (Sweet 1963).
The most convincing evidence that dental modification was practiced on living subjects comes from dental disease associated with excessive drilling or filing. For example, if teeth were drilled or filed too deeply, the pulp would be exposed, possibly leading to infection and in some cases producing alveolar abscesses (Fastlicht 1948; Romero 1970; Rubín de la Borbolla 1940). In addition, if teeth were filed or drilled extensively, cold and pressure would be more easily transmitted to the pulp and nerve fibers within, likely leading to disuse of the affected tooth. This is corroborated by heavy calculus deposits found on “over-filed” teeth, indicating that they were not used for mastication (Evans 1973; Fastlicht 1948). Dental modification is found predominantly in the anterior teeth, commonly the maxillary incisors and occasionally the maxillary canines (Fastlicht 1948; Rubín de la Borbolla 1940). Consequently, the posterior teeth, primarily involved in mastication, were not impaired by dental filing.
Status and the Ancient Maya
Ancient Maya society is generally considered to have been a kinship- or lineage-based hierarchy with both regional and local rulers (Chase and Chase 1992). Ancient Maya society has traditionally been viewed as two-tiered: nobles (elite) and commoners (non-elites) (Flannery 1983; Sanders 1981; Webster 1985), but recent investigations argue for a multitiered system consisting of different levels of elite individuals (Chase and Chase 1992; Morley et al. 1983) or secondary elites (Webster 1992). For example, there was probably a “middle class” of merchants and administrators who enjoyed many of the privileges of elite status but may have occupied residential areas classified as lower status (A. Chase 1992; D. Chase 1992; Morley et al. 1983).
The idealized model of Maya spatial organization has traditionally been a ceremonial core surrounded by residences, with the lowest-status individuals residing farthest from the core, but there is evidence that the peripheral areas were not necessarily occupied by low-status individuals (e.g., A. Chase 1992). It is generally accepted that the ceremonial center was used by the elite. However, those buried in the site core could also have included lower-ranked individuals such as servants or retainers (Reed 1999). Consequently, burial location should not be used as the sole indicator of status.
A traditional marker of status is architecture. According to David Pendergast (1992:63), there are three criteria for the identification of a structure as an elite residence: location, form, and construction type. The latter two criteria are more important than location (for the reasons outlined earlier). Height, overall size, masonry construction, and/or corbel-vaulted ceilings (Haviland and Moholy-Nagy 1992) demonstrate control over a large labor force and elaborate planning and therefore indicate elite residence (Tourtellot et al. 1992). In contrast, low status residences are characterized by small platforms supporting perishable superstructures (Haviland and Moholy-Nagy 1992; Pendergast 1992; Rathje 1970; Tourtellot et al. 1992). Elite residences are usually the most visible archaeologically due to the masonry construction and sheer size. Consequently, according to the hierarchical/concentric theory of Maya site organization, many studies of ancient Maya sites are biased toward the high-status sector of the population (Danforth 1999).
Sex may be an additional source of differences in social status. Various forms of evidence suggest that Maya women exercised considerable power (Joyce 2000) and may have shared a similar social status to men. Consequently, if diet is linked to social status, males and females may have consumed similar foods. However, chemical analyses of various Maya populations provide conflicting evidence for dietary differences between males and females. Significant sex-based dietary differences exist at Altun Ha (White et al. 2001), Pacbitun (White et al. 1993); and Copan (Reed 1999; Whittington and Reed 1997), but not at Lamanai (Coyston et al. 1999; White and Schwarcz 1989; White et al. 1994), or Marco Gonzalez and San Pedro (Williams et al. 2006).
Evidence for Diet
In stratified societies, such as that of the ancient Maya, differential food-access and -consumption patterns may indicate attempts to establish, legitimize, and maintain differences in social status (Bryant et al. 1985; Hayden 1990). Support for this assumption comes from stable-isotope analyses, where dietary differences are found between elites and individuals of lower status in many Maya sites, such as Lamanai (Coyston et al. 1999; White et al. 1994), Pacbitun (White et al. 1993), Copan (Reed 1999; Whittington and Reed 1997), Altun Ha (White et al. 2001), and at Late Classic period sites in the Pasión region in Guatemala (Wright 1997). In addition, high-status Maya both in ancient (Haviland 1967) and modern populations (Bogin et al. 1992) are taller than low-status individuals, presumably because access to better nutrition enabled them to reach their full genetic potential for height (Danforth 1999).
Dental pathology such as caries and calculus are particularly useful for studying ancient Maya diet because bone preservation is limited in the tropical environment characteristic of Belize. Caries is a lytic dental disease that depends on multiple factors and is related to the level of carbohydrate consumption. In general, a high-carbohydrate diet produces higher caries rates (reviewed by Hillson 1996 and Navia 1994). Although high levels of protein and fat may provide some protection against caries (Bowen and Pearson 1993; Costa 1980; Hillson 1996; Magennis 1999), caries in Maya populations consistently have been associated with the carbohydrate-rich diet of maize (Evans 1973; Whittington 1999). The amount of carbohydrates consumed, however, appears to be less important than consistency and the frequency of consumption (Magennis 1999; Whittington 1999).
Calculus (or calcified plaque) is an additional indirect indicator of diet. Factors that affect its formation include calcium, phosphate, and alkalinity levels in oral fluid; the mineral content and amount of fluid ingested; saliva flow; the amount of fibrous/abrasive food; the amount of dietary protein; and cultural practices such as betel or coca chewing (Hillson 1979; Lieverse 1999; Magennis 1999). Calculus and caries may be negatively correlated because the presence of extensive calculus can inhibit the formation of caries (Hillson 1996). Because diet may not be the primary factor in calculus formation, other lines of evidence, such as zooarchaeology and stable-isotope analysis, should be used to corroborate dietary interpretations.
Stable-isotope analysis is well established in paleodiet reconstruction using human skeletal remains (for a recent review, see Katzenberg and Harrison 1997). Carbon-isotope analysis is based on the premise that plants fall into specific categories (C3, C4, Crassulacae acid metabolism [CAM]) depending on how they use carbon dioxide during photosynthesis (Bender 1971; Smith and Epstein 1971). The δ13C values for C3 plants (e.g., legumes, root crops) average −26‰ (Deines 1980:335), with a range from −34‰ to −22‰ (Vogel 1980:6). The δ13C values for C4 plants (e.g. maize, sorghum, tropical grasses) average −13‰ (Deines 1980:335), with a range from −16‰ to −9‰ (Smith and Epstein 1971:380). The isotopic ratio of CAM plants encompasses the range of both C3 and C4 plants; the exact value depends on their environmental surroundings (Deines 1980; Troughton et al. 1974). These plants would not have been dietary staples for the Maya. The distinct isotope ratios in the various plants are passed on to consumers and recorded in tissues with some isotopic enrichment (DeNiro and Epstein 1978; van der Merwe 1982). The unique isotopic composition of various types of plants (C3 versus C4), plus the enrichment between diet and tissue, enables researchers to determine which dietary staples (e.g., maize versus manioc) were consumed by past populations. However, when the diet contains marine foods (which generally have a carbon-isotope composition within the range of C4 plants) or equal proportions of C3 or C4 foods, it is difficult to identify the various components of the diet (Chisholm et al. 1983; Walker and DeNiro 1986). Nitrogen-isotope analyses help to separate marine from terrestrial resources (e.g., fish versus maize) and some C3 from C4 (mainly legume vs. non-legume) resources.
A stepwise enrichment of 3–4‰ in δ15N values of bone collagen has been well established between trophic levels (e.g., omnivore to carnivore, primary carnivore to secondary carnivore; DeNiro and Epstein 1981; Minagawa and Wada 1984; Schoeninger and DeNiro 1984). This trophic level effect can be used to distinguish between a maize- or marine-dependent diet because marine resources (fish, shellfish, and most plants) are enriched in 15N relative to terrestrial resources, such as maize (reviewed in DeNiro 1987 and Schwarcz and Schoeninger 1991). Nitrogen isotopes can also be used to distinguish between legumes (with an average δ15N value of +1‰) and non-legumes (with an average δ15N value between +2‰ and +4‰; Virginia and Delwiche 1982).
Site and Sample
Lamanai is a large ceremonial center located in northern Belize on the western shore of the New River Lagoon, approximately 70 km from the Caribbean coast (Figure 3). More than 4.5 km2 have been investigated, and 718 structures have been recorded by David Pendergast, formerly of the Royal Ontario Museum (Pendergast 1981, 1990). The site represents one of the longest continuous occupations in Mesoamerica, spanning the Preclassic to the Historic period (300 b.c.–a.d. 1670; Pendergast 1986). Approximately 5% of the structures (N = 37) have been investigated. Because this sample is heavily biased toward ceremonial and other large structures, the elite sector of the population is likely overrepresented (Pendergast 1981). The “menu” at Lamanai was diverse, according to archaeological, palynological and artifactual evidence (Pendergast 1981). Stable-isotope analysis has indicated that although maize was the carbohydrate staple, its consumption at Lamanai was not as great as that found at sites with less heterogeneous environments (White and Schwarcz 1989; White et al. 1994). Maize consumption is estimated to have been moderate based on chemical analyses of bone (White et al. 1994), and its potential abundance in the environment makes it unlikely that it was restricted to elite individuals. Chemical analyses indicate a dramatic increase in the consumption of maize and marine resources in the Postclassic period that was sustained into the Historic period (Coyston et al. 1999). The highest elites likely consumed animals (deer or dog) fed C-4 resources such as maize (Coyston et al. 1999).

Map of Mesoamerica showing location of sites mentioned in text (modified from White 1999:xvii).
The skeletal sample from Lamanai represents one of the larger and more continuous Lowland Maya samples. The sample included the remains of 82 individuals representing the Postclassic period. The Postclassic portion of the Lamanai sample was chosen because dental filing was most common during that period, consistent with Javier Romero's (1970) findings. All individuals were excavated in the southwestern section (grid position N10) from Structures 1 (N = 3), 2 (N = 32), 3 (N =3), 4 (N = 39), 5 (N = 1), and 9 (N = 3), plus one burial not assigned to any structure. Structures 1, 2, and 9 were ceremonial, and Structure 4 was a Classic-period structure later used as a burial mound (Pendergast 1981). Structures 3 and 5 were residential plazas and can be considered lower status than the other four structures (Pendergast 1985). Based on similarities in grave offerings and grave type, these individuals likely represent elites (Pendergast 1985), with the previously mentioned caveat that some interments may have included servants or retainers. Differences in the grave locations, however, could reflect different social identities or levels.
The sample includes 61 individuals aged 16 or older. Of the adults, 25 are male and 17 are female, a sex ratio of 1.5:1 (Table 1). Because of the poor preservation of the samples and the common practice of cranial modification, which obscures many of the sex-related cranial features, sex determination was not possible for many individuals (Table 1). Caries and calculus were previously quantified by Christine White (1986, 1994). Carbon-isotope ratios in bone carbonate were analyzed by Shannon Coyston and colleagues (1999). Carbon- and nitrogen-isotope ratios in collagen were analyzed by White and Henry Schwarcz (1989). Isotope data from collagen and carbonate were available for 14 and eight individuals, respectively (Table 1).
Postclassic skeletal sample from Lamanai, Belize

METHODS
Age and sex determinations were done by Herman Helmuth of Trent University, using standard forensic morphological features (Bass 1984; McKern and Stewart 1957; Phenice 1969). All data on dentition (including dental modification) were collected by White. The classification system created by Romero (1958, 1970) was used to identify modification types because it constitutes the Mesoamerican standard for categorization (see Figure 1). Age categories were condensed into four groups for this analysis: (1) 16–21; (2) 22–50 years; (3) older than 50; and (4) adult (exact age indeterminable). Individuals younger than 16 were excluded from these analyses because dental modification is rare in subadults and absent in the subadult sample from Lamanai. The caries rate was determined by adding the total number of caries observed in all teeth of an individual (following Kerr et al. 1990), and dividing this by the total number of teeth scored for that individual (Table 1). The fragmentary nature of the sample precluded an accurate analysis of postmortem tooth loss, a potential source of error in caries calculation (Hillson 1996). Calculus was scored on a scale of zero to four (Baer et al. 1961). Zero corresponds to its absence and four to severe deposits covering the entire crown. A single calculus score for each individual was determined by adding the calculus value for each tooth present, then dividing by the total number of teeth observed (Table 1).
Stable carbon- and nitrogen-isotope values from bone collagen for eight of 22 individuals (36%) with dental modification, compared with six of 39 individuals (15%) without dental modification (Table 1). This bias toward individuals with dental modification means that interpretations about social status and dental modification based on isotopic data must be viewed with caution and may not be representative of the entire Postclassic sample. Statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS), and all significant values are reported to probability (p) < .05, unless otherwise specified. To compare data between paired samples of normally distributed data (determined using SPSS), a Student's t-test was used. If the samples were not normally distributed, a Mann Whitney U-test (reported as Z score) was used.
RESULTS
Age and Sex
Twenty-two individuals (36%) age 16 or older showed dental modification. Only one individual (Structure 4, individual 4) had both dental filing and inlay; this individual was older than 50.
Dental modification was more common in females (10/17; 58%) than males (10/25; 40%), but this was not statistically significant (Z = −0.132; p = .911). The most common types of dental modification were B4, C4, and C5 (Figure 4). Four individuals (two male, two female) showed the pattern C4/B4, which may have led to the increased representation of these two styles. Four types of dental modification—A2, B1, B3, and C7—were present in only one individual each, all of whom were female (Figure 5). Type C6 was found in two individuals, also both female. Two types, A4 and G2, were present in only one individual each; both were male (Figure 5). Type C3 was found in three individuals, also all male (Figure 5). The two most common styles, C4 and B4, were present in almost equal numbers of males and females (Figure 5).

Number of individuals with the various types of dental modification (following Romero Molina 1986). Number of individuals along the Y axis. Individuals were counted only once, even if the type of modification was present on numerous teeth.

Distribution of the various types of dental modification between males and females (following Romero Molina 1986).
Dental Pathology and Stable Isotopes
Individuals with dental modification had a higher caries rate than individuals without modification (14% versus 11%), but the difference was not statistically significant (Student's t = 0.618; degrees of freedom (df) = 59; p = .539). Individuals with dental modification and without modification both had similar average calculus scores (Student's t = −0.777; df = 59; p = .440). There were no significant differences in the isotope values between individuals with modification and those without (Table 2). The average δ13Cap value for individuals with dental modification was much greater than for individuals without modification, but the sample is extremely small (Table 2).
Average isotope values including the results of statistical tests

Structure
Dental modification was found exclusively in individuals from Structures 1, 2, and 4 (Figure 6a). Dental modification was equally common in all three of these structures (analysis of variance [ANOVA] F = .126; df = 2,54; p = .882). There was overlap for many of the modification types between the structures, but five types (A4, B1, C3, C7, G2) were unique to Structure 2, and six types (A1, A2, B3, B5, C6, G1) were unique to Structure 4 (Figure 6b). However, this distribution is distorted by the fact that many of these types (A4, B1, C7, G2, G1, A2, B3) were represented by one individual each.

(a) Frequency of dental modification by structure; (b) distribution of the various types of dental modification by structure.
DISCUSSION
The rate of dental modification was not significantly different between males and females at Lamanai which is consistent with other studies in the Maya area (Havill et al. 1997; Massey and Steele 1997; Saul and Saul 1997, but see Tiesler Blos 2001). Similarities in the frequency of dental modification between males and females at Lamanai corroborate ethnographic evidence that both had equal access to positions of power. It is possible that differences in the styles of dental modification found between males and females may have been related to marriage practices and lineages or to social roles, but this hypothesis needs to be tested further.
Individuals with dental modification had slightly more caries and a more positive average δ13Cap isotope value than individuals without modification. This could be interpreted as differential access to maize; however, this conclusion needs to be tested further with a larger sample. It is unlikely that dental filing caused the differences in caries rates between individuals with modification and those without for two reasons: (1) only one of the 22 individuals with dental modification had a carious lesion on a modified tooth; and (2) for all individuals, dental modification occurred in the anterior teeth, mainly the central and lateral incisors, which are less predisposed to caries than posterior teeth (Whittington 1999). The differences in caries rates may be related to postmortem tooth loss. Individuals with modification retained significantly more teeth than individuals without modification (Student's t = 4.277; df = 59; p = .001); however, the remains were very fragmentary. This finding lends further support to Simon Hillson's (1996) caution that caries rate is heavily biased by postmortem tooth loss. Access to protein (indicated by the calculus score, δ13C and δ15N values from bone collagen) did not differ between individuals with dental modification and those without.
Overall, this analysis has demonstrated that there are no statistically significant dietary differences, as inferred from chemical and dental analyses, between individuals with dental modification and those without. However, it is possible that individuals with dental modification did have access to special food items, but the level of consumption was not great enough significantly to alter their isotopic composition and/or dentition. Although some dietary differences have been found among Postclassic elites (White et al. 2001), there is evidence that during the Postclassic period, social divisions within elites and between elites and commoners were not as pronounced as earlier time periods (Smith and Berdan 2000; Williams et al. 2006). This could explain the similarity in diet between the various individuals at Lamanai. An alternative explanation is the fact that our sample does not include skeletons from “commoner” sectors of Lamanai. Since there are no significant differences in dietary indicators among the sampled groups from Lamanai, we can propose that dental modification was not related to social status during the Postclassic period, but this needs to be tested further with isotopic data from a larger and more representative skeletal assemblage. Vera Tiesler Blos (2001) also suggests that, during the Postclassic period, dental modification lost its connotation of social distinction.
Comparison of dental modification by structure appears to suggest a relationship between dental modification and site layout. Dental filing was limited to individuals buried in Structures 1, 2, and 4. All three of these structures were ceremonial in nature and likely housed the burials of nobility. This is corroborated by the rich burials in Structures 2 (Burial N10-2/9) and 4 (Burial N10-4/46) that represent nobles (Pendergast 1992: 75). In addition, burials in Structures 2 and 4 contained high-status grave offerings, including painted pottery vessels, copper, gold, jade, and shell (Pendergast 1981). The absence of dental filing in individuals buried in the residential plaza (Structure 3) may represent a differential distribution of dental filing within the site. At Copan, for example, Tiesler Blos (2001) found that Romero's (1970) Type C was more common in burials from the outlying areas, and Type E was more common in burials from the central ceremonial area. It has previously been suggested by White (1994) that there could be different levels of elites or lineages of elites who might choose a certain type of dental modification as a means of identification. Tiesler Blos (2001) further suggests that dental modification may be a means of local family organization. However, it must also be noted that the burial samples from Structures 3 and 9 were extremely small relative to Structures 2 and 4.
There is support for the general idea that dental and cranial modification are a means of identification, perhaps for the region or a lineage or of a political leader (Blom et al. 1998; Gertzen 1993; Lichtenfeld 2001; Patterson 1987; Tiesler Blos 1999; Torres-Rouff 2002). For example, “The [Inca] state required newly encapsulated groups to adopt practices that would distinguish them from neighboring groups—e.g., members of the Huancavelicas extracted six of their front teeth” (Patterson 1987:123; for a comprehensive review of regional differences in dental modification within Mexico, Guatemala, and Honduras, readers are referred to Romero 1958; Romero Molina 1986; Tiesler Blos 2001). Table 3 highlights examples of local differences in dental modification within Belize and regional differences between Belize and Guatemala. Although there is considerable overlap between the two regions, Romero's (1970) types E, F and G are more common in Guatemala and the Peten region (López Olivares 1997:Table 8.5:114) relative to Belize. Type E was not found in any of the Belizean sites included in Table 3; however, Romero (1958:62) noted its presence at Baking Pot and San José, Belize. These regional differences, in addition to those found by Tiesler Blos (2001), support the idea that the type of dental modification may represent identification with a local or regional polity, or familial lineage.
Studies of dental modification from other Maya sites

Temporally uncontrolled regional comparisons are problematic, however, because the presence and types of dental modification appear to have changed with time (Romero 1970; Tiesler Blos 2001), and many samples are extremely small or come from tooth caches (Lubaantun; Saul and Hammond 1973) and sacrifices (Colha; Massey and Steele 1997). Nevertheless, these data suggest that certain types of dental modification (e.g., types A, B, C) predominated at Lamanai during the Postclassic, while other types (e.g., E, F, G) were more common in other regions at different times.
CONCLUSION
The purpose of this analysis was to investigate whether dental modification was differentially distributed by sex or social status within the Postclassic burial sample from Lamanai, Belize, where the frequency of dental modification (36%) is similar to that at other Maya sites. Consistent with evidence from other Maya sites, no significant sex differences existed in the frequency of dental modification. However, five types of dental modification (A2, B1, B3, C6, C7) were present exclusively in females, and three types (A4, C3, G2) were present exclusively in males. Dental modification was not linked with any particular dietary regime as indicated by caries rate, calculus, and δ13C and δ15N values, although a δ13Cap value may indicate differential maize consumption. In contrast, architecture and burial location do suggest an association between dental modification and social or status differences relating to site layout. Of the six structures with burials, only three structures contained individuals with dental filing. All three of these structures were ceremonial in nature and likely housed the remains of nobility or elite individuals. Structures 3 and 5 were residential platforms, and the remains discovered there were presumed to be of lower-elite status; no individuals from these structures showed dental filing. Although the number of individuals was not equal among all six structures, the differences in frequency do support the theory that dental modification may have been differentially distributed within Lamanai. The most popular types of dental modification at Lamanai during the Postclassic period were C4 and B4, and these were present in both males and females. B4 was the most common type of dental modification found within other Maya sites in Belize, but C4 was relatively uncommon. Compared with the Peten region of Guatemala, where Romero's (1970) types E, F, and G are more common, the types of dental modification within Belize appear to be distinct. This is similar to the regional differences in the types of dental decoration between Mexico, Guatemala, and Honduras documented by Tiesler Blos (2001). This study demonstrates that dental modification during the Lamanai Postclassic was most likely a symbol of social or political affiliation. Better understanding of the relationship between status and dental decoration would come from analysis of skeletal assemblages from all sectors of this or other sites representing both elite and commoner living spaces from multiple time periods. Ultimately, this promising line of evidence, together with new isotopic techniques such as oxygen and strontium isotopic analysis of dental enamel, which reconstruct geographic relocation, may enable a greater understanding of the relationship between social organization and population movement among the ancient Maya.
RESUMEN
La modificación dental, presente en 36% de la muestra adultos funeraria (N = 61) que se fecha al período posclásico en Lamanai, Belice, fue analizada para determinar su asociación con estrato social y el sexo. Se usaron indicadores biológicos de la dieta (los isotopos estables y la patología dental), así mismo como los indicadores arqueológicos (ubicación de la tumba y la forma arquitectonica). No se encontró ninguna relación entre la dieta y la modificación dental. Los hombres y las mujeres comparten algunos de los tipos de modificaciones, a diferencia de otras modificaciones que fueron sin distinción de genero. La frecuencia de las modificación es varia por el contexto arqueológico, por ejemplo, solamente fue presente en la muestra de estructuras ceremoniales, la cual se supone representa el estrato social. Los altos tipos de modificación varien entre los sitios pertenecientes a Belice y aquellos de la otro regiónnes. Esta información apoya la hipótesis de que la modificación dental podría ser una forma de identificación con un linaje y un gobierno, un soberano, o una región.
ACKNOWLEDGMENTS
The authors acknowledge the generosity of David Pendergast, formerly of the Royal Ontario Museum, in supplying information and site reports from Lamanai, Belize, Jaime Awe, the Department of Archaeology, Belize and Dr. Herman Helmuth, Trent University, for allowing access to the skeletal material. Isotopic analyses were supported by the Natural Science and Engineering Research Council of Canada and Trent University. The authors thank Jerome Rose, Paul Sciulli, Tiffiny Tung, and an anonymous reviewer for helpful comments and references. The authors also thank Carolusa González Tirado, Subdirectora de Conservación, Biblioteca Nacional de Antropología e Historia, Mexico City, for photocopying and sending additional reference material.











