In many parts of the southeastern United States,Footnote 1 wild turkeys (Meleagris gallopavo) were sources of meat, eggs, bones, and feathers for Indigenous Americans. At Mississippian period (AD 1000–1450) sites in the Southeast, they were a common food (Peres Reference Peres2017) and often associated with contexts related to prestige, ritual, and feasting (Jackson and Scott Reference Jackson and Scott2003; Ledford and Peres Reference Ledford, Peres, Peres and Deter-Wolf2018; Reitz et al. Reference Reitz, Williams and Dalton2020). Given their importance as both utilitarian and ritual resources, and the successful domestication of the species in both the American Southwest and Mesoamerica (Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010), Indigenous peoples in Eastern North America (eastern United States and Canada) may have practiced flock management or small-scale captive rearing to promote turkey abundance.
Previous suggestions for turkey management or rearing in the Southeast are based primarily on sex ratios observed in zooarchaeological assemblages. Peres and Ledford (Reference Peres and Ledford2016) argue that an overabundance of large-bodied male turkeys in Mississippian deposits at the Fewkes site (40WM1) demonstrates potential flock management. Wild-kill assemblages typically contain more females and subadults than large adult males. Similar evidence suggests that wild poults (juvenile turkeys) were reared at Moundville (1TU500; Jackson and Scott Reference Jackson and Scott2003:566). Selective hunting or elite provisioning, however, could also explain the observed overrepresentation of adult male turkeys in these assemblages. Other lines of archaeological evidence for turkey management or rearing are lacking.
Ethnographic and ethnohistoric accounts provide additional evidence for potential turkey management and rearing in the Southeast. The De Soto chronicles (AD 1539–1543) report that large quantities of turkeys, or “hens,” were given to Spaniards by Indigenous peoples (Rangel Reference Rangel, Clayton, Knight and Moore1993:280–281; Robertson Reference Robertson, Clayton, Knight and Moore1993:83, 86, 165). Although these could be wild hunted turkeys, these large gifts raise the possibility that turkeys were penned or reared to ensure sufficient numbers were available for ceremonial or political events. Cherokee and other southeastern ethnohistoric accounts describe using scattered maize (Zea mays) to lure wild turkeys during hunting and rearing turkey poults from eggs to ensure reliable access to meat and feathers or to lure other wild turkeys (Lawson Reference Lawson1966 [1709]:149; White Reference White1980; Whitthoft Reference Whitthoft1946:377). Similar ethnohistoric accounts of the provisioning or taming of wild animals exist for other parts of Eastern North America (e.g., Galton Reference Galton1865; Sagard Reference Sagard and Hornby1939), and a stable isotope study by Morris and colleagues (Reference Morris, White, Hodgetts and Longstaffe2016) suggests maize provisioning of wild turkeys in Late Woodland (AD 900–1600) southwestern Ontario.
Neither turkey domestication, defined as long-term controlled breeding, nor management, have been explored extensively in Eastern North America. Throughout this article we use the term “management” to broadly refer to human behaviors that intentionally promote increased turkey abundance and availability (Zeder Reference Zeder2015). These could include selective hunting, seasonal provisioning of wild flocks with maize, or captive rearing. We specifically use the term “rearing” to acknowledge the potential for nonintensive feeding and tending of turkeys without controlled breeding (Vigne Reference Vigne2011; Zeder Reference Zeder2015).
Turkeys are highly tolerant of anthropogenic environments. In both the American Southwest and Mesoamerica, turkey management led to domestication, but it is currently unknown whether turkey management was practiced in other parts of the species’ natural range, such as the Southeast. Small-scale, nonintensive rearing could be largely invisible in the zooarchaeological record because it may not significantly increase the number of turkeys in archaeological assemblages. We therefore used stable isotope (δ13C, δ15N) and ancient DNA (aDNA) analyses to investigate whether turkeys were managed or captively reared at seven Mississippian period sites in the southeastern states of Tennessee and Georgia. Stable isotopes test for extensive maize consumption, a trait that distinguishes domestic and captive turkeys in both the American Southwest and Mesoamerica from their wild counterparts (Conrad et al. Reference Conrad, Jones, Newsome and Schwartz2016; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; McCaffery et al. Reference McCaffery, Tykot, Gore and DeBoer2014; Rawlings and Driver Reference Rawlings and Driver2010; Thornton et al. Reference Thornton, Emery and Speller2016). Our genetic analyses assess the degree and nature of mitochondrial DNA (mtDNA) variation among the birds, which might indicate the degree of selective breeding (if any), the introduction of domestic turkeys from either Mesoamerica or the American Southwest, or both.
Turkey management would be consistent with other examples of complex human-environment interactions in the Southeast, such as plant domestication (Smith Reference Smith2006) and fire and forest management (Abrams and Nowacki Reference Abrams and Nowacki2008; Delcourt et al. Reference Delcourt, Delcourt, Ison, Sharp and Gremillion1998). The Mississippian period is characterized by widespread maize agriculture, population growth, construction of large earthen mounds, and some degree of social inequality. Within this context, feasting and differential access to resources were important in negotiating and displaying status (Blitz Reference Blitz1993; Jackson and Scott Reference Jackson and Scott2003). In other parts of North America, turkey management and eventual domestication seem to have been motivated initially by controlling access to their feathers (Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; McKusick Reference McKusick2001) or by their use as status or ceremonial items (Thornton and Emery Reference Thornton and Emery2017). Increased demand for political or ceremonial events also could have motivated turkey management in the Southeast. Alternately, Mississippian peoples in Eastern North America may not have experimented with turkey management or rearing, despite adopting other Mesoamerican domesticates (e.g., maize, beans [Phaseolus spp.], and squash [Cucurbita spp.]) and the contemporary rearing of turkeys in both Mesoamerica and the American Southwest. Determining whether turkeys were managed or reared in the Southeast is thus relevant to a broader understanding of the cultural and environmental factors associated with the decision to invest in animal management or domestication. This line of inquiry also contributes to broader discussions of human-animal interactions beyond wild or domestic dichotomies (e.g., Zeder Reference Zeder, Gepts, Famula, Bettinger, Brush, Damania, McGuire and Qualset2012, Reference Zeder2015).
North American Turkeys: Genetic and Dietary Diversity
Six subspecies of wild turkey are found in central and northern Mexico and the eastern and southwestern United States (Figure 1). The subspecies native to the eastern United States and Canada (M. g. silvestris) has the broadest geographic distribution but is not thought to have been domesticated. In contrast, subspecies native to the American Southwester and Mesoamerica were domesticated by approximately 300–100 BC (Badenhorst and Driver Reference Badenhorst and Driver2009; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Thornton and Emery Reference Thornton and Emery2017).

Figure 1. Map of North America showing the geographic ranges of the six subspecies of Meleagris gallopavo (M. g. silvestris, M. g. osceola, M. g. merriami, M. g. intermedia, M. g. mexicana, M. g. gallopavo) and the Central American ocellated turkey (Meleagris ocellata).
Mitochondrial DNA analysis confirms that the southern Mexican subspecies (M. g. gallopavo) gave rise to the domestic turkeys bred and reared throughout the world today (Canales et al. Reference Canales, Manuel, Landi, Delgado Bermejo, Martínez, Acosta and Barro2019; Monteagudo et al. Reference Monteagudo, Avellanet, Azón and Teresa Tejedor2013; Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010). Genetic evidence also supports the independent domestication in the American Southwest of at least one other subspecies of wild turkey (Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010). Turkeys domesticated in the Southwest, however, do not appear to have contributed mtDNA to the genetic stock of modern domestic turkeys (Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010).
Within populations of Southwest archaeological turkeys, Speller and colleagues (Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010) identified two major mitochondrial DNA haplogroups. The most common haplogroup (referred to as H1) has low genetic diversity and is genetically distinct from wild and domestic Mesoamerican turkeys and from the Merriam's subspecies (M. g. merriami), which is native to the Southwest. Speller and colleagues (Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010) conclude that the H1 haplogroup represents a population of managed/domesticated turkeys introduced to the Southwest from outside the region, whereas the other major haplogroup (H2) corresponds to local/wild turkeys. Lipe and colleagues (Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016), however, indicate that turkeys from both haplogroups were heavily maize-fed and kept within human settlements, clarifying that both haplogroups contributed to precolumbian domestic flocks. Domestic turkeys from Mesoamerica belong to haplogroup H3 (Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010). The genetics of archaeological eastern wild turkeys (M. g. silvestris) have not been documented but are expected to be similar to those previously reported for their modern counterparts (Mock et al. Reference Mock, Theimer, Rhodes, Greenberg and Keim2002; Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010).
Dietary Shifts Associated with Management or Captive Rearing
When animals are brought under human control, their diets often change due to range restrictions or consumption of human-provided fodder, food waste, or both. Stable carbon (13C/12C) and nitrogen (15N/14N) isotope ratios serve as proxies for dietary shifts. Stable isotope analysis identifies management or captive rearing because dietary shifts may not be accompanied by morphological or genetic changes if breeding is not controlled, if the captive rearing and breeding process is in its early stages, or if there is extensive introgression between wild and captive-reared populations. Isotopic shifts associated with turkey husbandry and domestication have been identified in Mesoamerica and the American Southwest (Conrad et al. Reference Conrad, Jones, Newsome and Schwartz2016; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; McCaffery et al. Reference McCaffery, Tykot, Gore and DeBoer2014; Rawlings and Driver Reference Rawlings and Driver2010; Thornton et al. Reference Thornton, Emery and Speller2016, Reference Thornton, Emery, Steadman, Speller, Matheny and Yang2012). Dietary shifts also indicate management or captive rearing of nondomesticated white-tailed deer (Odocoileus virginianus), rabbits/hares (Leporidae), golden eagles (Aquila chrysaetos), and large felids (Panthera onca, Puma concolor) in Mesoamerica (Somerville et al. Reference Somerville, Sugiyama, Manzanilla and Schoeninger2016; Sugiyama et al. Reference Sugiyama, Fash and France2018, Reference Sugiyama, Somerville and Schoeninger2015; White et al. Reference White, Pohl, Schwarcz, Longstaffe and Emery2004), scarlet macaws (Ara macao) in the American Southwest (Somerville et al. Reference Somerville, Nelson and Knusdon2010), and hutias (Geocapromys ingrahami) in the Caribbean (LeFebvre et al. Reference LeFebvre, deFrance, Kamenov, Keegan and Krigbaum2019).
Wild turkeys have an omnivorous diet including fruits, flowers, seeds, nuts, insects, terrestrial gastropods, small lizards, and the leaves of shrubs, forbs, and grasses (Hurst Reference Hurst and Dickson1992). Most foods consumed by wild turkeys are C3 plants (e.g., fruits, shrubs, nuts, and flowers), but native C4 grasses (e.g., Panicum virgatum, Andropogon gerardii) are also available in the Southeast. Although turkeys often are considered crop pests, turkeys in maize fields primarily consume insects and waste grain (i.e., grain left over from the previous harvest) instead of seedlings or ripening maize grains (Groepper et al. Reference Groepper, Hygnstrom, Houck and Vantassel2013). Turkeys will consume maize when it is made available by people or when crop pests such as deer, squirrels (Sciuridae), blackbirds (Corvidae), and raccoons (Procyon lotor) knock down stalks or pull off cobs to obtain grain (MacGowan et al. Reference MacGowan, Humberg, Beasley and Rhodes2006; Otieno and Frenette Reference Otieno and Frenette2017). Turkeys, therefore, had access to maize, but large quantities were not likely consumed unless it was provided to them as bait or fodder (Morris et al. Reference Morris, White, Hodgetts and Longstaffe2016). Wild southeastern turkeys are expected to have largely C3-based diets (δ13Cco < −18‰), whereas captive-reared turkeys would have C4-based diets (δ13Cco ≥ −12‰) reflecting heavy maize consumption. Mixed C3/C4 diets would indicate that wild turkeys consumed maize-eating insects, foraged in maize fields or middens, or consumed maize used as a hunting lure. Captive-reared turkeys fed C3 plants, such as acorns (oak nuts), would be isotopically indistinguishable from wild birds. However, the strong maize signature observed in domestic turkeys elsewhere in North and Central America (Conrad et al. Reference Conrad, Jones, Newsome and Schwartz2016; Jones et al. Reference Jones, Conrad, Newsome, Kemp and Kocer2016; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; Rawlings and Driver Reference Rawlings and Driver2010; Thornton et al. Reference Thornton, Emery and Speller2016) suggests that similar patterns could be expected in the Southeast where maize was a staple resource.
Slightly higher δ15N in domestic turkeys is reported for both the American Southwest and Mesoamerica (Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; McCaffery et al. Reference McCaffery, Tykot, Gore and DeBoer2014; Thornton et al. Reference Thornton, Emery and Speller2016). Higher δ15N in captive-reared turkeys could reflect increased carnivory of animal pests associated with human settlements (DeNiro and Epstein Reference DeNiro and Epstein1981; Schoeninger and DeNiro Reference Schoeninger and DeNiro1984), rearing conditions that promote protein or water stress (Hobson et al. Reference Hobson, Alisauskas and Clark1993), consumption of human or animal waste, or consumption of crops enriched in 15N due to fertilization or nitrogen-cycle processes associated with crop or land management practices (Bogaard et al. Reference Bogaard, Heaton, Poulton and Merbach2007; Fraser et al. Reference Fraser, Bogaard, Heaton, Charles, Jones, Christensen and Halstead2011; Guiry et al. Reference Guiry, Beglane, Szpak, Schulting, McCormick and Richards2018, Reference Guiry, Orchard, Royle, Cheung and Yang2020; Hart and Feranec Reference Hart and Feranec2020; Hwang et al. Reference Hwang, Millar and Longstaffe2007; Szpak Reference Szpak2014). These possibilities may increase captive/domestic turkey δ15N by ~1 to 4‰ over wild turkeys.
Materials and Methods
Stable Isotope Analysis
We analyzed bone collagen δ13Cco and δ15N in 83 archaeological turkeys from seven Mississippian sites: three mound centers containing multiple flat-topped earthen mounds arranged around a central plaza, two towns containing one or more platform mounds, one small village site, and one site interpreted as a chiefly compound (Figure 2; Table 1). Twenty-two white-tailed deer and four canids (Canis sp.) were included for comparison. Deer are primarily browsers and are expected to have a C3-based diet, but their potential to feed in maize fields makes them a good comparison as a wild and potentially garden-hunted species. Canids presumed to be domestic dogs are used as a proxy for animals feeding largely within human settlement areas. Bone apatite δ13Cap was analyzed in a subsample of remains (61 turkeys, 5 deer, and 4 canids), but the results are not emphasized due to the greater potential for diagenesis in bone apatite compared to collagen (King et al. Reference King, Tayles and Gordon2011). Contextual information is lacking for many samples (see Supplemental Text 1; Supplemental Table 3), but turkey remains primarily came from middens or trash pits with smaller quantities from structure floors (n = 2), and burial fill layers (n = 6).
Figure 2. Map showing study sites. Middle Cumberland River Valley sites include Fewkes, Mound Bottom, Sandbar Village, Gordontown, and Inglehame Farm. Key: AL, Alabama; GA, Georgia; KY, Kentucky; MS, Mississippi; NC, North Carolina; SC, South Carolina; TN, Tennessee.
Table 1. Study Sites According to Chronology and Number of Individuals per Taxa Sampled for Isotopic Analysis.

a Sandbar village (40DV36) lacks an earthen mound but is currently interpreted as a peripheral section of the larger Mississippian town known as the Widemeier site (40DV9) (Smith and Moore Reference Smith and Moore2012).
Within each site, we ensured the sampling of discrete individuals by restricting our sample to single skeletal elements from the same side of the body. When skeletal elements were nonredundant, we relied on element age and size comparisons to prevent redundant sampling of individuals. Isotopic sampling was limited to adult individuals because very young turkeys consume large quantities of arthropods and shift to eating more plants as they mature (Hurst and Stringer Reference Hurst and Stringer1975). By only including adult turkeys, we controlled for age-based dietary variations.
Most samples (n = 97) were processed at Washington State University (WSU), with a subset (n = 12) processed at the Center for Applied Isotope Studies (CAIS) at the University of Georgia. Nearly identical procedures and equipment were used at both locations using a modified Longin (Reference Longin1971) method (see Supplemental Text 2 for a full description of the methods). Bone collagen δ13C and δ15N values were accepted when atomic C:N ratios were 2.9–3.6 and when collagen yield was >1% of dry weight (Ambrose Reference Ambrose1990). Data from samples not meeting these criteria are reported but not included in data plots and statistical analyses. We assessed differences in mean δ13C and δ15N across categories via independent sample two-tailed t-tests assuming unequal variance.
Ancient DNA Extraction and Analysis
Ancient DNA was extracted from 31 turkey skeletal elements in two laboratories: WSU and the University of York (BioArCh). At WSU, DNA extraction followed methods described by Kemp and colleagues (Reference Kemp, Monroe, Judd, Reams and Grier2014) and Moss and colleagues (Reference Moss, Judd and Kemp2014). At BioArCh, extraction methods followed those established by Yang and colleagues (Reference Yang, Eng, Waye, Dudar and Saunders1998) and modified as described in Speller and colleagues (Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010; see Supplemental Text 3 for full description of the laboratory methods).
At both laboratories, overlapping amplicons were sequenced to cover a maximum of 506 bp of the turkey mtDNA D-loop spanning nucleotide positions 15507–16013 (based on a complete mtDNA genome of GenBank specimen EF153719; Guan et al. Reference Guan, Silva, Gyenai, Xu, Geng, Tu, Samuels and Smith2009). DNA extracts were PCR amplified using primers described in Kemp and colleagues (Reference Kemp, Judd, Monroe, Eerkens, Hilldorfer, Cordray, Schad, Reams, Ortman and Kohler2017) and Speller and colleagues (Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010). Successfully amplified PCR products were sequenced using forward or reverse primers or both at Eurofins Genomics (Ebersberg, Germany), Elim Biopharm (Hayward, California) or MC Lab (South San Francisco, California). Canid samples were amplified for various stretches of the D-loop using primers and conditions described by Kemp and colleagues (Reference Kemp, Judd, Monroe, Eerkens, Hilldorfer, Cordray, Schad, Reams, Ortman and Kohler2017).
Turkey sequences were visually edited, and multiple sequences from the same bone were compiled into consensus sequences using ChromasPro software (www.technelysium.com.au) or Sequencher (version 4.8). The 25 turkey consensus sequences were submitted to GenBank under Accessions: MN587233-MN587257. The obtained ancient DNA sequences were BLAST-compared through GenBank to evaluate their identification as M. gallopavo. Multiple replicates of amplification and sequencing were used to confirm novel mutations and haplotypes and to resolve postmortem nucleotide damage. The obtained sequences were authenticated based on multiple criteria, including (a) the use of dedicated aDNA facilities, (b) no amplifications of expected length within the blank extracts and PCR negative controls, (c) multiple haplotypes observed within the dataset, and (d) amplification and sequencing conducted in independent laboratories yielding consistent results.
Initially, sequences were truncated to 435 bp (position 15567–16002) to remove primer sequences and make them comparable to published sequences. The obtained D-loop sequences were compared with 502 M. gallopavo sequences, including archaeological turkeys from the American Southwest (Kemp et al. Reference Kemp, Judd, Monroe, Eerkens, Hilldorfer, Cordray, Schad, Reams, Ortman and Kohler2017; Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010) and Mesoamerica (Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018), modern commercial breeds (Monteagudo et al. Reference Monteagudo, Avellanet, Azón and Teresa Tejedor2013), and North American wild turkeys (Mock et al. Reference Mock, Theimer, Rhodes, Greenberg and Keim2002; Szalanski et al. Reference Szalanski, Church, Oates, Bischof and Powers2000). Multiple alignments of the haplotype sequences and published Meleagris mtDNA reference sequences were conducted using ClustalW (Thompson et al. Reference Thompson, Higgins and Gibson1994) through BioEdit (Hall Reference Hall1999). Median-joining networks were created using Network (v. 5.0) and Network Publisher (Bandelt et al. Reference Bandelt, Forster and Röhl1999). Haplotype (h) and nucleotide (π) diversity were assessed based on a 309-bp fragment (positions 15651–15960) for which the majority of individuals contained sequence data and for which no polymorphisms could be observed within the larger 435-bp fragment. Diversity values were calculated for the Fewkes samples, contemporaneous archaeological turkey populations from the American Southwest (Kemp et al. Reference Kemp, Judd, Monroe, Eerkens, Hilldorfer, Cordray, Schad, Reams, Ortman and Kohler2017), and modern eastern wild turkey populations (Mock et al. Reference Mock, Theimer, Rhodes, Greenberg and Keim2002) using DnaSP v 5.10 (Librado and Rozas Reference Librado and Rozas2009). To ensure consistency with the Fewkes assemblage, diversity values for the comparative populations were assessed based on the same 309-bp fragment.
Genetic distances between populations of wild North American turkeys (Mock et al. Reference Mock, Theimer, Rhodes, Greenberg and Keim2002), American Southwest archaeological turkeys (Kemp et al. Reference Kemp, Judd, Monroe, Eerkens, Hilldorfer, Cordray, Schad, Reams, Ortman and Kohler2017; Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010), and the Fewkes archaeological turkeys were calculated on this same 309-bp fragment, using Arlequin 3.5 software (Excoffier and Lischer, Reference Excoffier and Lischer2010). FST pairwise comparisons were obtained with the Reynold's coancestry coefficient calculation, and associated p values were calculated on 1,023 repetitions. Negative indices and distances that were not significantly different at a 0.05 threshold were considered as null. A neighbor joining tree of the distances matrix was created using the “ape” library (Paradis et al. Reference Paradis, Claude and Strimmer2004) implemented in R 3.3.3 (R Core Team 2017).
The canid samples yielded no amplicons, so we could not confirm species/subspecies (i.e., domestic dog [Canis lupus familiaris], wolf [Canis lupus], or coyote [Canis latrans]). Given that coyotes expanded into the Southeast in recent times (Hody and Kays Reference Hody and Kays2018), the canids are likely dogs or wolves. Regardless of subspecies, δ13C can be used as a proxy for human interaction or management with C3-based diets expected in wild canids and more C4-based diets in tame or domesticated canids (Monagle et al. Reference Monagle, Conrad and Jones2018).
Results
Isotopic Evidence of Paleodiet
With the exception of one deer, all archaeological samples were well preserved, yielding acceptable atomic C:N ratios (2.9–3.6) and collagen yield weights (>1%). Accuracy of measured δ13C and δ15N was better than ± 0.2‰ based on replicate analysis (n >10) of laboratory standards. Precision of δ13C and δ15N measured from repeated chemical isolation of collagen from archaeological samples (n = 8) was ± 0.15 and ± 0.10‰ for δ13Cco and δ15N, respectively. Full isotopic results appear in Supplemental Table 1.
All southeastern turkeys had relatively low δ13Cco (mean = −20.1‰; range = −15.4 to −22.2‰; Table 2), which distinguishes them from archaeological domestic turkeys from the American Southwest and Mesoamerica that consumed a maize-based diet (δ13Cco ≥ −12‰; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; Rawlings and Driver Reference Rawlings and Driver2010; Thornton et al. Reference Thornton, Emery and Speller2016; Figure 3). Instead, turkeys from the Southeast resemble archaeological turkeys reported from southern Ontario (mean δ13Cco = −20.6‰; Figure 3; Guiry et al. Reference Guiry, Orchard, Needs-Howarth and Szpak2021; Morris et al. Reference Morris, White, Hodgetts and Longstaffe2016). Turkey δ13Cco did not vary significantly between sites in Tennessee and Georgia (t-test p = 0.24) nor between sites with earlier (e.g., Mound Bottom) and later (e.g., Fewkes) Mississippian occupations (t-test p = 0.29). Eight turkeys had slightly higher δ13Cco (−15.4 to −18.0‰) representing some consumption of C4 resources. Except for a turkey from Inglehame Farm (δ13Cco = −15.47‰), all turkeys with higher δ13Cco (≥−18‰) were from Fewkes (δ13Cco = −16.6 to −18.0; Figure 4). Available contextual information is limited, but the turkeys with mixed C3/C4 diets do not appear to be restricted to any particular context; instead they came from various site areas and deposit types including middens, structures, and burial fill deposits (see Supplemental Text 1; Supplemental Table 3). Moreover, Fewkes turkeys recovered within the same feature, including refuse pits and burial fill deposits, show variable δ13Cco, indicating that turkeys with varying diets were disposed of in the same location.

Figure 3. Comparison of mean (± 1 standard deviation) δ13Cco of archaeological fauna from the Southeast (SE) including turkeys (triangles), deer (diamond), and canids (square) compared to published values of archaeological turkeys from southern Ontario, Canada (Guiry et al. Reference Guiry, Orchard, Needs-Howarth and Szpak2021; Morris et al. Reference Morris, White, Hodgetts and Longstaffe2016), domestic turkeys from Mesoamerica (MesoAM) (Thornton et al. Reference Thornton, Emery and Speller2016) and the American Southwest (SW) (Conrad et al. Reference Conrad, Jones, Newsome and Schwartz2016; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Kellner et al. Reference Kellner, Schoeninger, Spielmann, Moore and Morgan2010; McCaffery et al. Reference McCaffery, Tykot, Gore and DeBoer2014; Rawlings and Driver Reference Rawlings and Driver2010), and Mississippian period (AD 1150–1550) humans (circles) from Irene Mound (Hutchinson et al. Reference Hutchinson, Larsen, Norr, Schoeninger and Lambert1992), and four sites in Tennessee's Cumberland River Valley (Mound Bottom, Goodlettsville, Arnold, and Averbuch; Buikstra et al. Reference Buikstra, Autry, Breitburg, Eisenberg, van der Merwe, Kennedy and LeMoine1988).
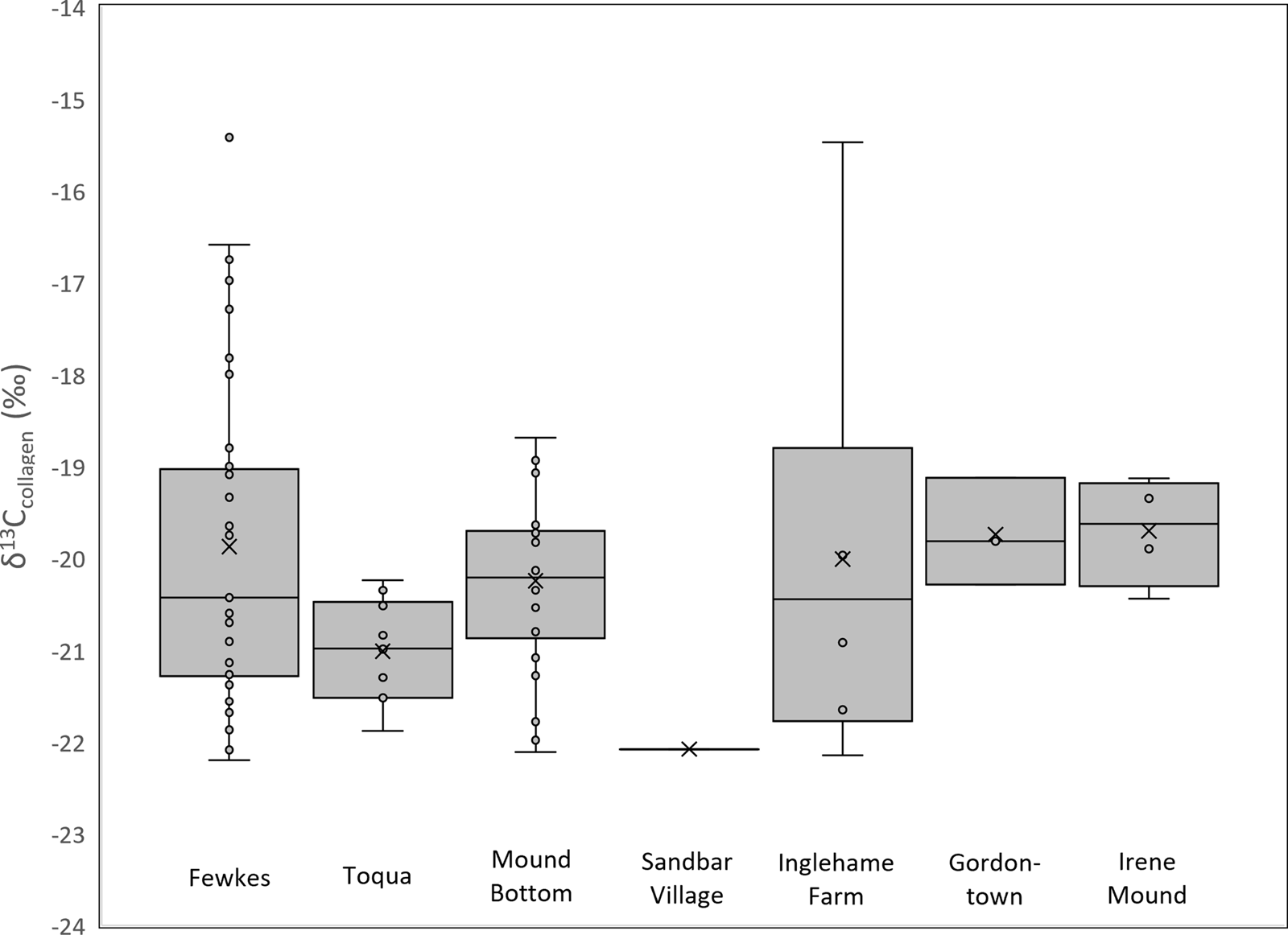
Figure 4. Median (horizontal line), mean (X) and interquartile range (box) of Southeast turkey δ13Cco.
Table 2. Summary Statistics for Southeast Archaeological Turkeys, Deer, and Canids.

a Number of collagen samples per site. 61 samples also were run for δ13Cap including turkeys from Fewkes (n = 37), Mound Bottom (n = 14), Sandbar Village (n = 1), Inglehame Farm (n = 4), Gordontown (n = 1), and Irene Mound (n = 4), deer from Irene Mound (n = 5), and canids from Fewkes (n = 4).
b STDEV = standard deviation.
Among the archaeological turkeys, there is a weak positive correlation between δ13Cco and δ15N (r = 0.264; Figure 5). Elevated δ15N has also been observed in archaeological domestic turkeys and other taxa consuming crops (e.g., Barton et al. Reference Barton, Newsome, Chen, Wang, Guilderson and Bettinger2009; Guiry et al. Reference Guiry, Beglane, Szpak, Schulting, McCormick and Richards2018, Reference Guiry, Orchard, Royle, Cheung and Yang2020; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; Thornton et al. Reference Thornton, Emery and Speller2016). This association lends some support to the contribution of maize to diets of southeastern archaeological turkeys with elevated δ13Cco, but other explanations including the consumption of maize-consuming insects, and protein or water stress cannot be ruled out.

Figure 5. δ13Cco and δ15N for archaeological turkeys, deer, and canids.
Southeastern archaeological turkeys have slightly higher δ13Cco than archaeological deer from the same site (deer mean δ13Cco = −22.1‰; t-test p < 0.01), which could be due to turkeys’ greater omnivory (i.e., trophic level increases in δ13C; Caut et al. Reference Caut, Angulo and Courchamp2009) or their greater consumption of mast and seeds that have slightly higher δ13C in comparison to leaves that deer consume in greater quantities (Cernusak et al. Reference Cernusak, Tcherkez, Keitel, Cornwell, Santiago, Knohl, Barbour, Williams, Reich and Ellsworth2009). The diets of archaeological turkeys and deer contrast with those of southeastern archaeological canids tested in this study (mean δ13Cco = −9.7‰; mean δ15N = 6.8‰) and humans reported from published sources (n = 69, mean δ13Cco = −9.9‰; n = 21, mean δ15N = 10.2‰), which consumed more maize and fed at higher trophic levels (Figure 3; Table 2). High δ13Cco in the archaeological canids suggests that they were tame or domesticated animals feeding within human settlements. The isotopic similarity of southeastern archaeological turkeys to deer and their pronounced isotopic separation from southeastern canids and humans contrast with isotopic patterns observed at sites in the American Southwest and Mesoamerica where domestic turkeys were reared on maize (Conrad et al. Reference Conrad, Jones, Newsome and Schwartz2016; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; McCaffery et al. Reference McCaffery, Tykot, Gore and DeBoer2014; Rawlings and Driver Reference Rawlings and Driver2010; Thornton et al. Reference Thornton, Emery and Speller2016).
The preferential routing of carbon from dietary protein to bone collagen (Ambrose and Norr Reference Ambrose, Norr, Lambert and Grupe1993) allows for the potential underestimation of maize consumption in δ13Cco if the protein component of the diet is largely C3 (Froehle et al. Reference Froehle, Kellner and Schoeninger2010, Reference Froehle, Kellner and Schoeninger2012; Harrison and Katzenberg Reference Harrison and Katzenberg2003). This could be expected in turkeys eating maize and invertebrates that fed on C3 resources. The contribution of C4 resources to southeastern turkey diets is somewhat more evident in bone apatite (δ13Cap = −8.00 to −15.66‰), but the amount is less than that consumed by southeastern canids (δ13Cap = −5 to −6.9‰, this study; see also Emerson et al. Reference Emerson, Hedman, Simon and Fort2020; Guiry et al. Reference Guiry, Orchard, Needs-Howarth and Szpak2021; Hogue Reference Hogue2003) and Mesoamerican domestic turkeys (δ13Cap = −0.6 to −7.8‰; Thornton et al. Reference Thornton, Emery and Speller2016; Figure 6). Although some southeastern turkeys consumed a mixed C3/C4 diet, δ13Cap does not indicate extensive consumption of maize or other C4 plants. The isotopic results thus do not support extensive maize provisioning or captive rearing of turkeys at the sampled Mississippian sites.

Figure 6. Collagen and apatite δ13C for archaeological turkeys, deer, and canids plotted with reference to C3-based (dashed line) and C4-based (solid line) protein models by Kellner and Schoeninger (Reference Kellner and Schoeninger2007).
Ancient mtDNA Results
We recovered mitochondrial DNA from 25 of the 31 turkey bones (81%), which yielded DNA sequences consistent with M. gallopavo. Five of the samples yielded the entire 435-bp sequence, and an additional 10 samples yielded partial mtDNA profiles (309 bp) sufficient for haplotype identification (Table 3). From these observations, four distinct haplotypes were recovered from the remains: eHap1 (seven individuals), eHap2 (six individuals), eHap3 (one individual), and eHap4 (one individual). The remaining 10 samples produced sequences too short in length to be used to confidently assign membership in one mitochondrial lineage or another (these are indicated as “partial” in Table 3).
Table 3. Summary of Turkey mtDNA Results.

Note: Sequences and mutational positions are relative to the turkey mtDNA reference sequence (EF153719; Guan et al. Reference Guan, Silva, Gyenai, Xu, Geng, Tu, Samuels and Smith2009).
a WSU = Washington State University Ancient DNA Lab; BioArCh = University of York Ancient DNA Lab.
The two more common haplotypes identified in the archaeological remains, eHap1 and eHap2, are observed in modern eastern wild turkeys (M. g. silvestris), whereas eHap3 and eHap4 are unique, differing by a single mutation from haplotypes observed in eastern wild turkey and Rio Grande wild turkey (M. g. intermedia) populations, respectively (Supplemental Figure 1). Fewkes turkeys group closely with most of the eastern wild turkeys, and the population is not significantly different from neighboring wild modern eastern populations from the Black Warrior and Scotch wildlife management areas in Alabama and the Ozark Mountains in Missouri (Figure 7). The recovered haplotypes, however, are distinct from turkeys recovered from archaeological sites in the American Southwest (Kemp et al. Reference Kemp, Judd, Monroe, Eerkens, Hilldorfer, Cordray, Schad, Reams, Ortman and Kohler2017; Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010) and Mexico (Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; Supplemental Figure 1).

Figure 7. Unrooted neighbor-joining tree displaying the relationship between the Fewkes turkeys and North American modern (Mock et al. Reference Mock, Theimer, Rhodes, Greenberg and Keim2002) and archaeological (Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010) turkey populations. Comparative sequences were obtained from GenBank. Fewkes turkeys are compared with archaeological samples from the American Southwest and modern North American wild subspecies. The first letter of each population abbreviation refers to the subspecies designation (E = M.g. silvestris (Eastern); F = M.g. osceola (Florida); R = M.g. intermedia (Rio Grande). Additionally, MWT = M.g. merriami (Merriam's), MGM = M.g. mexicana (Gould's), and SW dom. = archaeological Southwest domestic turkeys.
Genetic diversity indices for Fewkes turkeys indicate they are more similar to modern wild turkey populations than to managed or domestic archaeological turkey stocks. The haplotype diversity of the Fewkes assemblage is 0.657, within the range (0.556–0822) of modern eastern wild turkey populations from surrounding states (Figure 8; Supplemental Table 2). In contrast, haplotype diversity for contemporaneous archaeological turkey stocks in the American Southwest (Kemp et al. Reference Kemp, Judd, Monroe, Eerkens, Hilldorfer, Cordray, Schad, Reams, Ortman and Kohler2017) is much lower, ranging from 0 to 0.222. The turkeys from southwestern sites such as Shields Pueblo, Sand Canyon, Arroyo Hondo, and Albert Porter Pueblo display reduced genetic diversity associated with captive rearing (Figure 8) and, in the case of Shields Pueblo, evidence for enriched δ13C associated with maize provisioning (Rawlings and Driver Reference Rawlings and Driver2010).
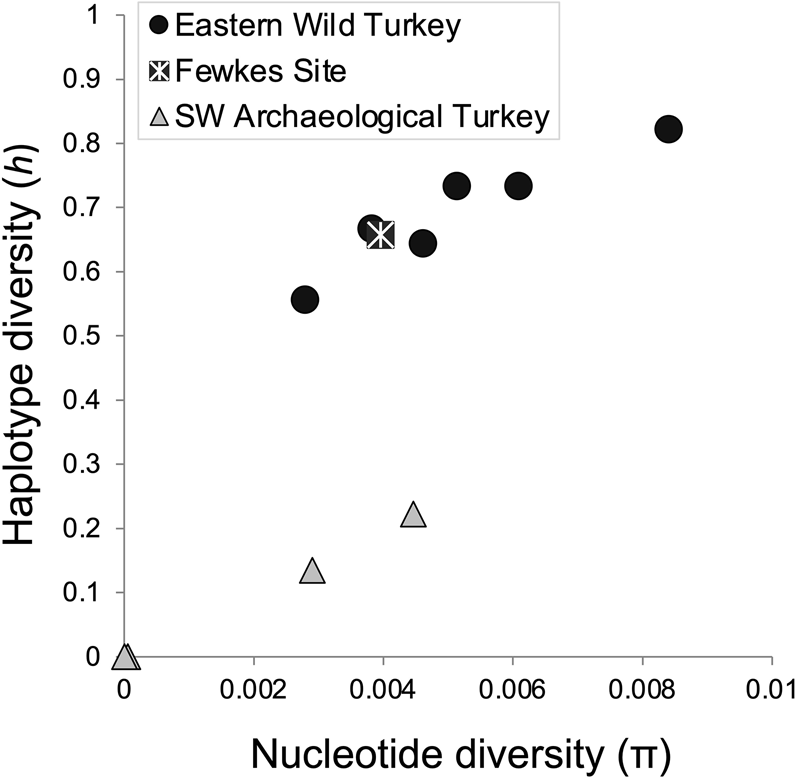
Figure 8. Haplotype and nucleotide diversity of the Fewkes turkey assemblage compared to modern eastern wild turkey (M.g. silvestris) populations and archaeological turkeys from sites in the American Southwest (SW) listed in Supplementary Material Table 2.
Discussion and Conclusions
Isotopic and genetic analyses of Mississippian turkeys show no evidence of prolonged or intensive captive rearing at the southeastern sites tested. Unlike archaeological turkeys from the American Southwest and Mesoamerica, southeastern turkeys show no evidence of extensive maize consumption or evidence of genetic management. Moreover, genetic analysis does not indicate domestic turkeys were introduced from other regions.
Isotopic Indicators of Rearing, Provisioning, and Garden-Hunting
C4/maize-based diets of archaeological canids in this and other studies (e.g., Emerson et al. Reference Emerson, Hedman, Simon and Fort2020; Guiry et al. Reference Guiry, Orchard, Needs-Howarth and Szpak2021; Hogue Reference Hogue2003) indicate that maize was an abundant food resource available to animals in Mississippian communities. If southeastern turkeys were reared in pens or were free-range village animals, they should have had higher δ13C and δ15N similar to archaeological domestic turkeys in Mesoamerica and the American Southwest (Conrad et al. Reference Conrad, Jones, Newsome and Schwartz2016; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; McCaffery et al. Reference McCaffery, Tykot, Gore and DeBoer2014; Rawlings and Driver Reference Rawlings and Driver2010; Thornton et al. Reference Thornton, Emery and Speller2016), as well as the isotopic outlier from southern Ontario (Morris et al. Reference Morris, White, Hodgetts and Longstaffe2016). Higher turkey δ13Cco (>−12‰) in these other regions is attributed to heavy maize consumption, whereas higher δ15N in captive turkeys is ascribed to the consumption of fertilized maize, greater ingestion of insects, consumption of human or animal feces, or some combination of these factors (McCaffery et al. Reference McCaffery, Tykot, Gore and DeBoer2014; Rawlings and Driver Reference Rawlings and Driver2010; Thornton et al. Reference Thornton, Emery and Speller2016). Substantially higher δ13Cco and δ15N was not observed in our southeastern turkeys nor in archaeological turkeys from other southeastern sites (Manzano et al. Reference Manzano, Pollack, Henderson, Erhardt and Munizzi2019; Price Reference Price2009; Rogers Reference Rogers2011).
Although no southeastern turkeys fell within the range of domestic turkeys from other regions, slightly higher δ13Cco (−18 to −15.42‰) indicates a mixed C3/C4 diet in eight southeastern archaeological turkeys. Similar levels of δ13Cco (−18.3 to −14.7‰) were observed in 10% of the archaeological turkeys from southern Ontario (Guiry et al. Reference Guiry, Orchard, Needs-Howarth and Szpak2021; Morris et al. Reference Morris, White, Hodgetts and Longstaffe2016). Morris and colleagues (Reference Morris, White, Hodgetts and Longstaffe2016) interpret such intermediate values as evidence of turkey management through intentional maize provisioning. However, the amount of maize available to wild-foraging turkeys in the absence of intentional human provisioning remains difficult to quantify, and some wild, nonprovisioned taxa have been shown to consume large quantities of maize (Guiry et al. Reference Guiry, Orchard, Needs-Howarth and Szpak2021, Reference Guiry, Orchard, Royle, Cheung and Yang2020).
In the American Southwest, small subsets of archaeological turkeys also yield δ13Cco, indicating a mixed C3/C4 diet. These individuals are either free-range domestic turkeys eating a mix of human-provided maize and wild foods (Jones et al. Reference Jones, Conrad, Newsome, Kemp and Kocer2016) or wild turkeys that occasionally raided maize fields or consumed wild C4/CAM resources (Conrad et al. Reference Conrad, Jones, Newsome and Schwartz2016; McCaffery et al. Reference McCaffery, Tykot, Gore and DeBoer2014). Similar uncertainty exists for interpreting archaeological Mesoamerican ocellated turkeys (Meleagris ocellata) with intermediate δ13Cco (−18 to −13‰). These could be wild garden-hunted birds or intentionally provisioned animals (Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; Thornton et al. Reference Thornton, Emery and Speller2016).
Accurately reconstructing where precolumbian turkeys fall on the wild to domestic continuum is crucial for understanding how people influenced and interacted with animal populations through direct (e.g., provisioning) and indirect means (e.g., landscape or land cover modification). Unfortunately, determining the human agency or intentionality in managing and promoting turkey populations is not as simple as observing the degree to which isotopic ratios deviate from an expected wild, C3-based diet. Although stable isotope analysis can readily identify domestic or captive-reared birds consuming almost exclusively human-provided maize (δ13Cco >−12‰), isotopic analyses less readily document lower levels of human provisioning because wild turkey diets can vary greatly and regions differ in the availability of wild C4 resources. The method also cannot detect captive or managed turkeys provisioned with C3 resources such as acorns because their δ13C would be identical to wild turkeys.
Highly diverse and variable wild turkey diets are a complicating factor. As opportunistic omnivores, turkeys may show great intra- and interannual dietary variation sensitive to local environmental factors, such as the amount and configuration of forest cover and proximity to water (Otieno and Frenette Reference Otieno and Frenette2017). Wild turkeys could display a broad range of isotopic values reflecting this dietary diversity. Across their broad geographic range, modern North American turkeys exhibit δ13Cco indicative of pure C3 to highly mixed C3/C4 diets, which argues in favor of wild turkey isotopic diversity (δ13Cco: −21.5 to −14.7‰, reflecting 1.5‰ correction for modern burning of fossil fuels to make modern δ13Cco comparable to archaeological δ13Cco; Jones et al. Reference Jones, Conrad, Newsome, Kemp and Kocer2016; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016; Morris et al. Reference Morris, White, Hodgetts and Longstaffe2016). Significant changes in land cover and configuration, wildlife management practices, hunting pressure, and the shift to mechanized agriculture further complicate the comparison of modern and archaeological turkey diets because they alter the balance of C3 and C4 resources in turkey habitats and diets.
Seasonal maize provisioning of turkeys is one of several possible explanations for slightly higher δ13C observed in southeastern archaeological turkeys. Other explanations include turkey consumption of insects or other invertebrates, native C4 grasses, or maize damaged by other crop pests. Slightly higher turkey δ13C should be interpreted cautiously until we have a better idea of the full range of nonprovisioned turkey diets, as these values could reflect a diversity of human-animal interactions from wild hunting to active provisioning.
Implications for Human-Turkey Interactions in Eastern North America
Increasing data indicate past human-animal interactions in North America that defy simple classifications of species as either wild or domestic (e.g., Jones et al. Reference Jones, Conrad, Newsome, Kemp and Kocer2016; LeFebvre and deFrance Reference LeFebvre, deFrance and Reid2018; LeFebvre et al. Reference LeFebvre, deFrance, Kamenov, Keegan and Krigbaum2019; Morris et al. Reference Morris, White, Hodgetts and Longstaffe2016; Somerville et al. Reference Somerville, Sugiyama, Manzanilla and Schoeninger2016; Sugiyama et al. Reference Sugiyama, Somerville and Schoeninger2015, 2017; Thornton and Emery Reference Thornton and Emery2017; Valadez Azúa Reference Valadez Azúa2003). It is likely that Mississippian populations occasionally provisioned turkeys with dried maize and tolerated or even promoted their presence in fields or middens as a means of pest control, thereby increasing local wild game populations. Higher δ13C in archaeological turkeys broadly indicates turkeys’ tendency to tolerate anthropogenic habitats, which inevitably brought them into greater and more complex interactions with human populations. This mirrors recent observations of domesticated millet (Panicum miliaceum, Setaria italica) consumption by wild pheasants (Phasianus colchicus) at the Dadiwan site in China 5,900–7,900 years ago (Barton et al. Reference Barton, Bingham, Sankaranarayanan, Monroe, Thomas and Kemp2020). In the case of turkeys, provisioning them with maize would promote higher winter survival rates, larger brood sizes, and smaller home ranges while decreasing fear of humans, all of which would increase local access to turkeys and promote further human-turkey interactions.
Regardless of whether Mississippian populations intentionally provisioned turkeys to improve hunting or to manage wild populations, our sample does not indicate long-term captive rearing or controlled breeding. Our sample, however, is limited in size and geographic scope and may not be representative of all Mississippian sites in the Southeast. Current research on North American plant and animal domestication reveals that the domestication process was characterized by prolonged periods of low-intensity cultivation or rearing and that regions varied greatly in the timing and intensity of food production (Smith Reference Smith2011, Reference Smith, Bovin, Crassard and Petraglia2017). For example, turkey domestication in the American Southwest originally focused on low-level rearing primarily for feathers, with more intensive rearing for subsistence purposes emerging centuries later and only in areas of highest population pressure (Kohler et al. Reference Kohler, Bocinsky, Cockburn, Crabtree, Varien, Kolm, Smith, Ortman and Kobti2012; Lipe et al. Reference Lipe, Bocinsky, Chisholm, Lyle, Dove, Matson, Jarvis, Judd and Kemp2016). In the Maya region, domestic turkeys were first adopted from northern Mesoamerica in the Late Preclassic (~350 BC) but were reared in very small numbers at select sites for use in elite ceremonial display until more widespread adoption after AD 1000 (Thornton and Emery Reference Thornton and Emery2017; Thornton et al. Reference Thornton, Emery, Steadman, Speller, Matheny and Yang2012). In both cases, turkey rearing was not initially accompanied by substantial increases in the numbers of turkeys in zooarchaeological assemblages, nor was turkey rearing necessarily present at all contemporary sites.
Wild turkey provisioning or captive rearing of poults hatched from wild-collected eggs may have occurred in Eastern North America on a limited basis at a few sites or in specific regions. Future research may reveal evidence for small-scale and patchily distributed turkey rearing in Eastern North America. The single maize-fed turkey identified by Morris and colleagues (Reference Morris, White, Hodgetts and Longstaffe2016) from southern Ontario supports the need for expanded isotopic testing to document the existence and extent of this practice. Additional lines of evidence, such as demographic profiles and paleopathology, should also be explored in more depth because of the potential for managed or captive turkeys to be provisioned with foods other than maize.
The wild turkey's tameness and tolerance for anthropogenic environments predispose it to greater and more complex interactions with humans and their built environments. The well-established history of plant cultivation in Eastern North America also provides a cultural context for the emergence of other complex human-environment interactions including animal management or rearing beyond domestic dogs. Finally, it remains possible that the idea for turkey rearing diffused to Eastern North America from the American Southwest or Mesoamerica. Our study found no evidence of turkeys being introduced from these confirmed centers of turkey domestication, but the regions share some cultural foundations and subsistence practices, and there is limited evidence of economic interaction (Blitz Reference Blitz2010; Carpenter Reference Carpenter2020; Washburn et al. Reference Washburn, Washburn, Shipkova and Pelleymounter2014).
Although the potential for turkey rearing and domestication existed in the Southeast, there are many types of intensified human-animal interactions that would not lead to either outcome (Vigne Reference Vigne2011; Zeder Reference Zeder2015). The decision to engage in more controlled use of animal resources is highly complex and is influenced by many factors, including the local diversity, abundance, seasonality, and sustainability of wild faunal resources; the amount of surplus crops available for use as animal fodder; and the social demand for particular animal resources. Eastern North America differs from other regions where turkey domestication emerged (i.e., American Southwest and Central Mexico) in terms of the overall diversity and abundance of faunal resources used by past societies. In particular, the greater availability of aquatic taxa and overall ecological productivity of the Eastern Woodlands offered more options for protein acquisition. Within this ecological context, specialization or greater reliance on turkeys may have been less likely. The wetter and mixed landscape mosaic of forest and agricultural fields in Eastern North America also could sustain higher populations of turkeys near human settlements. Maize provisioning, or fallow field and forest management could be an effective means of promoting local turkey populations without investing resources in animal rearing.
Reconstructing the nature of human-turkey interactions in Eastern North America is critical to understanding the overall process of turkey domestication throughout the Americas. Through comparative assessment of spatial and temporal variation in the types and intensity of turkey use, management, or domestication practiced, it will be possible to assess the social and environmental contexts that influenced region-specific interaction with this potential animal domesticate. The current study, by expanding research on turkey domestication in the American Southeast, moves us toward this goal, but additional detailed studies throughout the turkey's natural range are needed before we can advance more formal theories.
Acknowledgments
This research was completed through the collaborative efforts of numerous individuals and institutions. Primary funding for the study was provided by Washington State University (WSU), which also provided access to mass spectrometers and lab facilities. Personnel within the WSU Stable Isotope Core, particularly Ben Harlow and Dave Evans, were instrumental to generating the isotopic data. Supplemental funding was provided by a European Research Council Grant awarded to Aurelie Manin (MSCA-IF-2016, 748679) who collaborated with Lauren Basnett and Krista McGrath under the supervision of Camilla Speller to conduct a portion of the genetic analyses at the University of York. Thanks to Cara Monroe for assistance in the DNA laboratories at WSU and the University of Oklahoma. Archaeological samples were generously provided by Florida State University, the Tennessee Division of Archaeology, the Tennessee Department of Transportation, the University of Georgia, and the McClung Museum of Natural History and Culture with the assistance of Meagan Dennison.
Data Availability Statement
Ancient DNA sequences were submitted to GenBank under Accessions MN587233–MN587257. Stable isotope data are reported in the supplementary material and are available from the WSU Department of Anthropology Stable Isotope Lab. Archaeological faunal materials analyzed as part of this research are curated at Florida State University, the Tennessee Division of Archaeology, the Tennessee Department of Transportation, the University of Georgia, and the McClung Museum of Natural History and Culture in Knoxville, Tennessee.
Supplemental Material
For supplemental material accompanying this article, visit https://doi.org/10.1017/aaq.2021.58.
Supplemental Text 1. Archaeological site and sample descriptions.
Supplemental Text 2. Stable isotope analysis laboratory methods.
Supplemental Text 3. Ancient DNA extraction and analysis laboratory methods.
Supplemental Table 1. δ13C and δ15N of Archaeological Turkeys, Deer, and Canids.
Supplemental Table 2. Haplotype and Nucleotide Diversity for the Fewkes Assemblage, Other Archaeological Turkeys, and Modern Turkeys.
Supplemental Table 3. Context Descriptions for Archaeological Turkeys.
Supplemental Figure 1. Median-joining network displaying the relationships between the Fewkes turkeys and existing archaeological (Manin et al. Reference Manin, Corona-M, Alexander, Craig, Thornton, Yang, Richards and Speller2018; Speller et al. Reference Speller, Kemp, Wyatt, Monroe, Lipe, Arndt and Yang2010) and modern (Mock et al. Reference Mock, Theimer, Rhodes, Greenberg and Keim2002; Monteagudo et al. Reference Monteagudo, Avellanet, Azón and Teresa Tejedor2013; Szalanski et al. Reference Szalanski, Church, Oates, Bischof and Powers2000) turkey sequences obtained from GenBank. Fewkes turkeys (purple) are compared with archaeological samples from the American Southwestern (gray), Mexico (black), modern breeds (white), and wild subspecies (various colors).













