Introduction
While it is found worldwide that on average women live longer than men, there is no fixed relationship between duration of life and health at successive ages. Women are reported to be more likely than men to experience poor health and functional disabilities (Crimmins, Hayward and Saito Reference Crimmins, Hayward and Saito1994; Nusselder and Loomale Reference Nusselder and Loomale2004; Wang and Ye Reference Wang and Ye2006; Zeng, Liu and George Reference Zeng, Liu and George2003). The relationship between age and gender differentials in health is poorly specified in China, however, partly because recent results have been inconsistent. Anson and Sun (Reference Anson and Sun2002) investigated gender differentials in six health indicators among six age-groups across the entire life span in rural areas of the province of Hebei, and found poorer health among women than men.Footnote 1 The gender differential was apparent in early adulthood (ages 25–44 years), peaked among older adults (45–59 years), and at older ages narrowed. In contrast, from an examination of gender differences in the Activities of Daily Living (ADL), physical performance, self-reported health and life satisfaction, and cognitive functions among three age groups of the oldest old in China (80–89, 90–99 and 100–105 years), Zeng, Liu and George (Reference Zeng, Liu and George2003) found that the oldest Chinese women had significantly worse health than men and that the gender difference increased at older ages.
Beyond these contrasting findings, there is very little information about age differences in gender differentials in health among older people in China. That gender differentials exist, however, has been well established for China, Japan and western societies in recent years (Crimmins, Hayward and Saito Reference Crimmins, Hayward and Saito1994; Nusselder and Loomale Reference Nusselder and Loomale2004; Wang and Ye Reference Wang and Ye2006; Zeng, Liu and George Reference Zeng, Liu and George2003). The Chinese government is promoting ‘active ageing’, for which it will be useful to have a better understanding of age and gender differentials in health. With respect to the high prevalence of ill-health among older people, it has been shown that not only does this reduce their quality of life, but also that ill-health and disability increase the burden on families and societies. The policy agenda and the information gaps that have been described were the stimulation for the study reported in this paper. It aimed to specify and analyse variations in gender differentials in health by age among people in China aged 65 or more years.
Methods
Sampling design
The data were drawn from the Chinese Longitudinal Health Longevity Study (CLHLS) of older people. The baseline survey was in 1998, and there were follow-up surveys in 2000, 2002 and 2005 (for details see http://www.geri.duke.edu/china_study). The present analysis has used the third-wave (2002) data. The CLHLS used a stratified sample that randomly-selected 50 per cent of the nation's counties and cities in 22 of its 31 provinces.Footnote 2 The surveyed provinces had a population of 985 million, 85 per cent of China's total population. A special feature of the 2002 wave of CLHLS was that it sought to interview all centenarians in the selected counties and cities. For each centenarian who gave consent and was interviewed, the survey used quotas by age and gender to sample several subjects living nearby who were aged 65–79 years, and one octogenarian and one nonagenarian. ‘Nearby’ was loosely defined as the same street, village, town, city or county. The quotas ensured approximately equal numbers of males and females in each of the defined age groups tied to each centenarian. The overall sample size in 2002 was 16,057 people aged 65 or more years. After excluding cases with incomplete responses, the analysis sample for this paper was 15,789. Of this total, 6,781 (43.95%) were male and 9,008 (57.05%) were female. Approximately 54 per cent of the sample lived in rural areas, 22 per cent in towns, and 24 per cent in cities. When stratified by age group, 4,845 were aged 65–79 years, 4,239 aged 80–89 years, 3,747 aged 90–99 years, and 2,967 were aged 100 or more years.
Quality checks of the data
The CLHLS questionnaire had 92 questions and 180 items, including health indicators such as Activities of Daily Living (ADL), Instrumental ADLs (IADL), the Mini Mental State Examination (MMSE), visual function (VF), auditory function (AF), number of natural teeth (NNT), self-reported health (SRH), self-reported quality of life (SRQOL), and others. The questionnaire had self-evaluation questions and was administered using face-to-face interviews. The diligence and consistency of the interviewers was decisive for the survey's success (Zeng, Liu and George Reference Zeng, Liu and George2003). Training workshops were held in each province on the survey objectives, survey design, questionnaire items, interview skills and other requirements, and attended by about 300 interviewers. The interviewers were organised into 120 teams, each with one interviewer, one recorder and a clinician. Officials from the county or township authority and from older people's associations accompanied the survey teams on their home visits. Auditory and visual functions were tested by qualified medical staff. For each informant, the interviewers were required to ‘check the questionnaire themselves, have it checked by another member of the team, and check the internal consistency by sampling’. Where gaps and out-of-range and inconsistent responses were found, they were corrected immediately ‘in the field’. Every survey team was required to approach every household in the sample and not miss a single one or any person in the household. The completed questionnaires were checked by the survey supervisors, by provincial organisers, and by the project's academic members who also made site visits to monitor and supervise the data collection process. All reasonable efforts were made to ensure the high quality of the survey variables (e.g. the reliability coefficient for the ADL score is 0.88) (for further details, see Zeng, Liu and George Reference Zeng, Liu and George2003).
Measures
This study has examined the functional and psychological dimensions of health status and self-evaluated health. The ADL and IADL scales were adopted as relatively objective and comprehensive measures of functional health status (Katz et al. Reference Katz, Ford, Moskowitz, Jackson and Jaffe1963). Following international practice, six ADL items were included: eating, dressing, indoor mobility, bathing, toileting and continence. Each item was scored ‘1’ for ‘no assistance needed’, ‘2’ for ‘needs partial assistance’, and ‘3’ for ‘needs full assistance’, so lower scores represent greater independence in ADL, and the range of possible scores was from six to 18. There were eight IADL independent living items: socialising; shopping; cooking; laundry; walking for two kilometres without stopping; lifting up to 10 kilograms; repeated squatting and standing; and using public transport alone. IADL was also categorised as: ‘1’ for ‘can do’, ‘2’ for ‘have difficultly doing’, and ‘3’ for ‘cannot do’. Lower scores represent greater independence in IADL, and the possible range of scores was from eight to 24. The visual, auditory and dental measures are all regarded as important components of the functional health status of older people (Chou and Chi Reference Chou and Chi2004). Visual function was measured using an eye chart and scored: ‘1’ for ‘can see and distinguish breaks in a circle’; ‘2’ for ‘can see, but cannot distinguish breaks in a circle’; ‘3’ for ‘cannot see’; and ‘4’ for ‘blind’. Auditory function was scored: ‘1’ for ‘can hear without a hearing aid’; ‘2’ for ‘can hear with a hearing aid’; ‘3’ for ‘can partly hear with a hearing aid’; and'4' for ‘cannot hear’. For both the visual and auditory measures, lower scores represented better functioning.
For psychological health, the MMSE was used, an accepted measure of a person's cognitive ability (Deb and Braganza Reference Deb and Braganza1999; Osterweil et al. Reference Osterweil, Mulford, Syndulko and Martin1994). A Chinese version was developed for the CLHLS and carefully tested in pilot interviews to ensure its cultural appropriateness, and the MMSE international standard parameters were applied (Zeng, Liu and George Reference Zeng, Liu and George2003). The range of scores in the present sample was from zero to 34, with lower scores representing higher cognitive impairment. Turning to the self-evaluation measures, the items on SRH and SRQOL were scored with a consistent five-point ordinal or Likert scale: ‘1’ for ‘very good’; ‘2’ for ‘good’; ‘3’ for ‘intermediate’; ‘4’ for ‘bad’; and ‘5’ for ‘very bad’. For both the self-evaluation indicators, lower scores represent a perception of better health or quality of life.
Analytical strategy
Descriptive analyses are used in this article. First, the gender differences in the median scores (female minus male) for functional status, psychological factors and self-evaluated health were calculated for each five-year age group from 65–69 to 95–99 and 100+ years. Mann-Whitney U tests for data with a non-normal distribution were used to test the significance of the differences, and Spearman's rank correlation was used to examine the relationships between age and the gender differentials (Kunter, Nachtsheim and Netter Reference Kunter, Nachtsheim and Netter2004). Analysis was performed using SPSS 13.0 software.
Results
Age-specific gender differences in the health indicators
Figure 1 plots the gender- and age-specific mean scores of all the health indicators. Panels A and B are the two self-evaluation measures, and show relatively high variability in the scores, particularly for quality of life. The lack of any consistent gender differential is striking on the quality-of-life diagram. By contrast, Panels C, D, F and G show exponential rises with increasing age for both males and females for the ADL, IADL, visual and auditory problem scores (in each case showing deteriorating function with increasing age). For all four, the gender differential increased with greater age to the disadvantage of women. Comparing Panels C and D, it is seen that the IADL scores were higher than those of ADL and that the amplitude of the increase from the youngest to the oldest ages was much greater absolutely and relatively for IADL (the range for ADL was approximately from 6 to 8, and for IADL from 8 to 23). A gender differential in IADL becomes apparent from around the age of 77 years, while that for ADL was negligible until after 84 years. Panels E and H respectively plot MMSE and the number of natural teeth, and show that the scores decreased with age. There is a clear female disadvantage for MMSE at all ages, and women were substantially disadvantaged in terms of the number of natural teeth when aged from the late sixties to around 80 years.
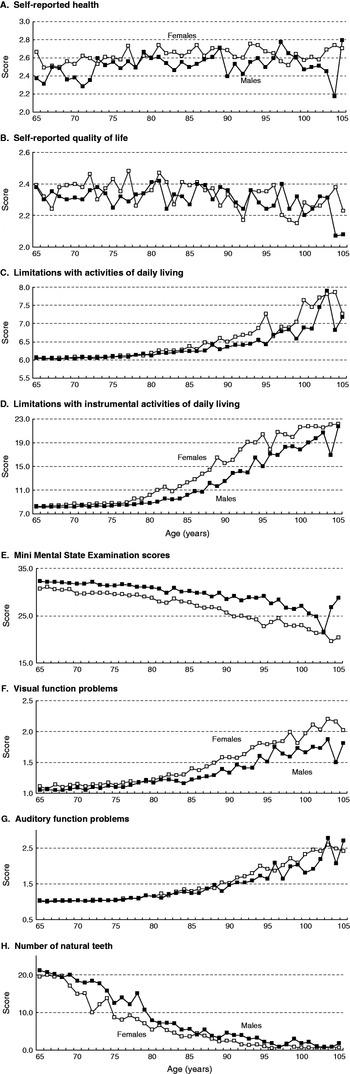
Figure 1. Health indicator median scores for older people by age and sex, China 2002.
Table 1 presents the results of the Mann-Whitney U tests of the age-group specific gender differences in the indicators. It shows that most of the differentials were statistically significant, but there were exceptions in certain age groups. For example, the differences were not statistically significant for the two self-evaluated measures at ages 75–79 and 95–99 years, for ADL at ages 65–69 and 75–79 years, for visual function at 75–79 years, and for auditory function at 70–74 and 95–99 years. Overall, however, it is clear that women were disadvantaged in most of the health indicators right across the studied age range.
Table 1. Mean scores of health indicators by sex and age among Chinese older people, 2002

Notes: M males, F females. ADL activities of daily living. A fuller table with standard deviations and medians is available from the authors.
Significance levels: Non-parametric Mann-Whitney U test: * p<0.05, ** p<0.01, *** p<0.001.
Trends by age in gender differentials in health
To examine the relationships between age and the gender differentials in health, for each indicator the gender differences in the scores were correlated with age (using single years of age). Table 2 presents the Spearman's rank correlation coefficients. There were statistically significant associations with age for ADL, IADL, MMSE, the visual and auditory function scores and the number of natural teeth, but not for the two self-evaluation measures.
Table 2. Correlation between age and gender differentials in various indicators of health among Chinese people aged 65 or more years in 2002

Note: Sample size 15,789.
Discussion
This analysis has shown that among older people in China, gender differentials in many health indicators increased with age, generally to the disadvantage of women. The one exception was that the gender differential in the number of remaining natural teeth decreased with age (Table 2). The endowment of natural teeth is the same for both sexes, but women's dentition degrades at younger ages than men's, and there is evidence that females present with more dental caries than males of the same age (e.g. Antunes et al. Reference Antunes, Junqueira, Frazao, Bispo, Pegoretti and Narvai2003). Moreover, dental health in adult women is compromised by poorer dental care, pregnancy and the menopause (Silk et al. Reference Silk, Douglass, Douglass and Silk2008; Brennan-Calanan et al. Reference Brennan-Calanan, Genco, Wilding, Hovey, Trevisan and Wactawski-Wende2008). In old age, however, men's dental health advantage is lost, and the gender differential in the number of natural teeth reduces with age. The gender differentials of ADL, IADL, MMSE, VF, and AF increased with age but that in NNT reduced, all of which indicates that Chinese elderly women had a health disadvantage compared to men. As previous studies have shown (e.g. Guralnik et al. Reference Guralnik, LaCroix, Branch, Kasl and Wallace1991), women have higher rates of disability before death than men who die at the same age. In sum, Chinese women live longer and suffer more.
Many studies have shed light on gender differentials in health in terms of both biological predispositions and life experiences. Life scientists suggest that genetic factors provide enhanced protection against mortality during infancy and early childhood for females when compared to males (Waldron Reference Waldron1983), and that the lower incidence of many diseases among females can be attributed to the protective effect of the hormone estrogen prior to menopause. During the post-menopausal phase, however, decreases in female reproductive hormones result in a sharp increase in cardiovascular disease and osteoporosis (or arthritis), which contribute to increased disability with age (Jordan, Gapstur and Morrow Reference Jordan, Gapstur and Morrow2001). In broad terms, the biological advantage of females in earlier life decreases with age and is lost in old age.
Social scientists interested in the lifecourse and longevity point to pervasive gender differences in underdeveloped countries where females are generally disadvantaged in both personal capital and social resources. For example, Fuse and Crenshaw (Reference Fuse and Crenshaw2006) noted that although sex differentials in infant mortality vary widely, in some countries a mortality disadvantage for females arises from social or behavioural factors that reflect deliberate discrimination in favour of boys. Among the current cohort of older people in China, women received as children and young adults appreciably less health care, education, nutrition and other valuable quality-of-life resources. Even after marriage, many women were required to engage in agricultural, industrial and domestic work while simultaneously caring for children and elderly relatives – reflecting the custom of extended-family households and filial support (Anson and Sun Reference Anson and Sun2002; Yu and Sarri Reference Yu and Sarri1997). Using United Nations data, Zeng and George (Reference Zeng and George2000) found that with increasing age, elderly Chinese women were more likely to be widowed, to live alone, and to experience a decrease in social relationships than their male counterparts.
Despite this diverse evidence, the current analysis found no clear or statistically significant trends in the relationships in old age between age and the gender differentials in the self-evaluated health status and quality-of-life measures. Previous research has shown that self-evaluation reports reflect past experiences, the history of diseases and illnesses and a person's general knowledge of health conditions (Sundquist and Johansson Reference Sundquist and Johansson1997). In addition, social networks and personal characteristics, including emotion and feelings of physical well-being, were important influences on an individual's self-evaluation of their health. According to Rahtz and Sirgy (Reference Rahtz and Sirgy2000), individual life satisfaction is associated with sense of community, personal health, and satisfaction with family relationships and one's job. Thorslund and Lunderg (Reference Thorslund and Lunderg1994) pointed out that people of different social class tend to evaluate their health using different foundations or criteria. As functional status declines through old age, it tends to over-ride such other considerations in self-evaluated health reports (Idler and Benyamini Reference Idler and Benyamini1997). It may be argued that human beings are adaptable to various environments including long-term differentials in personal capital and social resources. This attitude and behaviour is aptly reflected in an old Chinese saying, ‘What are the odds, so long as you are happy?’ Thus, despite women's many legal, material and social disadvantages inequalities (including the availability of health insurance and receipts of health care) (Lee and Xiao Reference Lee and Xiao1998; Li Reference Li1998), they draw on various adaptations to accommodate much of the structured disadvantage.
In China, the relationship in old age between age and the gender differential in health differs according to the indicator. IADL declines at a younger age than ADL, because it depends more on full cognitive functioning and makes higher physical demands (as with walking and lifting). The majority of the present findings are consistent with those of Zeng, Liu and George (Reference Zeng, Liu and George2003), but some differences with respect to self-evaluated health are noteworthy. Zeng and James (Reference Zeng and James2002) found that gender differentials in ‘self-reported health’ and ‘self-reported life satisfaction’ were significantly different by age group, but their sample was confined to those aged 80 or more years. Anson and Sun (Reference Anson and Sun2002) compared six age groups (0–5, 6–14, 15–24, 25–44, 45–59 and 60+ years) in a rural area of Hebei Province, China. Around 45–59 years-of-age, females experience the menopause and post-menopause and there is a high gender differential in health, but at older ages the differential declines.
The present study has a number of methodological shortcomings. The univariate analysis examined variations by only sex and age and other possible confounding factors were not controlled (although some multivariate results are available, seeZhang Reference Zhang2006). In spite of this, the study has been the first to examine gender differentials in health in a non-western country, and the findings have important implications for public health and health-care policies in China, namely that a high priority should be to improve the health status of women. As a final observation, it is suggested that more collaborative research between social scientists and life scientists would advance our understanding of the bases and aetiology of gender inequalities in health among older people.
Acknowledgments
The Chinese Longitudinal Health Longevity Study data collection was supported by the United States National Institute of Ageing (National Institutes of Health), China's Natural and Social Sciences Foundations, the United Nations Fund for Population Activities (UNFPA). The Max Planck Institute for Demographic Research provided support for international training. We also thank Professors Zeng Yi, Gu Danan (Duke University Center for Study of Aging and Human Development and Medical School and Peking University Center for Healthy Aging and Family Studies/China Center for Economic Research), and Liu Yuzhi (Center for Healthy Aging and Family Studies and Institute of Population Research, Peking University) for their kind personal support.





