Human skeletal remains constitute remarkably informative archaeological finds biologically and socioculturally. Their recovery, preservation, curation, storage, and analysis are complex issues that need to be addressed within any given biocultural context. Here, we give a brief overview of excavation, curation, and bioarchaeological practice within a Greek archaeological framework. We focus on the newly launched Phaleron Bioarchaeological Project as a case study to present our methodological approaches over a vast ancient necropolis that came to light during large-scale salvage excavations in the wider Athens region.
Given the country's geography and long history of human occupation, Greek field archaeology is intense and ongoing. Human burials in either organized cemeteries or scattered graves have been frequent occurrences in both rescue and systematic excavations. However, for most of the previous century, it was common practice to discard or rebury the skeletal remains from excavations or to preserve only the crania. Obviously, there have been notable exceptions (for the history of skeletal studies in classical archaeology, see Lagia Reference Lagia, Haggis and Antonaccio2015 and MacKinnon Reference MacKinnon2007). The systematic collection and preservation of human skeletal remains from excavations are more recent procedures resulting in the accumulation of skeletal remains in the storage areas of museums and government and academic archaeological departments. Even though the conservation of artifacts is often a standard post-excavation procedure, the conservation of osteological material continues to be optional and often becomes the responsibility of the researcher. Aspects like insufficient time, funding, and documentation in the field; conservation needs; and proper storage space—as well as the long time-gap between excavation and analysis—become common challenges. However, the abundance of skeletal remains can also be seen as the blooming of bioarchaeological research in Greece following the legacy of J. Lawrence Angel (for the history of bioarchaeology, physical anthropology, and forensic anthropology in Greece, see Buikstra and Prevedorou Reference Buikstra, Prevedorou, Buikstra and Roberts2012; Buikstra and Lagia Reference Buikstra, Lagia, Schepartz, Fox and Bourbou2009; Eliopoulos et al. Reference Eliopoulos, Moraitis, Vanna, Sotiris, Márquez-Grant and Fibiger2011; Lagia et al. Reference Lagia, Papathanasiou, Sevi, O'Donnabhain and Lozada2014; Moraitis and Eliopoulos Reference Moraitis, Eliopoulos, Groen, Márquez-Grant and Janaway2015; Roberts et al. Reference Roberts, Bourbou, Lagia, Triantaphyllou, Tsaliki and King2005; Schepartz et al. Reference Schepartz, Fox, Bourbou, Schepartz, Fox and Bourbou2009).
BACKGROUND
In Greece, archaeological field research can be either salvage (e.g., for construction) or systematic (e.g., research-driven and recurring), following a permiting procedure. Salvage excavations are conducted by the Greek Archaeological Service of the Ministry of Culture. Systematic excavations can be conducted by the departments of the Archaeological Service, scientific or academic Greek institutions, or foreign archaeological schools in collaboration with or under supervision by the Greek Archaeological Service. Human skeletal remains, like all archaeological finds from contexts in Greece, are considered cultural goods and portable monuments, and as such, they are owned by the state and managed by the Hellenic Ministry of Culture. All antiquities, including archaeological human remains, are regulated by the Law 3028/2002, “For the Protection of Antiquities and the Cultural Heritage in General” (http://www.tap.gr/tapadb/files/nomothesia/nomoi/n.3028_2002.pdf; Charalampopoulou Reference Charalampopoulou, Voutsaki and Maria Valamoti2013). Archaeological materials are administered by regional units of the Greek Archaeological Service called Ephorates. Attaining the rights for the study and publication of findings on archaeological materials and excavation finds requires an official permit administered by the regional Ephorate and the regional archaeological councils. Unless otherwise stated, the responsibility and cost of the conservation of the archaeological finds fall to the person or institution undertaking the study. Sampling and analysis of archaeological materials (e.g., radiocarbon dating, isotopic analyses, or ancient DNA, in the case of human remains) require a different permit that goes through the local Ephorates, the Department of Greek and Foreign Research Institutes, Organizations and International Affairs, and finally the Department of Applied Research of the Directorate of Conservation of Ancient and Modern Monuments (Charalampopoulou Reference Charalampopoulou, Voutsaki and Maria Valamoti2013).
SITE DESCRIPTION AND PROJECT HISTORY
During construction of the Stavros Niarchos Foundation Cultural Center, extensive salvage excavations took place by the Ephorate of Piraeus and Islands (formerly the Twenty-sixth Ephorate of Prehistoric and Classical Antiquities) under the direction of Dr. Stella Chryssoulaki (see Chryssoulaki Reference Chryssoulaki2017; Chryssoulaki et al. Reference Chryssoulaki, Alexandropoulou and Aggouras2014; Lobell Reference Lobell2018). The main excavation phase was funded by the Stavros Niarchos Foundation and was conducted between 2012 and 2016. Segments of the same cemetery had originally been excavated by the Greek Archaeological Service in the early 1900s (Pelekidis Reference Pelekidis1916).
The cemetery is located in the neighborhood of Phaleron, on the southern coast of Attica at the Bay of Phaleron, which served as the original port for the city of Athens before the development of Piraeus in the early fifth century. The date, size, density, and mortuary variability make Phaleron a truly unique site. According to ceramic finds, the cemetery dates from the eighth to the fourth century BC, with its major phase dated to the Archaic period. Thus, the lifespan of the cemetery coincides with major developments, including the formation of Athens as a polis (city-state), the first governmental institutions, the first consecrated sanctuaries for the major gods of Attica and the first temples and other landmark monuments, early codification of written laws (e.g., Draco's law), and intermittent feuding among the wealthiest families, eventually leading to the rise of democracy (for Archaic Athens, see Camp Reference Camp2001; Hall Reference Hall2006; Osborne Reference Osborne1996; Shapiro Reference Shapiro2007; Snodgrass Reference Snodgrass1980).
To date, more than 1,900 burials have been excavated at Phaleron. In this paper, we focus on the 1,100 burials excavated by the Ephorate between 2012 and 2013 (Chryssoulaki Reference Chryssoulaki2017; Chryssoulaki et al. Reference Chryssoulaki, Alexandropoulou and Aggouras2014). The majority were inhumations in simple pits in the sandy soil, usually minimally furnished or not furnished at all. The second largest burial category (about 30%) were pot burials of young infants. Other types of burials included primary cremations (less than 10%) and simple stone-lined graves (1%). Finally, horse burials were also found among the human graves. The cemetery generally lacks funerary architecture and inscribed monuments and shows no signs of spatial organization. Current evidence and preliminary observations may suggest that the cemetery population represents a wide range of social classes, including nonelite strata that are commonly missing from the bioarchaeological record.
The great significance of the Phaleron cemetery also stems from the large number of burials showing evidence for captivity (e.g., shackles), violent deaths, execution, and unorthodox burial treatments (e.g., prone or hog-tied), often referred to as the D-group from the initial of the Greek word for “bound” (desmotes). These occur either in single inhumations or in mass graves, raising questions regarding the identities of these individuals, their social roles, their inclusion in the cemetery space, and the nature and degree of persecution in ancient Athens (Chryssoulaki Reference Chryssoulaki2017; Chryssoulaki et al. Reference Chryssoulaki, Alexandropoulou and Aggouras2014; Lobell Reference Lobell2018).
In 2015 an international collaborative project was established for the contextualized bioarchaeological analysis and interpretation of the people of the Phaleron cemetery, and an interdisciplinary team of bioarchaeologists, forensic anthropologists, archaeologists, historians, biochemists, and paleogeneticists was assembled under the direction of Jane Buikstra. The Phaleron Bioarchaeological Project (PBP) is a collaboration among Arizona State University, the M. H. Wiener Laboratory for Archaeological Science of the American School of Classical Studies at Athens (ASCSA), the Ephorate of Piraeus and Islands of the Greek Ministry of Culture, the University of Tennessee Knoxville, and the Max Planck Institute for the Study of Human History.
METHODS
In this paper, we discuss approaches to a skeletal assemblage that reached the bioarchaeological team after excavation, without in situ documentation by a specialist or prior cleaning of the skeletal remains. Therefore, the methods discussed here apply to scholars working on skeletal collections excavated and stored in the recent or more remote past, which is a common occurrence in bioarchaeological practice. In cases like the one discussed here, bioarchaeologists need to rely on information recorded by the excavators. It is crucial to recover as much documentation as possible through field notes, photographs, and drawings. At the Phaleron cemetery, we were fortunate to have very detailed excavation methods and documentation. We strongly emphasize that the collaboration between archaeologists and bioarchaeologists should begin before excavation, and bioarchaeologists should participate in all stages of research design, excavation, recovery, and conservation treatment. Nevertheless, we stress the significance of studying human skeletal assemblages regardless of the excavation date, as there is still great potential in revisiting older collections. This should also remind excavators to record as much information as possible when excavating human skeletal remains, as those reports will be invaluable to future scholars.
In the summer of 2016, more than 1,400 crates and blocks of earth with human skeletal elements recovered from Phaleron cemetery were transferred to the facilities of the M. H. Wiener Laboratory (ASCSA) for the duration of this study. Each crate or block of earth was photographed and inventoried when they arrived to record the state of the material. We photographed the contents of crates as well as the original labeling to avoid potential issues with lost provenance. The material was organized according to burial type. The Phaleron assemblage consists of primary single or multiple burials (i.e., complete or nearly complete skeletons). However, the methodology described here can also be applied to secondary, commingled assemblages.
We worked closely with professional conservators to design appropriate treatment protocols following standard procedures on a case-by-case basis. First, we examined skeletal elements from different burial types to produce a conservation plan and an approximate time schedule. We further divided the assemblage in the following categories based on completeness and excavation method:
• loose skeletal elements (i.e., removed from the matrix during excavation) that required cleaning, conservation, and reassembling
• skeletons lifted from the field en bloc (within the soil) that required microexcavation in the lab before conservation, including segments (such as skull, pelvis, and thorax) and complete skeletons (the vast majority of the skulls and thoraxes were removed in blocks)
Cleaning and Conservation
We have adopted a minimalist approach to conservation (see Wills et al. Reference Wills, Ward, Sáiz Gómez, Korenberg, Phippard, Fletcher, Antoine and Hill2014). Our goal is to be noninvasive to the degree that time and cost constraints allow. We assess the condition of each case separately and adjust the conservation treatment according to the state of preservation and the degree of completeness. We employ dry brushing, using soft paintbrushes or toothbrushes or both. For more robust bone fragments, we employ wet brushing and make sure we never immerse the bones in water. The bones are then left to dry slowly on screens inside properly ventilated rooms and never under direct sunlight. When cleaning bone surfaces, the bones are always hand supported or placed on padded surfaces. Overall, we use paintbrushes of different sizes and soft toothbrushes. Metal tools should be avoided when working directly on bone surfaces, as they are very abrasive and can damage the bone cortex. If needed, dental tools can be useful for cleaning small foramina. Wooden tools, such as clay sculpting tools and thin wooden or bamboo skewers are very useful in bone excavation and conservation. While cleaning particular skeletal elements, it is also useful to have casts or printed diagrams of what the skeletal element should look like to avoid further damage.
When the soil is persistent, we apply ethanol dissolved in water (50%) to soft matrices and pure acetone (100%) to hard matrices using soft paintbrushes or cotton swabs adjusted on wooden skewers. When bones are covered by a hard crust resulting from the solidification of salts and sand from the nearby sea, mechanical cleaning is also required. We use electronic devices such as a dental ultrasonic scaler or a dremel rotary tool to remove the consolidated sand layer without affecting the bone cortex.
When needed, the soluble acrylic resin Paraloid B dissolved in acetone in low concentrations (3%–5%) is used to consolidate (i.e., strengthen) poorly preserved bone (Johnson Reference Johnson1994; Beiner and Rabinovich Reference Beiner and Rabinovich2013). In the cases of very fragile and fragmentary bones, we consolidate the soil that holds the bone together as well to preserve as much information as possible. The same resin in a higher concentration (30%−40%) is used as an adhesive. When reassembling bone fragments is required, we first consolidate the adjoining surfaces and let them dry before we apply the adhesive to avoid future softening of the porous edges and eventual breakage (Wills et al. Reference Wills, Ward, Sáiz Gómez, Korenberg, Phippard, Fletcher, Antoine and Hill2014). The joins need to be held in the proper position while the adhesive dries, otherwise they will be distorted. Different supporting methods exist, such as placing the bones in trays filled with sand. We have found lentils to be the most effective supporting material because they are soft and the least abrasive, they are large enough to avoid being glued to the joins, they do not produce bugs (contrary to rice), and they are inexpensive and easy to find. When working on skulls, it is important to use a supportive round base or small pillow. Small bicycle tires can be useful, as well as socks or tights filled with lentils or large beans.
It is important to allow enough time for drying, and multiple tasks can take place concurrently to maximize time management. Masking tape can be used to hold fragments in place; however, it should be used with great caution, as the bone surface can flake off when the tape is removed. Thus, it should only be used temporarily and never left on the bones for more than a couple of days (Wills et al. Reference Wills, Ward, Sáiz Gómez, Korenberg, Phippard, Fletcher, Antoine and Hill2014). In very fragile bone, we use Japanese tissue applied with Paraloid as an extra support. We avoid the use of filling materials, as the Phaleron remains are not intended for display.
One of the most challenging and time-consuming aspects for the conservation of the Phaleron skeletal assemblage is the microexcavation of large blocks of soil. During excavation, hundreds of complete and partial skeletons were lifted together with the supporting deposits in large blocks of earth, in most cases without supporting materials such as plaster. The skeletal elements commonly lifted en bloc are the torso, the hands, and the feet, which all contain small bones. This method is a common excavation technique for the removal of skeletal elements (Storch Reference Storch1983). The advantages are that it saves considerable time in the field and allows for the detailed excavation and documentation of the position of the skeleton to take place in the controlled environment of the laboratory. The procedure is particularly beneficial when specialists are not present at the excavation. The disadvantages are that the longer the skeletons remain inside the blocks, the drier and more brittle they become, increasing the time and effort needed in their conservation. The skeletal elements in blocks are first documented for burial position and then removed from the soil matrix and conserved independently (Figure 1).

FIGURE 1. Example of a thorax, removed from the field in a block, during microexcavation and conservation in the laboratory (courtesy of Lukas Waltenberger).
Sieving
Dry and wet sieving should be a standard practice in both the excavation and conservation of skeletal remains. We always work over small mesh screens to make sure we do not lose smaller bones or other finds (Figure 2). Everything is wet sieved, and the residue is sorted. We screen all soil removed during the conservation treatment, as well as any soil associated with the skeletal material that has reached the laboratory.

FIGURE 2. Golden bead of about 4 mm diameter recovered in the laboratory during the wet screening of soil masses from an otherwise poorly preserved jar burial of an infant (used with kind permission of the Ephorate of Piraeus and Islands).
Sampling Protocol
It is essential to preserve as much information as possible for macroscopic, microscopic, chemical, and paleogenetic analyses. To ensure the potential for biochemical analyses for all skeletons, regardless of the final sampling strategy, we established a strict sampling protocol for each skeleton during the conservation treatment:
• We select at least one long bone (preferably the femur) that we only dry brush.
• We keep the temporal bones completely untreated, when possible, especially the loose petrous bones, as they are the preferred samples for ancient DNA analysis designed to recover human genetic material (Jones et al. Reference Jones, Gonzalez-Fortes, Connell, Siska, Eriksson, Martiniano, McLaughlin, Gallego Llorente, Cassidy, Gamba, Meshveliani, Bar-Yosef, Müller, Belfer-Cohen, Matskevich, Jakeli, Higham, Currat, Lordkipanidze, Hofreiter, Manica, Pinhasi and Bradley2015; Mathieson et al. Reference Mathieson, Lazaridis, Rohland, Mallick, Patterson, Alpaslan Roodenberg, Harney, Stewardson, Fernandes, Novak, Sirak, Gamba, Jones, Llamas, Dryomov, Pickrell, Luís Arsuaga, de Castro, Carbonell, Gerritsen, Khokhlov, Kuznetsov, Lozano, Meller, Mochalov, Moiseyev, Rojo Guerra, Roodenberg, Vergès, Krause, Cooper, Alt, Brown, Anthony, Lalueza-Fox, Haak, Pinhasi and Reich2015; Pinhasi et al. Reference Pinhasi, Fernandes, Sirak, Novak, Connell, Alpaslan-Roodenberg, Gerritsen, Moiseyev, Gromov, Raczky, Anders, Pietrusewsky, Rollefson, Jovanovic, Trinhhoang, Bar-Oz, Oxenham, Matsumura and Hofreiter2015).
• Given the wealth of information derived from the dentition (e.g., isotopic analysis for dietary reconstruction, mobility, and pathogen and human DNA analysis), it is essential to not treat the teeth with any preservatives, consolidants, or adhesives (e.g., Knudson and Stojanowski Reference Knudson and Stojanowski2008; Price Reference Price and Schutkowski2008). Teeth, either in situ or loose, are cleaned gently with dry or wet brushing or sometimes both. For the same reasons, broken teeth and tooth roots should not be glued or marked with ink or pencil.
• When cleaning dentitions, great care should be taken so that dental calculus, the hardened plaque formed at the neck of the teeth, is not removed. When calculus is broken off by mistake or because of handling, it should be kept in a plastic bag with the identification of the tooth or position, awaiting microbiome analysis.
• We keep dry soil samples before any conservation treatment and after the sieving. When possible, we separately remove soil from the pelvic girdle area for dietary analysis, as well as control samples of soil from other areas, such as the cranium (Figure 3).
The same sampling protocol can be used for commingled assemblages.

FIGURE 3. Removal of a dry soil sample from the pelvic area before conservation treatment at the laboratory.
Packing and Storage
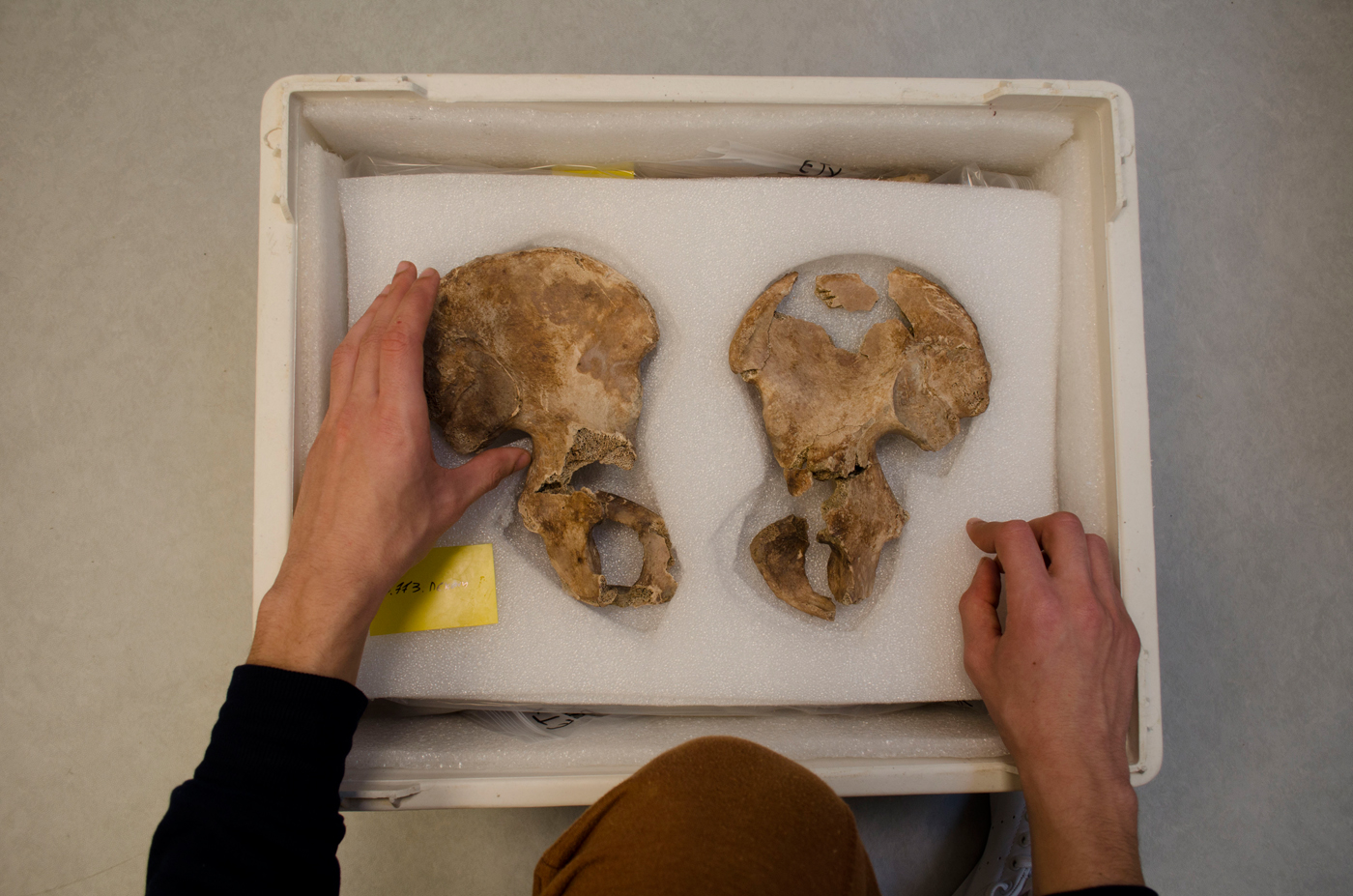
During conservation, it is important to consider short- and long-term storage as well as future transfers. We use ethafoam sheets, acid-free paper, and plastic ziplock bags to store the skeletal remains after conservation. Ethafoam sheets can be easily manipulated using cutting tools to form cases to hold the materials in place (Figure 4). We use Tyvek® tags for labeling, and we avoid the use of organic materials such as paper.

FIGURE 4. Storage of conserved skeletal elements using ethafoam sheets.
Documentation
We put a great deal of effort in documenting all steps in detail. For this purpose, the design of a customized digital database is in process and will include all curation and data collection information. Photographic documentation of all conservation stages is crucial, especially in handling such a large assemblage (see also Zejdlik Reference Zejdlik, Osterholtz, Baustian and Martin2014). As mentioned previously, we photograph each crate or item when it arrives at the laboratory, including the state of original container, the label, and the contents. As standard practice, we also photograph each skeleton or skeletal element before, during, and after conservation. When photographing loose teeth or fragmented bones, we have found placing them in trays filled with black sand particularly useful. We propose organizing photographic archives in the following categories: a) excavation, b) before conservation, c) during conservation, d) after conservation, e) data collection, and f) publication.
Moreover, we keep detailed notes during the conservation process. For each burial, we have a conservation form that describes the following information (supplemental document):
• type of conservation treatment: dry, mechanical, wet, or chemical
• chemicals, preservatives, or adhesives used
• taphonomic observations
• morphological observations (e.g., pathologies noted)
• information on other finds, such as pottery, jewelry, metal objects, charcoal, and animal remains (Figure 5)

FIGURE 5. Jar burial of an infant in situ. During conservation of the skeleton in the laboratory, we also discovered skeletal remains of a dove (Columba livia or Columba oenas) that was buried with the infant. Some of the avian elements were later identified in the excavation photo, traced here in blue (courtesy of Giannis Asvestas, used with kind permission of the Ephorate of Piraeus and Islands).
THE TEAM
The proper curation of skeletal remains requires a team effort. Bioarchaeologists need to closely collaborate with professional conservators to discuss and decide on the conservation treatment for each skeleton. It is important to note that not all conservators are familiar with human skeletal remains, so they may need to be trained in basic human osteology and the properties of osseous materials. The goals of bioarchaeological study and the potential for future exhibition will further determine the appropriate techniques.
In addition to our professional conservation staff, we collaborate with Departments for the Conservation of Antiquities in Greece and Europe (as study abroad students), and we involve student interns trained in the conservation of human remains under close supervision. For example, students in archaeological conservation in Greece need to complete a six-month practicum to obtain their degrees. By offering them training in conservation of archaeological skeletal remains, we gain a large number of interns who alternate every six months. We also have a volunteer program for undergraduate and graduate students in departments of archaeology who want basic training in handling human skeletal remains. Archaeology students participate in the microexcavation and curation of archaeological skeletal remains (e.g., inventorying, photographing, storing, cleaning, and archiving), while acquiring training in human osteology, which is not part of their curriculum. As a result, we have a large team of professionals and trainees from different fields, promoting the education of future generations of young professionals from Greece and providing a strong public benefit. In PBP, we are lucky to have the infrastructure and support of the M. H. Wiener Laboratory that provides us with fully equipped conservation and storage spaces. It is an example of how academic institutions and broad-scale collaborations can play vital roles in developing bioarchaeological research worldwide.
DISCUSSION AND CONCLUSIONS
Overall, our conservation approach is based on the minimal use of invasive techniques, minimal application of chemicals and preservatives, and minimal loss of information. The excavation and conservation of archaeological remains can be destructive processes. We try to be least intrusive, and we need to be time, labor, and cost effective. Thus, it is crucial to develop a conservation strategy that balances the time devoted, the available resources, and the desired outcome. The application of proper conservation methods determines the quality of future macroscopic, microscopic, and biochemical analyses and serves long-term preservation goals.
Management and curation of skeletal assemblages, though essential to a successful bioarchaeological study, are laborious and often difficult to maintain. We have found it easier to obtain funds for excavation and analyses than for laborious conservation tasks. We suggest that bioarchaeological projects, particularly outside of the United States, collaborate with local universities, small colleges, and institutions for the conservation of remains. Involving students and interns can provide great help in this process, and it serves educational purposes and broad impact goals.
It is important to be aware of the academic, educational, legal, and sociocultural system of the country where the curation takes place. Even in contexts wherein bureaucratic procedures can be burdensome, we encourage a positive collaborative effort among all involved parties and respect for the cultural ethics and archaeological laws of the region. Context is important not only for the analysis of the remains but also for all curation stages. It is crucial to remember that we, as bioarchaeologists, also serve long-term curation goals, and we all work toward a common goal to preserve our global cultural heritage (Cleere Reference Cleere1993).
Acknowledgments
We want to thank the excavator Dr. Stella Chryssoulaki and the Ephorate of Piraeus and Islands for the amicable collaboration. Our study and analysis of the human skeletal remains from the cemetery at Phaleron follows the permit issued by the Hellenic Ministry of Culture (ΥΠΟΠΑΙΘ/ΓΔΑΠΚ/ΔΙΠΚΑ/ΤΕΕΑΕΙ158386/93923/8033/777). We are grateful to our funding bodies: the Malcolm Hewitt Wiener Foundation, the Malcolm H. Wiener Laboratory for Archaeological Science of the American School of Classical Studies at Athens, the Paul and Alexandra Canellopoulos Foundation, the School for Human Evolution and Social Change at Arizona State University, the Desnick Family, the National Endowment for the Humanities (Collaborative Research Grant RZ-255623-17), and the National Science Foundation (Senior Archaeology Award BCS-1828645). We also want to thank the Training Vocational Institute Petras and Ilioupolis, the University of Athens, and our student interns and volunteers. We thank Lukas Waltenberger and the Austrian Academy of Sciences. Finally, we are grateful to Zoe Chalatsi, Dimitris Michaelidis, Panagiotis Karkanas, Jim Wright, and Jenifer Neils.
Data Availability Statement
The Phaleron skeletal assemblage, currently housed at the M. H. Wiener Laboratory for Archaeological Science, ASCSA, will be returned to the storage facilities of the Ephorate of Piraeus and Islands when the bioarchaeological and biogeochemical studies are complete. The M. H. Wiener Laboratory will provide long-term storage for 5% of the skeletal assemblage to be included in the laboratory's skeletal reference collections for scientific purposes. All data produced over the course of the Phaleron Bioarchaeological Project will be managed and organized in a custom PostgreSQL database. Public access to the data generated will depend on the Ministry of Culture's data-sharing policies and permissions.