Significant outcomes
-
∙ There exists a time-dependent natural improvement in visuospatial memory in patients with DAI.
-
∙ Neuroplasticity plays an important role in the natural course of DAI, particularly for visuospatial memory.
Limitations
-
∙ This was a single centre study and all inherent generalisation limitations apply.
-
∙ A lack of blinding of assessors could have resulted in bias.
Introduction
Traumatic brain injury (TBI) is the leading cause of sequelae and death in children and young adults in western industrial countries (Reference Kraus1,Reference Mcarthur, Chute and Villablanca2), and is the worldwide leading external cause of death (Reference Imai and Koizumi3). TBI results in persistent dysfunction, which is primarily characterised by cognitive impairment (Reference Whitnall, Mcmillan, Murray and Teasdale4). The most common type of TBI is diffuse axonal injury (DAI), the cause of which is angular or rotational acceleration and deceleration that stretches and shears brain axons, resulting in white matter damage (Reference Meythaler, Peduzzi, Eleftheriou and Novack5–Reference Goetz, Pappert and Schmitt10).Unlike focal lesions, diffuse injury affects widespread cortical and subcortical neural networks, which compromises the brain’s ability to compensate for damage. Both severe and moderate head injuries are associated with DAI (Reference Goetz, Pappert and Schmitt10), cognitive deficits (Reference Christensen, Colella and Illness6), and poor clinical outcomes (Reference Scheid, Walther, Guthke, Preul and Von Cramon7). Memory impairment is one of the most common residual deficits following TBI (Reference Levin8,Reference Palacios, Sala-Llonch and Junque11,Reference Sozda, Muir, Springer, Partovi and Cole12). The impairment is generally non-specific in patients, and can involve the encoding and retrieval of verbal and visual information equally, as DAI typically affects multiple brain networks (Reference Mapou9).
A large number of studies have investigated the effects of TBI on verbal memory, which is to be expected given the relevance of verbal learning and memory in education, academic performance, and everyday functioning. However, few studies have explored visuospatial memory after brain injury (Reference Shum, Harris and O’Gorman13,Reference Brooks14). This ability is crucial for everyday activities such as remembering an object’s location, operating devices, driving, and route finding. Many visuospatial tasks use familiar geometric signs, for which verbal bias may occur because such shapes can easily be encoded verbally. Thus, one of the most widely used instruments to evaluate visuospatial memory in clinical and experimental settings is the Rey–Osterrieth Complex Figure (ROCF) test (Reference Spreen and Strauss15,Reference Osterrieth16). The ROCF test is particularly sensitive to diffuse damage after TBI (Reference Ashton, Donders and Hoffman17), because the instrument is non-specific with respect to laterality (Reference Ariza, Pueyo and Junque18). The complexity of the ROCF also demands executive function, requiring strategies and organisational approaches for drawing, and is sensitive to dysfunction of frontal–striatal systems (Reference Scheid, Walther, Guthke, Preul and Von Cramon7,Reference Tomaiuolo, Carlesimo and Di Paola19).
Brooks (Reference Brooks14) was one of the first to explore visual memory following TBI. He reported worse performance for a TBI group versus a control orthopaedic group. Scheid et al. (Reference Scheid, Walther, Guthke, Preul and Von Cramon7) demonstrated worse performance in a group with TBI as compared with a healthy group in various cognitive domains, most notably in learning, and verbal and visuospatial memory. Thus, previous neuropsychological studies have shown the existence of visual memory impairment in those with TBI (Reference Brooks14,Reference Tomaiuolo, Carlesimo and Di Paola19). Nevertheless, the samples were usually heterogeneous, which could have influenced the aetiology and severity of the TBI. Further, most of the study designs were cross-sectional. Accordingly, longitudinal examination of visuospatial memory performance in patients with DAI has been little explored. Our primary aim was to detect cognitive changes in visuospatial memory between 6 and 12 months after TBI. Our secondary aim was to assess the role of executive function in visuospatial memory. We also carried out an exploratory analysis to verify the influence of potential confounding variables, namely age, intelligence quotient (IQ), years of education, and severity of the trauma. Our first hypothesis was that visuospatial memory would improve over time. Our second hypothesis was that visuospatial memory would depend on executive function, as the latter plays a role in memory processing. To test these hypotheses, we compared ROCF performance at 6 and 12 months after the trauma in a group of patients with moderate and severe TBI, who were diagnosed with DAI.
Method
This was a 1-year prospective one-arm longitudinal study with three levels of a repeated-measures independent variable. The project was approved by the Institutional Ethics Committee for Research (CAPPESQ: Comissão de Ética para Análise de Projetos de Pesquisa) of the University of São Paulo (USP; project number 0097/11) and by the Special Ethics and Research Committee of the Division of Psychology of the USP Hospital (project number 18/2010).
Subjects
The group consisted of 40 patients with moderate-to-severe traumatic DAI. The severity of DAI was defined using the Glasgow Coma Scale (GCS) (Reference Tomaiuolo, Carlesimo and Di Paola19). Inclusion criteria consisted of the following: the participant had sustained a TBI up to 3 months before taking part in this study, had experienced loss of consciousness for at least 30 min as a consequence of TBI, was between 18 and 55 years of age, had at least 4 years of education, and a GCS score of <13 points. Exclusion criteria consisted of the following: patients who had suffered more than one TBI, who exhibited evidence of abnormalities other than DAI according to 3-Tesla magnetic resonance imaging (MRI; e.g. contusions, epidural haematoma, subdural haematoma), or who had been diagnosed with neurological conditions (e.g. epilepsy, stroke, tumour) or psychiatric conditions (e.g. bipolar disorder, major depression, or other conditions requiring admission to a psychiatric ward).
Setting
All data collection took place at the USP Clinics Hospital, Brazil. The sessions were structured using a fixed assessment battery to characterise depression and anxiety symptoms and cognitive measurements. The patients were recruited during the first follow-up medical visit after the trauma. They were invited to participate and received details of the study as explained in the consent form. As soon as they agreed to enrol in the project, we began phase 1 (see below). The follow-up assessments were scheduled during the same day the patient had his or her regular medical follow-up.
Instruments
Beck Depression Inventory (BDI) (Reference Beck, Erbaugh, Ward, Mock and Mendelsohn20), Brazilian version (Reference Gorenstein and Andrade21)
The BDI is a 21-question tool that measures symptoms of depression (e.g. sadness, pessimism, failure, guilt, suicidal ideation) using an ordinal response scale.
State scale from State-Trait Anxiety Inventory (STAI-S) (Reference Spielberg, Gorsuch and Luschene22), Brazilian version (Reference Gorenstein and Andrade23)
The STAI-S consists of 20 questions that assess how patients feel at the moment, or felt in recent past, or what they anticipate their feelings would be in a specific situation that could happen in the future, or in a hypothetical situation.
ROCF test (Reference Spreen and Strauss15,Reference Osterrieth16,Reference Rey24,Reference Rey25)
In the ROCF test, the respondent first copies a complex geometric figure, which is shown on a stimulus card. Subsequently, after three minutes, he or she draws the figure again, from memory (ROCF recall). These tasks assess visuoconstruction and visual episodic memory, respectively. In addition to the traditional method of scoring the ROCF, we also used the Savage scoring system (Reference Savage, Baer, Keuthen, Brown, Rauch and Jenike26). This is a 0–6-point organisational scoring system that allows fast and accurate assessment of planning and organisation.
In the Savage scoring system (Reference Savage, Baer, Keuthen, Brown, Rauch and Jenike26), the ROCF is subdivided into five elements: the large rectangle, the diagonal cross, the vertical midline, the horizontal midline, and the vertex of the triangle on the right. Patients receive scores for reproducing each element as an unfragmented unit. Reproduction of the larger rectangle garners from 0 to 2 points to reflect its importance to the structure of the figure as a whole. All other elements are assigned 0 to 1 points, resulting in a range of scores from 0 to 6. The precision and accuracy of the drawing is not considered in this score. An organisational element receives a score when it is an unfragmented unit: (a) each side comprises a unit (e.g. each of the four sides of the rectangle) if drawn as a continuous line without interruption; and (b) all sides of the unit are drawn consecutively. The order in which the lines are drawn is unimportant, as long as they are drawn one after another. The patients do not receive points for organisation if part of the organisational unit is missing.
Digit span forward/backward from the Wechsler Adult Intelligence Scale-III (WAIS-III) (Reference Weschler27)
The forward task is considered to assess attentional verbal span capacity and the backward task assesses working memory. This test was applied in the 3 min between the end of the figure copy task and the start of figure recall task.
Grooved Pegboard Test (GPT) (Reference Matthews and Klove28)
GPT measures fine motor speed, visuomotor speed, and eye-hand coordination for the dominant hand (part A) and the non-dominant hand (part B). The test consists of 25 holes with randomly positioned slots. Pegs, which have a key along one side, must be rotated to match the hole before they can be inserted.
Intelligence quotient (IQ): vocabulary and matrix reasoning from WAIS-III (Reference Weschler27)
The Vocabulary subtest and Matrix Reasoning subtest of the WAIS-III are established methods for estimating general intelligence (IQ) (Reference Ringe, Saine, Lacritz, Hynan and Cullum29,Reference Coutinho and Nascimento30). The subtests assess verbal knowledge and concept formation, and visuoperceptual, abstraction, and visual reasoning abilities, respectively.
Procedure
All patients who completed the medical examination were submitted to 3-Tesla MRI and signed the consent form. Two experienced neuropsychologists performed the assessment, which had a maximum duration of 90 min and was completed during a single day. Patients were examined at three different points in time:
Phase 1
From 1 to 3 months after the injury. Patients completed the BDI and STAI-S and demographic questionnaires.
Phase 2
After 6 months of injury, the patients completed BDI and STAI-S questionnaires and received a cognitive assessment consisting of the ROCF, digit span forward and backward, GPT, and estimated IQ.
Phase 3
After 12 months of injury, the patients underwent the same procedure as in phase 2.
The study endpoints followed the pattern of those used in clinical trials for TBI (Reference Zaninotto, French and Paiva31), in which cognitive functioning was assessed at 6 and 12 months post trauma (Reference Bell, Temkin and Esselman32–Reference Wade, Walz, Carey and Williams36).
Statistical analysis
Classical descriptive statistics were used according to the type and distribution of the data, which was assessed by graphical and statistical methods. For variables with a normal distribution, effects over time were compared using repeated-measures ANOVAs, whereas for data that were distributed non-normally, the Wilcoxon signed-rank test was used.
To assess the visuospatial memory improvement between 6 and 12 months after the TBI, the ROCF recall score percentage variation was considered the dependent variable in a multivariate linear regression model. Age, IQ, hand coordination, and severity of the trauma were included as independent variables. In addition, the analysis was adjusted for the baseline ROCF recall absolute score.
All tests were two-tailed and the significance level was set at 0.05. The analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 20.0 for Windows (IBM SPSS statistics software, version 20; IBM Corp., Chicago, IL, USA).
Results
The sample consisted of 40 outpatients who were assessed prospectively for 1 year after the trauma. Table 1 provides basic demographic and clinical characteristics.
Table 1 Demographic information and clinical characteristics of diffuse axonal injury (DAI) patients

Data are presented as n (%), unless otherwise stated.
The majority of patients were young (28.7±9.4 years), predominately male (35, 87.5%) and nearly all were victims of road traffic accidents (38, 95%). Their TBI was classified as severe in 60% (Reference Gorenstein and Andrade23) of cases and moderate in 40% (Reference Rey24). Mean IQ was 86.2 (±8.5) as assessed at 6 months and 86.6 (±7.7) at 12 months. These values are ~1 SD below the normal average.
Mood symptoms, as measured by the BDI, did not differ among phases [F(1. 49)=0.731, p=0.451], with mean scores of 9.6±7.8, 9.1±7.0, and 7.5±7.2 at phase 1, 2, and 3, respectively. Anxiety symptoms, assessed by the STAI-S, also showed no differences between phase 1 (40.5±7.6), phase 2 (36.5±9.5), and phase 3 (38.1±9.0; F(2)=1.411, p=0.254).
Table 2 depicts the neuropsychological outcomes, comparing phase 2 with phase 3. IQ, attentional verbal span capacity, working memory, and executive functioning did not change over time. An improvement in non-dominant hand coordination, as measured by the GPT B, was detected [t(34)=−2.677, p=0.007]. Visuospatial memory recall scores, as assessed by ROCF recall, improved from phase 2 to phase 3 [t(38)=−2.611, p=0.013], but no differences were observed for ROCF copy (Fig. 1).
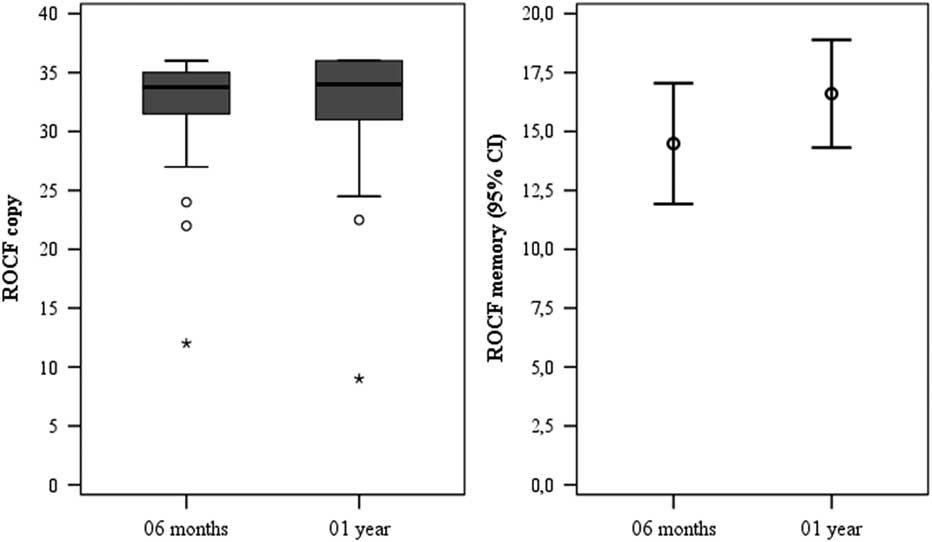
Fig. 1 Rey–Osterrieth Complex Figure (ROCF) copy and recall at 6 months and 1 year.
Table 2 Neuropsychological outcomes of diffuse axonal injury patients (n=40)

GPT, Grooved Pegboard Test; IQ, intelligence quotient; ROCF, Rey–Osterrieth Complex Figure.
Data are presented as mean (SD) or median (1st–3rd quartiles), as appropriate.
Table 3 shows the multivariate analysis results. Neither age, years of education, admission Glasgow Coma Scale (CGS), nor IQ influenced the ROCF recall improvement. The only significant variable was ROCF recall at 6 months. Each point of reduction in this score at 6 months predicted an improvement of 3.7% at 12 months (β=−0.037, 95% confidence interval (CI): −0.058 to −0.016, p=0.001), which may, at least partly, reflect a regression to the mean phenomenon.
Table 3 Multivariate analysis for predictors of visual memory improvement (ROCF recall variation) in diffuse axonal injury patients (n=40)

CI, confidence interval; GCS, Glasgow Coma Scale; IQ, intelligence quotient; ROCF, Rey–Osterrieth Complex Figure; Std., standardised.
Model summary: r 2 0.388, F(5)=4.056, p=0.006.
Discussion
Our follow-up study showed significant improvement in visuospatial memory as assessed by the ROCF test in DAI patients when comparing scores at 6 and 12 months after the trauma. This improvement appeared unaffected by executive function. There was a notable effect of time on visuospatial memory function. However, it is worth considering the possible effect of regression to the mean, as the greater the loss of visuospatial memory after TBI (lower ROCF recall in the early phase), the greater the improvement towards (hypothetical) original levels. This phenomenon can be related to neuroplasticity in chronic changes after TBI. In patients with brain injury, neuroplasticity is a mechanism that compensates for neural damage and re-establishes function (Reference Villamar, Portilla, Fregni and Zafonte37). In the chronic stage, axonal remyelination occurs accompanied by greater recovery.
There exists controversy regarding the most important period of cognitive recovery following TBI. Although 6–12 months post trauma has been proposed (Reference Lannoo, Colardyn, Jannes and De Soete38,Reference Kersel, Marsh, Havill and Sleigh39), others have suggested between 1 month and 1 year after the injury (Reference Dikmen, Temkin, Mclean, Wyler and Machamer40). Even following recovery, it is likely that patients do not return to their premorbid skill levels and some memory deficits can persist even after 10 years (Reference Scheid, Walther, Guthke, Preul and Von Cramon7,Reference Zec, Zellers and Belman41,Reference Vakil42). Despite improved visuospatial memory performance, patients continue to generate worse scores than normative data for healthy subjects (Reference Rey25).
Nevertheless, when comparing months 6 and 12 after the trauma with baseline, only visuospatial memory and manual motor performance were significantly improved, suggesting a therapeutic window for selected domains. High educational levels and genetic predisposition are assumed to be involved in higher cognitive performance. Patients with greater cognitive reserve have a higher threshold for brain injury before clinical deficits appear and ultimately may suffer less impairment (Reference LEE43). Indeed, although IQ estimates tended to be associated with higher ROCF recall at 6 months, and years of education was associated with higher ROCF recall at 12 months (data not shown), neither predicted the improvements on this test between 6 and 12 months.
Regardless of the role of executive function in visuoconstruction ability and planning, our secondary analysis did not show any relationships between the Savage scoring system and improvement on the ROCF recall test. We used the Savage score to assess whether patients were able to make a structured reproduction of a figure during the copy and recall task. We hypothesised that the Savage scoring system would provide a simple way of measuring executive function, which is one of the most complex cognitive abilities in humans. An organised reproduction ability was expected to be associated with better memory recall. Thus, Savage scoring was used to assess the role of executive function on memory recall as measured by the ROCF.
Difficulties with fine motor coordination could have disrupted patients’ performance. We measured such coordination via the GPT, to identify any potential bias in reproduction ability. Arm paresis may occur after TBI, but most patients show good recovery within the first 2 months (Reference Ashton, Donders and Hoffman17). Furthermore, we found improved fine motor coordination over time in the non-dominant hand, which would not lead to better drawing ability.
These results are of clinical relevance because visuospatial memory is independent of executive function, the former can be trained to increase performance even if the patient has a dysexecutive syndrome.
There is an association between TBI and an increased risk of developing depression and anxiety, which may lead to worse functional, social, and cognitive outcomes (Reference Fann, Katon, Uomoto and Esselman44). In our study, anxiety and depression symptoms were at the minimum level during the three phases of the studies. It is possible that these low scores were responsible for the lack of association between anxiety and depression symptoms and the cognitive outcome measures. Similarly, a previous study found no association between visuospatial memory and depressive symptoms (Reference Sozda, Muir, Springer, Partovi and Cole12).
Our study has several limitations. Although we cannot discard the possibility that practice influenced the ROCF test scores, we believe this to be unlikely. No studies have reported ROCF test–retest learning and there is some evidence that the same memory tests can be used successfully in follow-up assessments (Reference Tombaugh, Faulkner and Hubley45,Reference Levine, Miller, Becker, Selnes and Cohen46). ROCF is considered a difficult image to learn in a clinical setting, and in the current study, the ROCF figure was presented twice to the same patient with 6 months between presentations. Moreover, visual deficits following moderate and severe TBI are common (Reference Ventura, Balcer and Galetta47). These deficits are related to abnormal saccades, pursuit, convergence, accommodation (Reference Goodrich, Flyg, Kirby, Chang and Martinsen48), and perceptual organisation (Reference Costa, Zaninotto and Benute49). In our previous study (Reference Costa, Zaninotto and Benute49), we observed patients’ difficulties in combining elements into a whole by means of elementary grouping processes, as assessed by the Leunen perceptual organisation screening test. We analysed visuospatial memory using the ROCF test and investigated whether the scores were related to executive function, as measured by the Savage scoring system. However, we found no such association.
No reports exist to date regarding sex differences in visuospatial memory, visual organisation, or visual perception in patients with TBI. Between 70% and 85% of patients with TBI are male (Reference Herrera-Melero, Egea-Guerrero and Vilches-Arenas50,Reference Greenwald, Hammond, Harrison-Felix, Nakase-Richardson, Howe and Kreider51), which was also true of our sample, thus supporting the internal and external validity of our results.
There exists the possibility of bias in the current study due to a lack of blinding. We tried to avoid bias by using standardised, objective tests to assess mood and cognition. Possible sampling bias should also be considered. The ratio of patients screened to those followed was large, that is, a small and perhaps distinctive group of patients with DAI was able to participate in the study.
Overall, our results suggest that patients with DAI have an open window for neuroplasticity of visuospatial memory between 6 and 12 months after the trauma. Clinicians need to be aware of this natural improvement in visuospatial memory, as interventions during this phase could take advantage of the increased neuroplasticity.
In conclusion, our findings suggest that the ROCF test is suitable for assessing visuospatial memory following TBI and that neural reorganisation appears time dependent. The natural course of DAI recovery due to neuroplasticity remains unknown. Understanding this natural recovery course would be helpful when identifying effective rehabilitation strategies.
Acknowledgements
None. Authors’ Contributions: all authors contributed equally and fulfil the International Committee of Medical Journal Editors (ICMJE) authorship criteria.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare there are no conflicts of interest pertaining to this work.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







