Significant outcomes
-
The main hypothesis proved that the use of biofeedback is an effective intervention to help the reduction of depression levels compared to the control group.
-
Biofeedback is a complementary method for the treatment of moderate and severe depression and dysthymia.
Limitations
-
Further research is needed to evaluate the adherence of the participants to drug therapy during the follow-up period in both groups, considering that this study was not expected to verify the serum levels of substances.
-
The research did not identify how long the patients used oral antidepressants. In the pre-test, information about which medications the participants were using was collected.
-
There was a reduction in the range of scope data. The sample size was reduced by 4 in the experimental group and by 3 in the control group, from an initial sample of 43 individuals characterising losses of follow-up.
Introduction
Depression is a common disorder in the world, with over 264 million people affected, and the leading cause of disability. It has the potential to become a serious health condition and is also a contributing factor to suicide deaths (World Health Organization [WHO], 2020).
According to the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5), there are classifications for depressive disorders. Major depressive disorder (MDD) presents five or more symptoms for at least 2 weeks (most of the day/almost every day), changing the previous mode of acting: depressed mood; loss of interest or pleasure; loss or significant weight gain without being on a diet; insomnia or hypersomnia; psychomotor agitation or retardation; fatigue or loss of energy; feelings of worthlessness or excessive guilt; reduced ability to think or concentrate, or indecisiveness; recurrent thoughts of death, recurrent suicidal ideation, attempted suicide or specific plan to commit it. One of the symptoms should be depressed mood or loss of interest or pleasure (American Psychiatric Association [APA], 2013).
Furthermore, it must cause significant distress or impairment in functioning areas and should not be assigned to the effects of a substance or other health condition. A more chronic form of depression is known as dysthymia, which presents similar symptoms to MDD, however, lasting at least 2 years, and in the lighter form (APA, 2013).
Different health services offer support for people with depression, including psychiatric outpatient clinics, which provide care services in mental health at the secondary level and psychosocial care center, an open and communitarian health service of the Brazilian Public Health System, whose dynamic action constitutes the promotion, prevention, treatment and rehabilitation of mental health (Brazil, 2004, 2016).
Globally, there are effective treatments for depression, but in some countries, the percentage of people who receive treatment gets only 15% to 24% (WHO, 2020). Antidepressants are chosen based on minimising side effects, physical condition, lifestyle and individual’s temperament. There are several classes of antidepressants available, with different mechanisms of action (Sadock et al., Reference Sadock, Sadock and Ruiz2017).
Approximately two-thirds of people with major depression do not respond adequately to drug therapy, with remission rates between 30% and 35% (Rush et al., Reference Rush, Trivedi, Stewart, Nierenberg, Fava, Kurian, Warden, Morris, Luther, Husain, Cook, Shelton, Lesser, Kornstein and Wisniewski2011; Canadian Agency for Drugs and Technologies in Health [CADTH], 2014). To increase the efficacy of treatment and to solve the limitations of conventional methods, additional treatments have been proposed. Biofeedback, for example, is a method that provides a complementary approach in the treatment of people with symptoms of depression, aiming to improve the affective state of the individual (Schoenberg & David, Reference Schoenberg and David2014). Its first official definition was in 2008, as a process that enables people to change their physiological activity through measurements of heart and respiratory rate, and others, and provide feedback to them. The projected information can cause changes in emotions, thought, and behaviour of people (Schwartz et al., Reference Schwartz, Collura, Kamiya, Schwartz, Schwartz and Andrasik2016).
Studies demonstrate scientific evidence of biofeedback in clinical practice and, in 2016, Association for Applied Psychophysiology and biofeedback classifies the use of biofeedback technique as evidence level of four, effective for depression (Tan et al., Reference Tan, Shaffer, Lyle and Teo2016).
There are different types of biofeedback, one focuses on increasing the heart rate variability (HRV), mediated by the autonomic nervous system, through breathing regulation, usually every five to six breaths per minute (Hartogs et al., Reference Hartogs, Bartels-Velthuis, Ploeg and Bos2017). In all modalities, the individual is in contact with some sensors connected to electronic equipment, plugged into a computer, which allows the feedback of autonomic activities to the participant.
More research is needed to identify innovative therapies for treating depression. A reduction in the time of treatment and a resumption of the daily life of the participants is expected if the effectiveness of biofeedback training in reducing depression is confirmed.
Therefore, the research has a null hypothesis (H0: μ1 = μ2): the use of biofeedback does not improve depression levels. As an alternative hypothesis (H1: μ1 ≠ μ2), the use of biofeedback improves depression levels.
Aims of the study
This study aimed to evaluate the use of biofeedback intervention in the levels of depression. Thus, the research intended to answer the following question: Does biofeedback training improve depression levels?
Material and methods
Study design
A randomised, parallel, open, with two arms, on the effect of interventions with biofeedback in the level of depression in users assisted in a psychiatric outpatient clinic and psychosocial care centres. Participants in the experimental group were submitted to six training, once a week, from 10 to 30 min with biofeedback, keeping the conventional treatment used for services for each participant with depression. The control group received only conventional treatment for depression (psychiatric medication and/or psychotherapy).
The study followed the criteria established in the Consolidated Standards of Reporting Trials (CONSORT), which highlights the necessary items for conducting a randomised clinical trial (Moher et al., Reference Moher, Hopewell, Schulz, Montori, Gøtzsche, Devereaux, Elbourne, Egger and Altman2010).
Study site
Held in three locations: at a psychiatric outpatient clinic of a University Hospital (Hospital Universitário Professor Alberto Antunes, in Maceió, Alagoas, Brazil), in psychosocial care centres, and at the bioneurofeedback laboratory of a School of Nursing located in a Federal University (Universidade Federal de Alagoas, in the same city previously mentioned).
Participants
The sampling method was a simple random type. Sample calculated on 34 individuals (17 people per group) based on a clinical trial that used biofeedback for the treatment of MDD, showing greater reduction of depressive symptoms in the experimental group, assessed by Beck Depression Inventory (BDI) (Caldwell & Steffen, Reference Caldwell and Steffen2018), the same as the present research.
As shown in Fig. 1, from an initial sample of 43 individuals recruited (22 designated to the experimental group and 21 to the control group), 1 person in the first group (4.5%) did not carry the first training due to family members. Additionally, three patients (13.6%) in this group discontinued intervention: two patients referred difficulty to attend intervention once a week and one person refused to continue biofeedback training after the first training had been applied. This patient referred to feel uncomfortable due to asthma and anxiety. In the control group, three people (14.3%) characterised losses to follow-up; they did not return to the post-test or the researchers did not have success in contacting them. Thus, studies with follow-up time shorter than 6 weeks may be performed to reduce losses to the same outcome.

Fig. 1. Research flowchart.
The final sample was composed of 36 participants met in the services (18 in each group, experimental and control). Participants included individuals aged 18 years or older, males and females; under antidepressants; met the criteria of the Mini International Neuropsychiatric Interview 5.0.0 (MINI) for major depressive episode or dysthymia, and with scores from 20 to 63 in BDI, which correspond to the moderate and severe of depression.
Individuals were excluded under any of the following conditions: acute phase of dissociation and hallucinations; manic or hypomanic episode (current or past); in psychoactive substances dependence, except nicotine; psychotic syndrome; mood disorder with psychotic features; or antisocial personality disorder, after MINI instrument had been applied.
Procedure
Users from the services were recruited. Individual meetings to present the research to them were held. Eligible participants were informed about the study procedures. All of them provided the written consent before the application of the instruments. Randomisation was done in blocks, through opaque, sealed envelopes with numbers in sequence, which was specified the group where the participant would be allocated to.
In order to prepare these, the online programme Sealed Envelope was used, with number 245302110504878, in blocks of four envelopes, so that each one received a stamp glued on the outside of the envelope, with code numbers and letters, in the following sequence: block identifier, block size, envelope sequence within the block, and code, for example, 1,4,4, OR6. Inside the envelope, there was a card revealing which group the participant would be part of, so that the identifier ‘treatment’ was inserted, for example, 1,4,4, without biofeedback, OR6.
The preparation of the envelopes was conducted by research collaborators who did not participate in the recruitment, pre-test and post-test stages, aiming to minimise the selection bias.
The logistics of the research made it impossible for the study to be double-blind. However, researchers, during the pre-test application, did not know which envelopes represented the control or biofeedback group. At the end of the pre-test, after the person had been included in the study, researchers showed four envelopes randomly distributed and participants chose which envelope would be opened. At this moment, both patient and researcher identified which group the participant would be allocated to.
Furthermore, the researcher who analysed the data was blinded, so it was not possible to know from which group each person belonged to, to avoid biased analysis. Aiming to identify the eligible participants, some instruments were firstly applied: questionnaire of demographic data, MINI and BDI.
The questionnaire was adapted for this research, which was investigated gender, marital status, religion, colour or race, level of education, and other variables. MINI is an instrument of the standardised diagnostic interview, validated in Brazil, and explores the main psychiatric disorders of the Diagnostic and Statistical Manual of Mental Disorders IV (DSM IV) (Amorim, Reference Amorim2000; APA, 2013). BDI is an inventory of self-report validated in Brazil, adapted from Beck and Steer (Reference Beck and Steer1993), with estimated reliability between 0.79 and 0.90 in six samples of people with mental disorders (Cunha, Reference Cunha2001).
It was conducted through face-to-face interviews with the digital application ODK Collect (Open Data Kit), available on the Android system installed in Tablets. In the post-test, after 6 weeks of biofeedback training or conventional treatment, BDI was applied only for purposes of checking the change in the level of depression and to evaluate the outcome.
To avoid biased responses, the post-test was administered by research collaborators, who were undergraduate and postgraduate students and did not know which group the participant belonged to, thus ensuring the reliability of the answers. The training of biofeedback was applied by only one researcher who was not involved in the post-test application.
For the execution of training, the Nexus-32 equipment and two types of sensors were used: respiration sensor (RSP), to measure the respiratory rate and depth in real time; and blood volume pulse sensor (BVP), which determines the volume of blood pulse, heart rate, beats minute with parameters derived from the heart rate related to the blood flow, in the range of 40 to 240 beats per minute.
The RSP sensor is set in the abdominal circumference of the individual so that it is comfortable, and about two fingers above the umbilicus. BVP sensor is already in a digital non-dominant hand. The participant sat in a chair in a comfortable position, coupled with the sensors.
During the training, the participant was asked to breathe in his/her usual manner for 2 min, and from 2 min the researcher requested that maintained a nasal inspiration around 4 s, followed by exhalation through the mouth for about 5 s, stimulating diaphragmatic breathing. Adopted a biofeedback protocol from the NeXus-32, which allowed to select different images as flower, ocean, puzzle, during practice and enabled a correlation between heart rate and respiratory rate simultaneously and in real time.
The expected primary endpoint of the study was to reduce the score of depression, reported by the BDI, from the observation of at least 5% of the variation in pre-test and post-intervention measurements.
Outcome measure
The outcome measure was assessed in two stages, one pre-test (before the first training, in the experimental group; and at recruitment in the control group) and a post-test (at the end of the last training in the experimental group, and after 6 weeks with only the conventional treatment in the control group).
Data analyses
The factors and variables were presented in terms of descriptive statistics (Tables 1 and 2) and inferential statistics (Tables 3 and 4). For the counting variables, Fisher’s exact test (p < 0.05) was used to verify the existence of an association between them.
Table 1. Frequency by group and general of sociodemographic variables, Maceió-AL, 2019 (n = 36)
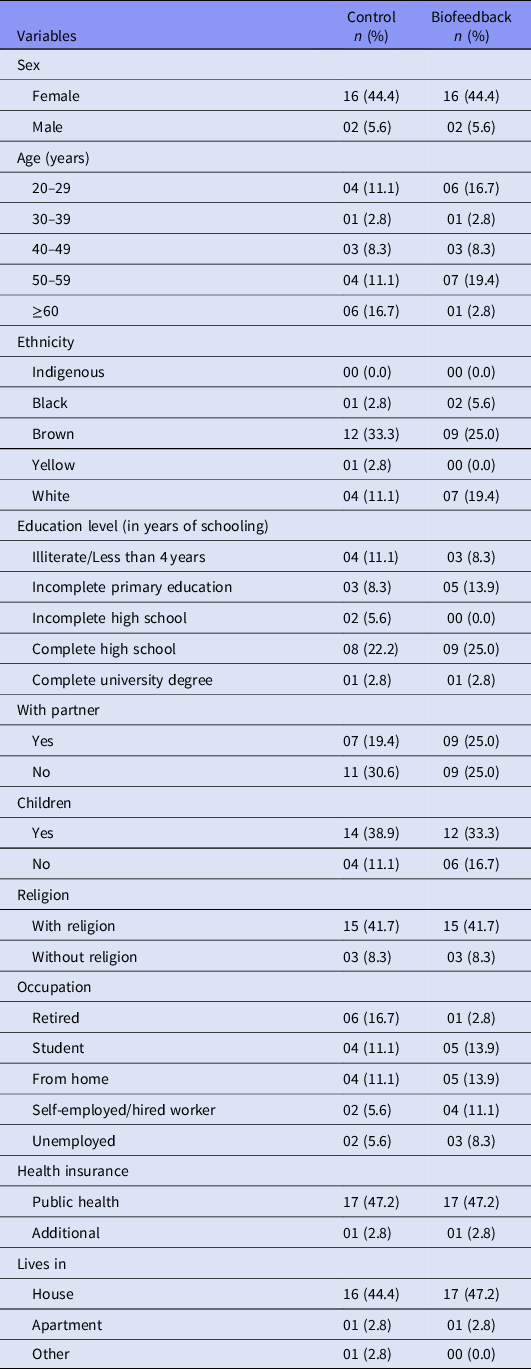
Table 2. Prevalence by group and general of the received intervention, 2019 (n = 36)

SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin and norepinephrine reuptake inhibitors; Other, other classes of psychiatric medication.
Table 3. Levels of depression in BDI pre-test and BDI post-test, by group, 2019 (n = 36)

BDI, Beck Depression Inventory.
a Fisher’s exact test (p < 0.05).
Table 4. Estimation coefficients (β’s) and odds ratio according to the levels of depression of the BDI, 2019 (n = 36)

SE, standard error; DF, degrees of freedom; OR, odds ratio; CI, confidence interval (Wald).
a p-value (Wald’s test).
b Pseudo-R 2Nagelkerke = 0.67.
The multinomial logistic regression model was adopted to verify possible confounding factors, with the depression levels of the groups analysed according to the BDI scale as the dependent random variable (outcome). To achieve it, the presence of multicollinearity (tolerance values> 0.1 and VIF < 10.0) was first verified among the factors studied. After such verification, a multinomial regression model was built including all the factors involved. Then, a second regression model was developed where all the factors that showed significant regression coefficients by the Wald test (p < 0.05) remained. Odds ratios were obtained by exponentiating the respective β-coefficients, as well as the 95% confidence intervals for OR by the Wald test (p < 0.05). The suitability of the fit of the final model was verified by the Pearson and Deviance method (p < 0.05). The determination coefficient obtained was Nagelkerke’s Pseudo R square. In both analyses, the Logit link function was used.
The software used was the RStudio version 3.6.2 (Core Team, 2019). Further, an intention to treat analysis was performed, considering the treatment to which the participant was allocated regardless of whether received it or not. Thus, this method includes the losses to follow-up in experimental and control groups and calculates the Number Needed to Treat (NNT), which evaluates the clinical significance by the average number of participants who should receive the particular intervention, rather than control, so that an unwanted event is avoided (Fletcher & Fletcher, Reference Fletcher and Fletcher2006; Guyatt et al., Reference Guyatt, Rennie, Meade and Cook2008).
The unwanted event was the non-reduction of BDI scores (cases in which participants presented their scores maintained or increased by comparing pre-test and post-test). Hence, a calculation of absolute risk reduction (ARR) for this event was performed, by subtracting the event rate in the control by the event rate in treated.
Ethical standards
The project was submitted to the Ethics Committee for Research with Human Beings of the Federal University of Alagoas (Brazil) and approved under the number 3.213.304. The project was also registered in the Brazilian Registry of Clinical Trials Platform (ReBEC) and obtained approval under the RBR-5prgcn registration number.
All participants were aware of the informed consent form and provided their consent. It has been signed in two copies: one copy would remain with the participant.
Results
The study included 36 users with major depressive episode or dysthymia: 26 followed in a psychiatric outpatient clinic and 10 in psychosocial care centers. And 88.9% (n = 32) were female, 30.6% (n = 11) were within the 50–59 years age range, 58.3% (n = 21) reported themselves as brown, 55.6% (n = 20) affirmed not having a partner and 72.2% (n = 26) had children. Participants who reported a religion totalled 83.3% (n = 30), and the predominant level of education was complete high school/incomplete higher education, corresponding to 47.2% (n = 17) (Table 1).
Moreover, 25.0% (n = 09) reported to work at home and 25.0% (n = 09) being a student, 91.7% (n = 33) lived at house, 58.3% (n = 21) said having their own house, and 72.2% (n = 26) had a family member as responsible for higher income. Most people, 94.4% (n = 34), only used public health care system as agreement (Table 1).
Data relating to the conventional treatment received by all participants in this study (Table 2) show that 30.6% (n = 11) attending therapeutic group and 50.0% (n = 18) psychotherapy; all of them had an antidepressant prescription, whereas only 13.9% (n = 05) used serotonin and norepinephrine reuptake inhibitors (SNRIs), 30.6% (n = 11) tricyclics, and 69.4% (n = 25) selective serotonin reuptake inhibitors (SSRIs). The majority, 58.3% (n = 21), used benzodiazepines, whereas 36.1% (n = 13) other medications, such as antiepileptic, antihistamine and mood stabilisers.
As the results found in the pre-test by the BDI (Table 3), the predominant level of depression was moderate in biofeedback and control groups of 44.4% (n = 16) and 38.9% (n = 14), respectively. There was no significance (p = 0.658) between the moderate and severe levels, so it is assumed that there was no statistical difference in the groups before the intervention. Consequently, the distribution of these groups is similar.
Adverse effects were not observed in the groups.
The calculation of event rates was conducted in controls and treated, to find the ARR and, finally, the NNT. Considering four losses and one failure in biofeedback, in the experimental group, five people filled in the field exposure and unwanted event (not reducing the BDI score). In the control group, there were three losses and six cases of failure in conventional treatment received, totalling nine cases of an unwanted event.
Applying the formula cited by Buehler et al. (Reference Buehler, Cavalcanti, Suzumura, Carballo and Berwanger2009), it is found an ARR of 0.20 and NNT of 05. Thus, it is suggested that it is necessary to treat five participants with biofeedback to avoid an unwanted event (maintenance or increase in BDI score at post-test).
The multinomial logistic model constructed in the study is shown in Table 4. The factors that remained in the final model were group, sex, partner, atypical antidepressants, benzodiazepines, mood stabiliser, antiepileptic and antihistamine, according to the BDI scale variable. The criterion for this was the statistical significance of its regression coefficients (p Wald < 0.05).
For odds ratios, its respective 95% confidence intervals were also presented (p Wald < 0.05). To present the results and discussion, the cut-off value of these OR was adopted.
For the group that did not undergo the biofeedback intervention, there was a synergistic effect in the order of 2.8 percentage points in the BDI scale related to the participants in the experimental group. The group that did not receive biofeedback intervention proved to be a risk factor with 16 times more chances of worsening the depressive state compared to participants in the experimental group.
For ‘Sex’, being a man had an antagonistic effect in the order of 3.3 percentage points on the BDI scale, representing as a protective factor in about 96.0% fewer chances of worsening the depressive state when compared to women.
For ‘Partner’, there was an antagonistic effect in the order of 2.5 percentage points in the BDI scale of participants who had a partner. It can be inferred that the chances of aggravating the depressive condition were 92.0% lower related to the participants who did not register about the marital relationship.
For ‘Atypical antidepressants’, there was a synergistic effect of 8.6 percentage points in the BDI scale for participants who did not use this medication class. The non-use of these was configured as a risk factor with 5,276.4 times more chances of aggravating the depressive condition when compared to those who used it.
For ‘Benzodiazepines’, there was an antagonistic effect in the order of 2.9 percentage points on the BDI scale for participants who did not use such medication. The non-use of this was configured as a protective factor with 95.0% fewer chances of worsening the depressive state in comparison to the participants who used this medication class.
For ‘Mood stabilizers’, there was a synergistic effect of around 4.4 percentage points in the BDI scale of participants who did not use this medication. The non-use of this medication class was found to be a risk factor with approximately 79 times more chance of worsening the depressive state when compared to the participants who used this.
For ‘Anti-epileptics’, the non-use of these represented a synergistic effect in the order of 4.7 percentage points in the BDI scale. The non-use of these proved to be a risk factor in about 113 times more likely to aggravate the depressive state compared to those who used it.
For ‘Antihistamines’, the non-use of these had an antagonistic effect in the order of 8.4 percentage points in the BDI scale. The use of this medication class was configured as a risk factor with 100 times more chances of worsening the depressive state related to the participants who did not use it.
The fact of people being under psychotherapy or in a therapeutic group does not represent a significant effect. Hence, it is not statistically considered as a confounding factor related to the dependent random variable (outcome) in this model.
Discussion
The results of this study showed a significant improvement in the levels of depression in the biofeedback group.
The literature indicates that social and economic factors are some of the determinants for mental health problems (WHO, 2020). Such factors may contribute to the multi-causality of depression; however, its true cause is still unknown (Klijs et al., Reference Klijs, Kibele, Ellwardt, Zuidersma, Stolk, Wittek, Mendes de Leon and Smidt2016). It is noticeable, in this study, the different contexts are experienced by individuals. The sociodemographic data that appear most in studies related to depression include sex and marital status.
The results of the sample in this study showed that women had depression more commonly than men, as the literature suggests. Worldwide, depression is generally twice more common in women than in men. Such difference is related to the gender gap in depression, and it is suggested that it is linked to gender differences in psychological and biological susceptibility, in addition to environmental factors that interfere at both micro and macro levels (Malhi & Mann, Reference Malhi and Mann2018).
In the United States, females have 1.5 to 3 times more depression than the opposite sex, beginning in adolescence (APA, 2013). Although women experience depression more commonly, being female does not indicate that it is a risk factor for depression, but rather the environment in which the woman lives in and the social support she receives in her culture. Additionally, men have the tendency to report fewer symptoms, which contributes to this number (Lima, Reference Lima1999).
Regarding marital status, the results of the survey revealed a higher prevalence of depression among those who stated that they did not have a partner (55.6%). The literature reveals that there is a higher frequency of depression in those who declare themselves divorced or separated than married and single (Lima, Reference Lima1999; Ziliotto, Reference Ziliotto, Marcolan and Castro2013).
In the context of depression, different approaches can be indicated for depressive disorders, including psychotherapy and pharmacotherapy. Over the decades, psychodynamic psychotherapeutic interventions and cognitive-behavioural approaches have gained prominence and influenced the treatment of depressive behaviour and its understanding (Souza & Lacerda, Reference Souza, Lacerda, Quevedo and Silva2013).
The therapeutic class of antidepressants most used in the study was SSRIs (69.4%). The literature claims that these are the most commonly used among psychopharmacological agents suitable for MDD and other disorders, including obsessive-compulsive disorder, post-traumatic stress disorder and panic disorder (Sadock et al., Reference Sadock, Sadock and Sussman2013, Reference Sadock, Sadock and Ruiz2017).
Benzodiazepines, used by 58.3% of the research participants in association with an antidepressant, have a rapid anxiolytic and sedative effect and are often used as an adjunct to SSRIs for chronic anxiety disorders (Sadock et al., Reference Sadock, Sadock and Sussman2013). The model presented in this research shows that those who did not take benzodiazepines had a lower risk of worsening depression, compared to those who used it. A meta-analysis pointed low response of anxious depression to benzodiazepines, which did not reveal any significant differences when compared to placebo. Furthermore, this meta-analysis identified a lack of information about the effects of this medicament class in long-term depressive symptoms treatment (Benasi et al., Reference Benasi, Guidi, Offidani, Balon, Rickels and Fava2018).
From the collected data, it is possible to affirm that six biofeedback training, once a week, help in treating depression. Research has shown the clinical efficacy and safety of biofeedback, demonstrating its potential for the treatment of depression (Cadth, 2014). A randomised clinical trial and a study of a single group, carried out to six HRV biofeedback protocol training in people with depression have shown a reduction in BDI scores during and after training, compared to pre-test, confirming the research findings (Caldwell & Steffen, Reference Caldwell and Steffen2018; Jester et al., Reference Jester, Rozek and Mckelley2018). Another study of three groups using five biofeedback training in one of the groups also shows a significant reduction in the levels of depression in the experimental group compared to the others (Caldwell, Reference Caldwell2015).
Karavidas et al. (Reference Karavidas, Lehrer, Vaschillo, Vaschillo, Marin, Buyske, Malinovsky, Radvanski and Hassett2007) conducted a study on a single group of HRV biofeedback for the treatment of major depression, in practice applying 10 training for each participant. It was found BDI scores decreased from the fourth training. Comparing the first to the fourth, there was a −9.11 difference in the mean of BDI scores; from the training 1 to 10, the difference increases with −10.25 value (p < 0.01). In addition to that, the intervention can help reduce some symptoms of depression, such as loss of energy, lack of motivation, sleep disturbances, and other neurovegetative aspects of MDD (Karavidas et al., Reference Karavidas, Lehrer, Vaschillo, Vaschillo, Marin, Buyske, Malinovsky, Radvanski and Hassett2007).
Although the inclusion and exclusion criteria are well defined, and the interventions had been completed in most participants of the experimental group, the study sample was small. However, other studies used a small sample size and showed important results for the clinical practice of using biofeedback for depressive disorders (Karavidas et al., Reference Karavidas, Lehrer, Vaschillo, Vaschillo, Marin, Buyske, Malinovsky, Radvanski and Hassett2007; Siepmann et al., Reference Siepmann, Aykac, Unterdörfer, Petrowski and Mueck-Weymann2008; Cheng et al., Reference Cheng, Lin, Huang, Tang, Lee and Lai2014; Kotozaki et al., Reference Kotozaki, Takeuchi, Sekiguchi, Yamamoto, Shinada, Araki, Takahashi, Taki, Ogino, Kiguchi and Kawashima2014).
The discussion of the variables related to depression levels allows us to understand that the primary endpoint was reached, from the finding of a change of at least 5% in the pre- and post-intervention measurements, in the experimental group. There was a reduction in the score of depression evaluated by BDI (scores average reduction of 48.6%).
On the presented results, the clinical implications of this research pointed out the use of biofeedback reduces depression, representing a complementary alternative for the treatment of moderate and severe depression levels, and dysthymia. As future research directives, other studies including adherence evaluation of the participants to drug therapy can be suggested.
Acknowledgements
We would like to thank all patients and professionals of the psychiatry outpatient clinic and psychosocial care centres for their support.
Author contributions
WHCM and MCSA were responsible for the design of the study. WHCM, JJS, CSGC, JDSS, COP, MCSB and FMPB were responsible for the data collection. RCSS, PAS and CRBC were responsible for the descriptive and inferential statistics. CRCJ conducted the multinomial logistic regression model adopted in this study. All authors contributed to the interpretation of data. All authors wrote the drafts of the manuscript, which was critically revised and approved.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Conflict of interest
None.








