Introduction
A patient with symptoms of depression is assessed by a psychiatrist and, when treatment is discussed, mentions reading about tryptophan being of potential benefit ‘because it turns into serotonin in the brain'. The patient wants to try a tryptophan-rich diet and asks for the psychiatrist's overall opinion about the matter and advice on how to achieve such a diet.
What advice should the psychiatrist give? This review focuses on dietary methods to influence tryptophan levels, the clinical applications this may have and the empirical research supporting the role of tryptophan in depression, particularly studies published in the last 10 years and also earlier relevant studies. The article also examines the information provided by the general English language media regarding tryptophan and mood over the past few years, but does not focus on the role of tryptophan in sleep disorders, behaviour, anxiety, attention or cognition.
Tryptophan's role in mood
Tryptophan's potential as a treatment for depression has been given much attention by the general media. A search using ‘tryptophan' and ‘mood' as keywords in Factiva, a search engine for general media publications, particularly newspapers and magazines, yielded 1461 citations from 1986 to February 2010. The Factiva search was for English language articles and these were published in newspapers from a wide range of countries and regions, including the United Kingdom, United States, Canada, the Philippines, India, Pakistan, Middle-East, Singapore, Australia and New Zealand.
Tryptophan is an essential amino acid; a subunit in protein molecules and a precursor to serotonin. It cannot be synthesised by the body and must be obtained only through diet; the average daily requirement for adult humans being 4 mg/kg body weight (Reference Elango, Ball and Pencharz1). A Cochrane Review of the efficacy of oral tryptophan and 5-hydroxytryptophan (5-HT) supplementation as treatments for depression concluded that there was insufficient evidence that either is more effective than placebo in unipolar depression (Reference Shaw, Turner and Del Mar2). However, because of the strictness of the inclusion criteria, there were only two studies, published in 1972 and 1982, in this meta-analysis. All the studies reviewed, including those rejected, were quite old and were published from 1964 to 1999, despite a search update in 2004.
Altering dietary intakes of protein and carbohydrate can theoretically modify mood by manipulating tryptophan uptake by the brain. The Wurtman hypothesis states that a carbohydrate-rich meal stimulates insulin secretion and causes most of the amino acids in the bloodstream, other than tryptophan, to be taken up by the peripheral tissues (Reference Benton and Donohoe3–Reference Wurtman and Wurtman5). The resulting higher ratio of tryptophan to other large neutral amino acids (LNAAs) (valine, isoleucine, leucine, tyrosine, phenylalanine, methionine and histidine) (Reference Young6) leads to a greater transport of tryptophan to the brain.
Changing tryptophan levels by diet
There are very few published studies in humans which manipulate plasma tryptophan levels by dietary means alone. Merens et al.'s 2007 review of empirical studies that manipulated tryptophan levels through supplementation or depletion techniques did not report any studies that used solely dietary methods (Reference Merens, Willem Van der Does and Spinhoven7). The study by Markus et al. (Reference Markus, Panhuysen, Tuiten, Koppeschaar, Fekkes and Peters8) is one of the few to use dietary techniques. Plasma tryptophan:LNAA ratios rose significantly on administering high-carbohydrate-low-protein diets (protein content 3.9–5% of caloric intake), by an average of 42% above the ratio when on high-protein-low-carbohydrate diets. Inclination to stress was measured through the Inadequacy Scale of the Dutch Personality Inventory, which assesses neuroticism, and in individuals highly prone to stress, high-protein-low-carbohydrate diets increased depression when stress was induced. However, in individuals less prone to stress, both high-protein-low-carbohydrate and low-protein-high-carbohydrate diets led to increased severity of depression. It is not known if the test diets influenced the fasting amino acid profiles as baseline plasma amino acid ratios were not measured, and also the test meals were administered only on the test day rather than as long-term diets. Wurtman et al.'s more recent study (Reference Wurtman, Wurtman, Regan, McDermott, Tsay and Breu9) showed that the protein and carbohydrate content of a meal can significantly alter plasma tryptophan:LNAA ratios in healthy adults. In this 2003 randomised crossover trial, a high-carbohydrate breakfast (69.9 g carbohydrate, 5.2 g protein) initially lowered tryptophan:LNAA ratios below baseline, with the ratio then increasing over the 3 h of the test, albeit not significantly. In contrast, the high-protein breakfast (15.4 g carbohydrate, 46.8 g protein) significantly lowered plasma tryptophan:LNAA ratios from baseline, by 35% at 3 h. Wurtman et al.'s test meals were designed to reflect typical breakfasts consumed in the United States, but the study had a small sample (n = 9) and did not include psychological measures; it is not known if the changes in amino acid profile were associated with mood changes. The authors highlighted that while the combination of various foods in a meal would mitigate changes to serotonin synthesis, snacks may be composed of a single food item and if it is very high or low in protein, its impact on brain serotonin would be mostly unmoderated. However, Wurtman et al. also noted that the effects would be confounded by the composition of prior meals (Reference Wurtman, Wurtman, Regan, McDermott, Tsay and Breu9). In this vein, the composition of subsequent meals, snacks or beverages and the time between consumption would also confound the effects of a ‘single-item snack' on serotonin levels. It is questionable how long the changes in brain serotonin would be sustained in daily life outside of the laboratory.
Energy restriction also lowers plasma tryptophan, but its impact on mood is not consistent. Attenburrow et al.'s study of 50 healthy women (Reference Attenburrow, Williams and Odontiadis10) found low calorie diets (1000 kcal/day) followed over 3 weeks significantly lowered total plasma tryptophan levels and total plasma tryptophan:branched chain amino acid ratios, but did not change levels of depression or anxiety symptoms. Further, in a randomised, double-blind placebo-controlled method, the women were supplemented with 1.8 g tryptophan/day derived from lactalbumin (Reference Attenburrow, Williams and Odontiadis10). The supplementation did not circumvent the fall in plasma tryptophan induced by the weight-loss diet. Attenburrow's group hypothesised that food restriction may upregulate tryptophan metabolism for which the tryptophan supplements could not compensate. In contrast, a much older study by Schweiger et al. (Reference Schweiger, Laessle, Kittl, Dickhaut, Schweiger and Pirke11) into weight-loss diets found that plasma tryptophan:LNAA ratios correlated with overall mood. However, unlike Attenburrow's study where the diet had a fixed macronutrient composition, participants in Schweiger's study were advised only on maintaining a ‘mixed' or a ‘vegetarian' diet and so the proportions of protein and carbohydrate intake were not controlled.
Changing tryptophan levels by supplementation and depletion methods
Most empirical studies use supplementation, intravenous infusions or tryptophan depletion techniques to alter tryptophan and serotonin levels. Supplementation methods include high-tryptophan protein dietary supplements, such as α-lactalbumin, and amino acid supplements. Acute tryptophan depletion is effected through administering amino acid mixtures without tryptophan, which stimulates protein synthesis by the liver and subsequently lowers the body's endogenous pool of plasma tryptophan (Reference Dougherty, Marsh-Richard and Mathias12); it is a popular research technique for modifying plasma tryptophan levels.
As tryptophan is a limiting amino acid in most food proteins (Reference Benton and Donohoe3), there are few foods that can be used as a source of tryptophan. One ‘food' that has been used as a supplement in studies is α-lactalbumin, a tryptophan-rich whey protein. To investigate the cognitive and mood effects of α-lactalbumin, Booij et al. (Reference Booij, Merens, Markus and Van der Does13) supplemented low-protein-high-carbohydrate diets with α-lactalbumin in individuals recovered from depression and in controls. Although the plasma tryptophan:LNAA ratio was 71.5% higher in those supplemented with α-lactalbumin compared with the casein placebo and was 77.5% higher than baseline, there was no significant improvement in mood from baseline or compared with the placebo group. Firk and Markus (Reference Firk and Markus14) found that individuals supplemented with α-lactalbumin had improved mood but this did not occur with casein supplementation; however, this study did not measure plasma amino acid profiles. Merens et al. (Reference Merens, Booij, Markus, Zitman, Onkenhout and Van der Does15) significantly increased the plasma tryptophan:LNAA ratio in individuals with a history of depression and in healthy participants by 21% from baseline, also using α-lactalbumin. α-Lactalbumin did not elicit a significant difference in mood compared with those taking casein supplements as a placebo, despite casein promoting a significant decrease in tryptophan:LNAA, and there were minimal differences when the participants were placed under stress. It is possible that a larger load of α-lactalbumin is required to influence mood (Reference Merens, Booij, Markus, Zitman, Onkenhout and Van der Does15).
An older study by Beulens et al. (Reference Beulens, Bindels, De, Alles and Wouters-Wesseling16) raised plasma tryptophan:LNAA ratios in young healthy men by 16% by supplementing a normal diet with α-lactalbumin. The researchers also found no significant difference in mood between supplement and placebo groups an hour after consumption. All trials were randomised, double-blind, placebo-controlled and crossover, and, like the studies by Markus et al. and Wurtman et al. (Reference Markus, Panhuysen, Tuiten, Koppeschaar, Fekkes and Peters8,Reference Wurtman, Wurtman, Regan, McDermott, Tsay and Breu9), the diets and supplements were administered only on test days and not as long-term dietary regimes. Thus, the findings can only represent acute effects. It is not clear whether the healthy status and/or youth of the participants rendered them insensitive to such changes in the tryptophan:LNAA ratio or whether a greater elevation of the ratio would be required in order for differences in mood to be detected. Murphy et al.'s placebo-controlled trial is notable in that it supplemented healthy participants with 3 g tryptophan/day for 14 days (Reference Murphy, Longhitano, Ayres, Cowen and Harmer17). This trial of 38 participants found that tryptophan induced a positive bias in women's processing of emotional material after 14 days, but not men's, indicating women may be more sensitive to changes in serotonin level. In a similar vein, Moreno et al.'s study of 59 individuals in remission for depressive symptoms found women had significantly greater depressive responses to acute tryptophan depletion compared with men (Reference Moreno, McGahuey, Freeman and Delgado18). However, neither study measured plasma or cerebrospinal fluid (CSF) tryptophan levels, and the background diet was not controlled for, which may be particularly pertinent in Murphy's lengthier trial.
The form in which tryptophan is administered also influences its in vivo effects. Markus et al. (Reference Markus, Firk, Gerhardt, Kloek and Smolders19) investigated the differences in administering tryptophan as intact α-lactalbumin, a protein hydrolysate, pure tryptophan and as a tryptophan dipeptide. Despite providing the same quantities of tryptophan, mood in healthy individuals improved significantly only with the protein hydrolysate and pure tryptophan supplements but not with the casein control or other forms of tryptophan. The plasma tryptophan:LNAA ratio increased significantly from baseline with all four versions of tryptophan (peak increases being 263% for the peptide, 255% for the hydrolysate, 191% for pure tryptophan and 67% for α-lactalbumin), although with α-lactalbumin the increase was significantly lower than the protein hydrolysate and pure tryptophan (Reference Markus, Firk, Gerhardt, Kloek and Smolders19). The source of tryptophan may have implications when trying to increase plasma tryptophan through dietary means alone.
Whether or not an individual has a history of mood disorders also influences their susceptibility to changes in tryptophan levels. Meren's 2007 review noted that in healthy subjects, tryptophan manipulation had minimal effect on mood and while mood did decrease in patients with a past or family history of depression, the results were inconsistent (Reference Merens, Willem Van der Does and Spinhoven7). Even in older individuals in remission and on medication, acute tryptophan depletion had no significant effect on mood and it was hypothesised that longer remission times may be protective (Reference Porter, Phipps, Gallagher, Scott, Stevenson and O'Brien20). Moreno et al.'s prospective study (Reference Moreno, Heninger, McGahuey and Delgado21) observed that individuals were more likely to experience a depressive episode when followed up over the course of a year, if they had a depressive response to acute tryptophan depletion. Moreno included individuals in remission for major depression and healthy controls and found no correlation between the number of previous major depression episodes and the occurrence of episodes on follow-up, although it was noted that the sample size was small (n = 24). The inconsistent impact of tryptophan depletion on the mood of those with a history of depression was also highlighted by Booij et al.'s meta-analysis (Reference Booij, Van der and Benkelfat22), where chronic depression, recurrent depressive episodes, being female, previous treatment with selective serotonin reuptake inhibitors (SSRIs) and a history of serious suicidal thoughts or attempts were independent predictors of a mood response in patients. In Klaassen et al.'s study of healthy individuals with and without a family history of depression, tryptophan depletion significantly lowered plasma tryptophan and tryptophan:LNAA ratios after 6 h compared with placebo, which were restored after 24 h, but there were no differences at baseline (Reference Klaassen, Riedel, Van, Deutz, Honig and van Praag23). There was no difference in mood at baseline between placebo and tryptophan-depleted groups, but mood significantly lowered in the latter at 6 h only in those with a family history of depression. Klaassen et al.'s study is notable as it also controlled background protein intake using low-protein diet for the 24 h of the study.
As highlighted by Merens et al. (Reference Merens, Willem Van der Does and Spinhoven7), there is a lack of studies in participants with current depression. Booij et al. (Reference Booij, Van der Does, Haffmans and Riedel24) studied acute tryptophan depletion in patients who were depressed and receiving antidepressant medication. Depletion of tryptophan and significantly lowering of tryptophan:LNAA ratios were associated with improved mood in patients receiving selective serotonin–noradrenaline reuptake inhibitors (SSNRIs) but there was no change in mood in those receiving other types of medication. The reason given for the improvement was that long-term treatment with SSNRIs desensitises and downregulates serotonin receptors 5-HT1, 1A and 2, but in patients who have not been medicated for very long, depletion of tryptophan would not yield the usual decrease in serotonin because of the SSNRIs, and thus may increase post-synaptive 5-HT receptor activity. However, the sample size was small (n = 14), with the authors acknowledging the difficulty of recruiting depressed participants. Delgado et al.'s 1994 study (Reference Delgado, Price and Miller25), although older, is remarkable for its large sample of currently depressed patients who were not on medication (n = 43). This double-blind, placebo-controlled crossover trial found that acute tryptophan depletion significantly reduced plasma-free and total tryptophan levels by 83%, but there was no significant change in depressive symptoms. A lag time was observed, where there were significant and heterogeneous changes in depressive symptom severity the following day, when plasma tryptophan levels had recovered. Individuals who had responded to previous antidepressant treatments reported improved mood according to the Hamilton Depression Rating Scale when compared with placebo, while those who had not responded to prior antidepressant treatments scored worse (increase by 5–9 points) or much worse (increase by 10 or more points) in their depressive symptomatology (Reference Delgado, Price and Miller25). It was speculated that acute tryptophan depletion and repletion may have sensitised some of the patients' serotonin function, hence their improved mood, while those who reported worse mood may have impaired serotonin metabolism and thus were less able to compensate for tryptophan depletion. The authors suggested that serotonergic antidepressants may not be efficacious in improving the mood of patients with impaired serotonin metabolism.
Plasma tryptophan versus brain tryptophan and serotonin
Most human studies measure plasma tryptophan levels, and thus do not directly show whether the techniques which alter tryptophan levels in the blood translate to changes in the uptake and metabolism of tryptophan by the brain. Roiser et al. (Reference Roiser, Levy and Fromm26) investigated regional blood flow in the brain following acute tryptophan depletion, but found no significant change in mood either in healthy participants or in patients with remitted major depressive disorder. There were differences in hemodynamic responses between the two groups depending on the treatment. However, the results could not confirm if the hemodynamic differences were mediated by serotonin and tryptophan. Blomstrand et al. (Reference Blomstrand, Møller, Secher and Nybo27) inferred tryptophan uptake by the brain in human subjects through the difference in blood concentration of tryptophan in the radial artery compared with that of the jugular vein, although this study's focus was on the impact of carbohydrate intake on amino acid uptake during exercise, rather than mood.
Radio-labelled 3-(11)C-amino-4(2-dimethylaminomethylphenylsulfanyl)benzonitrile has been used to study the binding potential of the serotonin transporter 5-HTT in the brains of healthy humans (Reference Praschak-Rieder, Wilson and Hussey28). Binding potential is an index of the expression and affinity of 5-HTT and this study found that there was no difference in 5-HTT binding potential between control and depleted states in any region of the brain assessed. This was despite significantly lowering plasma tryptophan by 90% through tryptophan depletion, indicating that the degree of transport of serotonin in the brain was not altered. Mood was not assessed but the absence of change in binding potential is congruent with the frequent observation that healthy individuals are protected against the mood-lowering effects of tryptophan depletion (Reference Praschak-Rieder, Wilson and Hussey28). It would be interesting to see this study repeated in individuals with depression or a history of depression.
Most studies in humans extrapolate changes in plasma tryptophan to changes in brain levels and very few studies assess CSF levels of serotonin or tryptophan. The validity of CSF serotonin levels as an index of changes in brain serotonin has also been questioned, with neuroimaging (using radioligands) offered as a more sensitive method of assessing serotonin metabolism in the brain (Reference Nishizawa, Benkelfat and Young29). Salomon et al. (Reference Salomon, Kennedy and Johnson30) measured CSF levels of tryptophan and 5-hydroxyindoleacetic acid (5-HIAA), a metabolite of tryptophan, during tryptophan depletion in individuals being treated with sertraline or bupropion. Tryptophan depletion elicited a relapse in mood in those whose CSF tryptophan fell below 40 nM and depletion led to a significant decrease in CSF tryptophan and 5-HIAA and in plasma tryptophan. There was a strong and significant inverse correlation between mood and CSF tryptophan levels during tryptophan depletion, but mood did not correlate significantly with CSF 5-HIAA or plasma-free tryptophan levels. The association between plasma and CSF levels was not consistent: at baseline and before commencing sertraline or bupropion, there was a significant and strong correlation between CSF and free plasma tryptophan, but no significant correlation between CSF tryptophan and 5-HIAA. Following 5 weeks of medication, both correlations were not significant. The researchers acknowledge that their sample size was small (n = 12), making it inappropriate to draw generalisations (Reference Salomon, Kennedy and Johnson30), and this also reflects the difficulties of recruiting participants for a study with such a demanding protocol.
Tryptophan, mood and the popular press
Much of the scientific research into tryptophan and mood has focused on elucidating mechanisms for mood disturbances, rather than trialling tryptophan manipulation as a clinical treatment. A complicating factor in the interpretation of these studies is that not all controlled the composition of the participants' background or baseline diets. The effects of lowering tryptophan levels may not be purely because of serotonin metabolism and may also be mediated through other neurotransmitter pathways (Reference Hood, Bell and Nutt31). Further, as tryptophan depletion induces or worsens depressive symptoms only in certain groups, depression may not be mediated solely by reduced serotonin (Reference Bell, Hood and Nutt32). In addition, a large epidemiological study of 29 133 men found no association between dietary tryptophan intake and self-reported depressed mood, hospital admission for depressive disorders or suicide (Reference Hakkarainen, Partonen, Haukka, Virtamo, Albanes and Lonnqvist33). In contrast, articles in the lay media emphasise ‘treating' mood through altering tryptophan levels and thus serotonin levels.
A pure carbohydrate load in fasting individuals will significantly increase the plasma tryptophan:LNAA ratio compared with placebo (Reference Markus34). Despite scientific commentary and empirical research showing the impracticality of using the Wurtman effect to alter tryptophan and serotonin levels, the Wurtman effect is constantly invoked by the general media. Implementing the Wurtman effect in practice is problematic. Benton and Donohoe (Reference Benton and Donohoe3) state that a mere 5% of calories as protein is sufficient to curb the increase in the tryptophan:LNAA ratio and that even ‘high-carbohydrate' foods typically still contain sufficient protein to prevent this increase. Young (Reference Young6) put the protein threshold at 4% of total energy. Benton and Donohoe (Reference Benton and Donohoe3) note that there is a clear increase in the plasma tryptophan:LNAA ratio when the meal contains less than 2% of its calories as protein, but there is little evidence that carbohydrate specifically benefits depressed individuals.
General media reports regarding the effects of tryptophan are often hyperbolic, misleading and ambiguous, with much of their advice and information being inconsistent with Wurtman's hypothesis and even basic biochemistry. The list of ‘tryptophan-rich foods' extolled by lay publications is wide-ranging and includes bananas, turkey, chocolate, dates, papaya, poultry, milk, oats, various nuts and beans, chickpeas, sunflower seeds, dairy foods, avocado, eggs, red meat, soybeans and soy foods, tuna, shellfish, brown rice, lentils, lobster, seafood, wholegrains, hummus, pineapple, asparagus, beetroot and chicken soup (Reference Hakkarainen, Partonen, Haukka, Virtamo, Albanes and Lonnqvist33–Reference Highfield52). Protein-rich foods such as meat, poultry, dairy products, nuts, beans and eggs contain tryptophan (Table 1), but tryptophan is the limiting amino acid in most protein sources (Reference Elango, Ball and Pencharz1,Reference Benton and Donohoe3,Reference Young6), meaning that it is the essential amino acid which is present in the lowest quantity in that food source. As can be seen in Table 1, fruits and vegetables are generally poor protein sources, including those recommended by the general media, and cannot be viewed as concentrated sources of tryptophan. Another consideration is that even if a food is high in protein and therefore high in tryptophan, it may not be normally eaten in sufficient quantities or frequency to be a significant dietary source.
Table 1 Total protein and tryptophan levels in various ‘tryptophan-rich foods' commonly cited by the general media
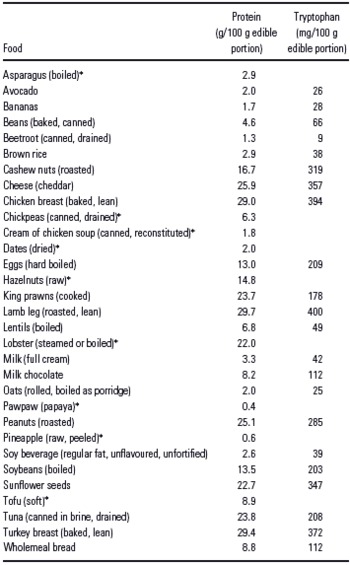
While a few lay articles provide sound and scientific information (54–Reference Rajiv56), many reports are ambiguous or misleading. Carbohydrate foods are also recommended to increase serotonin levels, although the way these foods do this is not always explained (Reference Caradas45,Reference Gan50). Recommending milk as a source of both protein (and thus tryptophan) and carbohydrate to increase serotonin (Reference Stanely, Milroy and Govindji57) shows a lack of understanding of the mechanism behind the Wurtman effect. One magazine article went so far as to say ‘brains can only make serotonin when we eat starchy carbs…without protein', only to then add ‘The more tryptophan you eat with carbohydrates, the cheerier you become… Tryptophan is enhanced with carbohydrates, so the key is to eat something that contains tryptophan with complex carbohydrates…'(Reference Bailey58). Unfortunately, the article did not explain how eating tryptophan could be achieved without eating protein in the daily diet. Some newspaper columnists advise eating protein and carbohydrate foods separately (59) or to eat low-protein-high-carbohydrate meals to improve mood (60), but neglect to explain that a protein food contains tryptophan along with other amino acids and that ‘carbohydrate foods' such as bread also contain protein. In the case of white bread, protein forms about 14% of its caloric content. General media articles also show a lack of understanding in biochemistry, in that tryptophan, as an amino acid, is a component of protein; they describe proteins as stimulatory and tryptophan as calming (61). Some published statements are profoundly misleading, such as ‘…tryptophan, which is plentiful in carbohydrate-rich foods, such as chocolate'(Reference Taylor62) and ‘starch- and sugar-rich breakfast cereals…are high in the amino acid tryptophan…'(Reference Jacobs63). Tryptophan is an amino acid, not a carbohydrate, and although chocolate does contain an appreciable amount of protein along with fat and carbohydrate, the protein content would reduce rather than increase brain uptake of tryptophan (Reference Benton and Donohoe3). As described in the studies reviewed above, the whey protein α-lactalbumin is rich in tryptophan but needs to be consumed as a supplement and not as an intact food and thus is still an artificial method for influencing plasma tryptophan levels. Lastly, published personal testimonials detailing success in treating depression by dietary manipulation often do not report what the pre-existing diet was like (Reference Mercieca35,Reference King64).
Toxicity, side-effects and caveats
The outbreak of eosinophilia-myalgia syndrome (EMS) in 1989 in individuals using over-the-counter l-tryptophan supplements resulted in these supplements being banned in the United States (Reference Fernstrom65,Reference Barrett66). EMS is characterised by elevated blood eosinophil count, myalgia and sometimes neurological and pulmonary complications (Reference Fernstrom65,Reference Barrett66). The syndrome was thought to be caused by contaminants, although it could not be convincingly established whether these compounds were responsible (Reference Fernstrom65).
Supplementation with tryptophan as an amino acid can have side-effects, including nausea, vomiting, headaches and drowsiness (Reference Leathwood and Pollet67); such side-effects also occur with consuming amino acid loads in tryptophan depletion studies (Reference Dougherty, Marsh-Richard and Mathias12,Reference Klaassen, Riedel, Van, Deutz, Honig and van Praag23). A 6 g oral load of tryptophan in healthy adults significantly increased lipid peroxidation and tryptophan metabolites of the kynureinine pathway after 5 and 7 h compared with baseline. This potentially promotes cellular oxidative stress, damage or death, which may have clinical implications in individuals either taking tryptophan supplements or following popular high-protein diets (Reference Forrest, Mackay and Stoy68).
Mood changes may not occur until plasma tryptophan has dropped below a certain level, and a 60% decrease has been suggested as a threshold (Reference Hood, Bell and Nutt31). Also, individuals with a family or personal history of depression or who are chronically stressed may be more sensitive to tryptophan changes induced through dietary means (Reference Markus, Firk, Gerhardt, Kloek and Smolders19,Reference Klaassen, Riedel, Van, Deutz, Honig and van Praag23), while healthy young individuals may be less susceptible (Reference Beulens, Bindels, De, Alles and Wouters-Wesseling16,Reference Markus, Firk, Gerhardt, Kloek and Smolders19). Robinson and Sahakian (Reference Robinson and Sahakian69) found that in healthy women, depletion of plasma tryptophan in itself was not associated with negative mood. However, when negative mood was induced during tryptophan depletion, this was associated with more pronounced negative mood on a subsequent episode of tryptophan depletion. Thus, individuals with concurrent depressed mood and reduced serotonin may be sensitive to changes in tryptophan levels.
Conclusion
Research into tryptophan must continue to elucidate its role in mood and mood disorders, and in the mechanisms behind SSRNIs. Most of the studies to date have been on small to modest sample sizes, and hence more studies with larger sample sizes are needed, along with studies in individuals with current depression. Empirical studies have mostly shown that healthy individuals are insensitive to changes in tryptophan levels and for those who do have a personal or family history of depression, the results are inconsistent.
It is unlikely that the highly artificial dietary regimens used in studies such as Markus et al. (Reference Markus, Panhuysen, Tuiten, Koppeschaar, Fekkes and Peters8) would be popular in the community and sustained for any length of time, if diet alone were used to alter brain serotonin levels. As Benton and Donohoe (Reference Benton and Donohoe3) state, ‘No normal meal will contain so little protein that the uptake of tryptophan will be increased' (p404). However, many general media articles give contradictory and impractical advice. The articles convey the impression that mood can be improved with ‘tryptophan-rich foods' or by eating carbohydrate to raise serotonin levels, but do not consider whether this would be of any use to those without depression. It is questionable whether dietary changes to manipulate tryptophan levels, even if achievable, would be of advantage to the general population. This does not detract from the importance of a nutritious diet for individuals with depression, as well as the general population, for overall health.
Acknowledgement
Over the past 48 months, Nerissa Soh and Garry Walter have had no competing interests.



