Significant outcomes
-
∙ Four electroconvulsive seizures (ECS) treatments cause substantial retrograde amnesia in the Morris water maze when memory is tested 7 days after the first treatment.
-
∙ A single ECS treatment is not sufficient to cause retrograde amnesia in the Morris water maze when memory is tested 24 h, 72 h, or 7 days after the treatment.
-
∙ The retrograde amnesia observed after multiple ECS treatments is possibly caused by an accumulated effect of repeated treatments.
Limitations
-
∙ Small sample sizes, mainly regarding electroconvulsive seizures (ECS)-treated animals in Group D
-
∙ This study adds to the literature about ECS-induced retrograde amnesia without suggesting new possible mechanisms.
Introduction
Electroconvulsive therapy (ECT) is one of the most efficient treatments available today for severe major depression (Reference Pagnin, de Queiroz, Pini and Cassano1), with the highest remission and response rates of all clinically established antidepressant treatments (2). Despite its effectiveness, the use of ECT has been limited due to concerns about adverse effects, most notably on cognition. Several reports describe both anterograde amnesia (inability to form new memories after ECT) (Reference Semkovska and McLoughlin3) and retrograde amnesia (difficulty in remembering events from before the treatment) (Reference Lisanby, Maddox, Prudic, Devanand and Sackeim4). Although most memory disturbances are temporary and reversible (Reference Semkovska and McLoughlin3, Reference Meeter, Murre, Janssen, Birkenhager and van den Broek5), some patients experience permanent memory loss (Reference Fink6). In an animal model of ECT (electroconvulsive seizures (ECS)), rats given a series of five treatments displayed spatial retrograde amnesia in the hippocampus-dependent memory task Morris water maze (MWM), when tested 24 h (Reference Kyeremanteng, MacKay and James7) and 72 h (unpublished observations) after the last treatment.
The mechanisms behind ECT/ECS’s antidepressant and amnestic effects are not well understood. It has repeatedly been shown that ECS (both a single and multiple treatments) as well as different classes of antidepressant drugs stimulate neurogenesis in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) (Reference Madsen, Treschow, Bengzon, Bolwig, Lindvall and Tingström8–Reference Scott, Wojtowicz and Burnham10), and it has been proposed that this may contribute to the antidepressant effect (for review, see Bolwig (Reference Bolwig11)). Indeed, recent studies suggest that hippocampal neurogenesis is necessary for these treatments to exert an antidepressant effect (Reference Santarelli, Saxe and Gross12–Reference Schloesser, Orvoen and Jimenez14).
Apart from its potential antidepressant role, extensive research posits that adult hippocampal neurogenesis is important for learning and memory functions (such as spatial pattern separation (Reference Clelland, Choi and Romberg15–Reference Nakashiba, Cushman and Pelkey17), consolidation of spatial long-term memory (Reference Snyder, Hong, McDonald and Wojtowicz18–Reference Ben Abdallah, Filipkowski and Pruschy20) and cognitive flexibility (Reference Burghardt, Park, Hen and Fenton21, Reference Swan, Clutton, Chary, Cook, Liu and Drew22)). It has recently been shown that new neurons compete with existing neurons and form new synaptic connections that (over time) replace older connections (Reference Yasuda, Johnson-Venkatesh, Zhang, Parent, Sutton and Umemori23). It is therefore possible that the functional integration of newly formed neurons may result in forgetting or degradation of existing memories (Reference Frankland, Köhler and Josselyn24, Reference Akers, Martinez-Canabal and Restivo25). Similarly, the amnestic effect of ECT/ECS could possibly be a result of an increase in neurogenesis.
Aim of the study
The aim of this study was to investigate whether the memory impairment seen after multiple ECS treatments is a cumulative effect of repeated treatments, or if it is the result of a delayed effect after a single ECS.
Materials and methods
Animals and experimental design
A total of 62 adult (7 weeks old), naive, male Lister Hooded rats (Charles River, Sulzfeld, Germany), weighing ~200 g at the beginning of the study, were housed two or three per cage (Type III H cages), with ad libitum access to food and water and kept on a 12 h light-dark cycle. Experiments were carried out during the light period. The rats were randomly divided into four groups (Group A, B, C, or D) and assigned to ECS or sham treatment within these groups.
All animals were first trained for 8 consecutive days to find the hidden platform in the water maze. Two hours after the last training session, the treatment regimens (a single or four (multiple) ECS/sham treatments) started. Animals in Group A–C were treated with a single ECS (n Group A=7Footnote 1 , n Group B, C=8) or sham (n=7), and animals in Group D were treated with multiple ECS (n=5Footnote 2 ) or sham (n=7) treatments. The memory for platform location was evaluated in retention tests performed at three time points as noted below and depicted in Fig. 1.
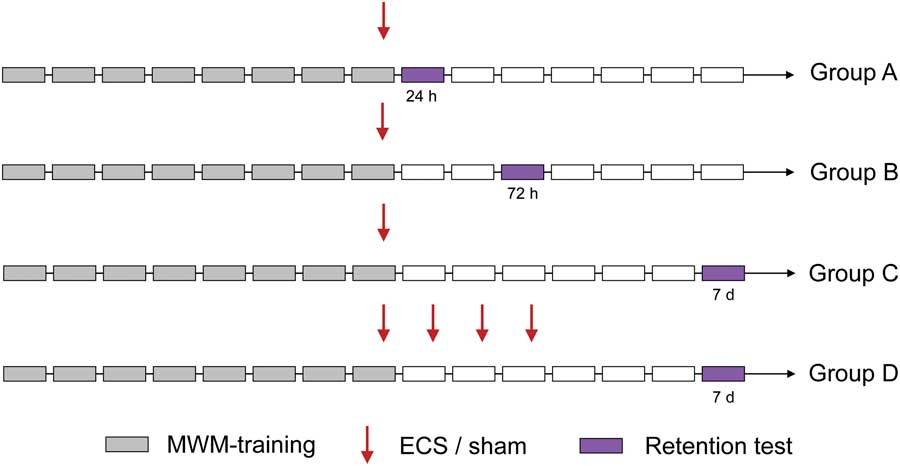
Fig. 1 Study design. Animals were subjected to an 8-day training period in the MWM. After training the last day, a single ECS or sham treatment (Group A–C) was given. In Group D, one treatment per day for 4 consecutive days was given. Retention tests (where the platform was removed to evaluate memory for platform location) were conducted 24 h, 72 h or 7 days after the single ECS treatment in Group A, B, and C, respectively, and 7 days after the first treatment in Group D. ECS, electroconvulsive seizures; MWM, Morris water maze.
MWM navigation task
Spatial memory and learning were evaluated using the hippocampus-dependent MWM task (Reference Morris26). The water maze consisted of a 45 cm deep, circular tank (180 cm in diameter) filled with water (20±1°C) to a depth of 30 cm. Imaginary lines divided the maze into four equal-sized quadrants (25% of the pool surface area each). A platform was submerged 1.5 cm in the centre of the southeastern (SE) quadrant. Non-toxic white paint (Swingcolor, Bauhaus, Sweden) was added to make the water opaque. Visual extramaze cues (such as abstract figures and light sources) were kept strictly constant during the experiment.
Hidden platform training was performed for 8 consecutive days for all groups (A–D), with four trials per day and 20 s between trials. During each trial, the animal was placed in the water facing the pool wall at one of four starting points (N, W, S, E). Every day the order of these four starting points were pseudorandomly varied, such that the same order did not appear more than once during the learning period. Animals finding and climbing onto the platform had to stay on it for 20 s. If an animal failed to find the escape platform within 90 s, the experimenter guided them to it. Regarding learning trials in the MWM, the escape latency (the time required to reach the platform) was recorded and data from the four daily trials were averaged.
During the retention test, the platform was removed and the animal was allowed to search for it during 90 s. The retention tests were performed 24 h (Group A), 72 h (Group B), or 7 days (Group C and D) after the single (Group A – C) or first (Group D) ECS treatment (Fig. 1). In the retention tests, we evaluated four different measures of platform memory; time spent (%) in the platform quadrant [platform quadrant], mean distance (cm) to the former position of the platform [proximity], latency (s) to first crossing over the former platform position [first platform crossing] and number of crossings over the former platform position [crossings]. These are commonly used measures of memory performance in MWM, and proximity was recently shown to be the most sensitive one (Reference Maei, Zaslavsky, Teixeira and Frankland27). Regarding platform quadrant, proximity, and crossings, the first 30 s were used to evaluate memory performance. In our opinion, this is the best time period to evaluate memory for platform location, since we have observed that animals that remember the position of the platform, start searching other areas of the pool when they realise the platform is not in the position they remembered it to be in. To investigate whether potential group differences in memory performances could be explained by a difference in total distance swum or swim velocity during the retention tests, these measures were evaluated as well. The entire 90 s was used in statistical analyses regarding the measure first platform crossing (since we did not want to limit the time allowed to search for the platform), total distance swum, and swim velocity.
Apart from spatial learning of platform location, the MWM task also includes learning about the task, which is to swim away from the sidewall, finding the hidden platform and staying on it (Reference Morris26). When first introduced to the water maze, most animals swim around the pool close to the sidewall (a phenomenon called thigmotaxis). Under normal conditions, they soon learn to search over the entire pool area. We investigated whether the ECS-treated animals in Group D (whom displayed a substantial retrograde amnesia, Figs 3 and 4) had an intact memory for the task by comparing thigmotaxis behaviour (i.e. time spent (%) in a corridor within 15 cm from the sidewall) during the retention test with thigmotaxis during the first learning trial.
ECS
Bilateral ECS treatments were delivered via ear clip electrodes (Ugo Basile, Gemonio, Italy). Animals in Group A–C were given a single ECS, and animals in Group D were given one daily ECS for 4 consecutive days (with 24 h interval). The ECS apparatus (57 800 ECT Unit, Ugo Basile) delivered unidirectional square wave pulses (current, 75 mA; pulse width, 0.5 ms; pulse train duration, 0.5 s; frequency, 100 Hz). Each ECS treatment gave rise to a tonic-clonic seizure and the length of the tonic phase was defined as in Jansson et al. (Reference Jansson, Wennström, Johanson and Tingström28). Control animals were sham-treated (handled identically to the ECS animals except that no current was applied).
Data collection and statistical methods
The swim paths were recorded using a computerised video-tracking system (Ethovision 3.1; Noldus Information Technology, Wageningen, the Netherlands).
A full factorial three-way mixed-design analysis of variance (ANOVA) with the within-subject factor Time and the between-subject factors Treatment (ECS/sham) and Group (A–D) was used to analyse weight gain. Weight was recorded twice; in the beginning and at the end of the experiment. We have in our previous studies used this method to analyse weight gain, and by ‘mixed-design’ we mean that the ANOVA consisted of both within-subject and between-subject factors (Reference Svensson, Grahm, Ekstrand, Movahed-Rad, Johansson and Tingström29). In statistical follow-up analyses, a linear regression analysis was used to calculate weight gain. A second full factorial three-way mixed-design ANOVA with the within-subject factor Time (8 days of learning trials) and the between-subject factors Treatment and Group was used to analyse escape latency. Since it turned out that all groups of animals learnt equally well, we used accumulated data of all animals per day when calculating the slope of the learning curve using a centred quadratic polynomial regression analysis.
An ANOVA with the within-subject factor Time (four days of treatments) was used to analyse tonic ECS seizure duration over administration days for animals assigned to multiple ECS treatments (Group D). A one-way ANOVA (with the between-subject factor Group) followed by Tukey’s multiple comparisons test was used to compare single tonic seizure duration means between ECS-treated animals in Group A–C.
Independent samples t-tests were used to analyse differences between ECS and sham groups regarding the four measures of platform memory (platform quadrant, proximity, first platform crossing, and crossings) as well as total distance swum and swim velocity. Since our a priori aim was to analyse the four measures of memory for the four groups (A–D) independently, no global analysis test was performed. In order to control for the family-wise error rate within these groups, we used the Holm–Bonferroni method.
Regarding platform quadrant, a one-sample t-test was used to compare actual group means to a hypothetical mean of 25% (representing the amount of time an animal would spend in the platform quadrant by chance).
A paired t-test was used to analyse thigmotaxis in ECS- and sham-treated animals. A full factorial two-way mixed-design ANOVA with the within-subject factor Time (thigmotaxis was evaluated during the first learning trial and the retention test) and the between-subject factor Treatment was used to analyse differences in this behaviour between ECS- and sham-treated animals in Group D.
Heat maps illustrating swim paths of all groups of animals during the 90 s retention test and during the first learning trial for animals in Group D were generated in MATLAB (2014b, The MathWorks Inc., Natick, MA, USA). The output from Ethovision (x, y coordinates for each time point during the tracking) was processed using a custom written MATLAB script. The rat’s position was recorded in a matrix with 240×240 elements. Each time the rat visited a specific position, the corresponding cell in the matrix was incremented by 1. The value of each cell was then divided by the total number of time points to get the proportion of the total time spent in the different positions. The matrices for the rats in each group were averaged. In order to increase the resolution for heat map generation, the matrices were resized by a factor of 4 (using bicubic interpolation) resulting in 960×960 pixel images. To generate the heat maps, a two-dimensional rotationally symmetric Gaussian filter (window size, 160×160 pixels; standard deviation, 20) was applied. A MATLAB colour map (jet) was applied. Red colour indicates that more time was spent in that area while blue colour indicates less time.
Greenhouse-Geisser adjustment was used when data violated the assumption of sphericity, and corrected p-values are reported together with uncorrected degrees of freedom. A t statistic not assuming homogeneity of variance was used when Levene’s test for equality of variances was violated, and corrected p-values are reported. Data are presented as mean±SEM. Statistical significance level was set at p=0.05. Statistical analyses were performed using IBM SPSS Statistics 23 and GraphPad Software, Prism 6.
Results
Weight gain
Weight was recorded in the beginning and at the end of the experiment. The three-way mixed-design ANOVA revealed a significant effect of Treatment (F(1,49)=4.78, p=0.033) but not Group (F(3,49)=0.329, p=0.805), indicating a difference in weight between ECS- and sham-treated animals irrespective of group belonging (ECSbeginning=191±2.17 g, shambeginning=197±2.19 g, p=0.075; ECSend=275±2.85 g, shamend=285±2.76 g, p=0.013). However, no significant difference in weight gain was observed between animals receiving ECS or sham treatments (no significant interaction between Time×Treatment; F(1,49)=2.40, p=0.128), or belonging to groups A–D (no significant interaction between Time×Group; F(3,49)=1.58, p=0.207), as also indicated by the non-significant interaction between Time×Treatment×Group (F(3,49)=0.152, p=0.928). All animals gained weight over time (F(1,49)=3190, p<0.001; ECSincrease=83.4±3.58 g; shamincrease=88.0±3.52 g).
Learning trials
The rats were subjected to 8 days of training before ECS treatments. The three-way mixed-design ANOVA revealed a significant progressive decrease in escape latency for all animals over the 8 training days (as indicated by a significant effect of Time, F(7378)=309, p<0.001), which was interpreted as learning (Fig. 2). As expected, no significant effects of Treatment (F(1,54)=2.12, p=0.151) or Group (F(3,54)=2.54, p=0.066) were observed. Neither were the interactions between Time×Treatment (F(7378)=1.30, p=0.276), Time×Group (F(21 378)=1.06, p=0.393) or Time×Treatment×Group (F(21 378)=0.483, p=0.870) significant, indicating no difference in learning between animals assigned to ECS or sham treatment in Group A–D (mean learning curve of all animals: −7.04±0.221 s/day).

Fig. 2 Learning curves. The three-way mixed-design ANOVA revealed a significant effect of Time (interpreted as learning) over the 8 days of training in the Morris water maze (p<0.001). No significant differences in learning were observed between the groups. ECS, electroconvulsive seizures.
Seizure durations
The mean lengths of the tonic seizure durations were 13.5±0.535 s for Group A (1 ECS), 14.9±0.479 s for Group B (1 ECS), 15.5±0.779 s for Group C (1 ECS), and 16.2±0.350 s for Group D (4 ECS). The one-way ANOVA revealed no differences in tonic seizure duration between Groups A, B, or C (F(2,21)=2.80, p=0.084), and the ANOVA with the within-subject factor Time revealed no difference in tonic seizure duration over administration days for animals in Group D (F(3,12)=0.578, p=0.512).
Analysis of retention tests
Heat maps illustrating the swim paths (during the 90 s swims) of ECS- and sham-treated animals in Groups A–D are depicted in Fig. 3.

Fig. 3 Density plots (heat maps) of swim paths during the 90 s retention tests for ECS- and sham-treated animals in Group A–D. The white circle illustrates the former platform location. The heat maps are based on the tracking data from Ethovision where the rat’s position is given by an x, y coordinate for each time point. The heat maps were generated as described in the ‘Materials and Methods’ section. Red colour indicates that more time was spent in that area while blue colour indicates less time.
Group A
Animals in Group A were given a single ECS or sham, and spatial retrograde memory was evaluated 24 h after the treatment. The independent samples t-tests revealed no significant differences between ECS- and sham-treated animals regarding any of the measures of platform memory ([p platform quadrant=0.191; meanECS=59.2±3.48%; meansham=51.9±4.01%], [p proximity=0.166; meanECS=34.5±2.46 cm; meansham=40.8±3.50 cm], [p first platform crossing=0.047; meanECS=4.89±0.418 s; meansham=13.9±3.59 s], [p crossings=0.126;meanECS=3.29±0.286 crossings; meansham=2.29±0.522 crossings]). Both ECS- and sham-treated animals spent significantly more time in the platform quadrant compared to chance levels (p<0.001, respectively), indicating a memory for the former platform location for both groups (Fig. 4, Group A).
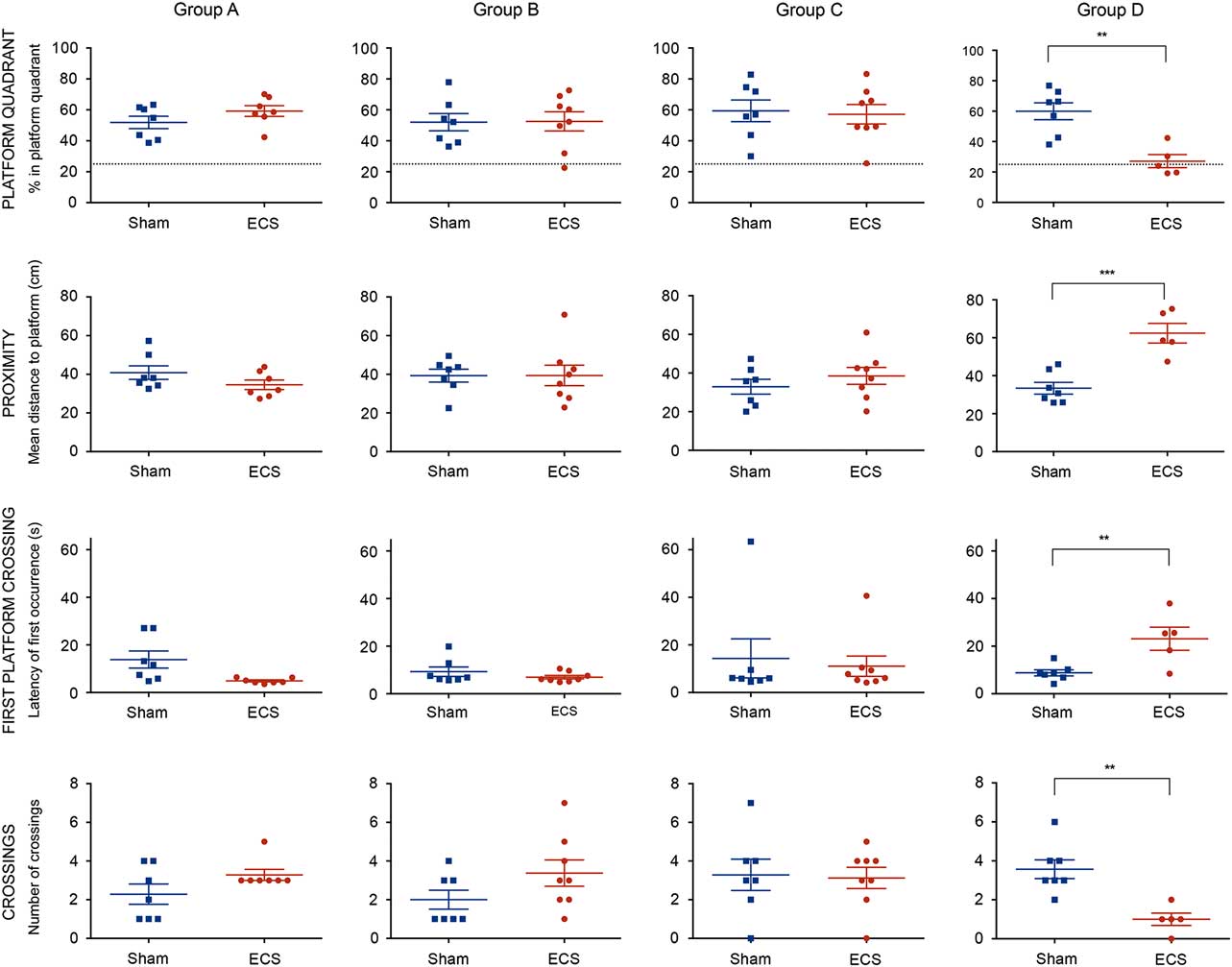
Fig. 4 Differences between ECS- and sham-treated animals in Group A–D in the four measures of platform memory (platform quadrant, proximity, first platform crossing, and crossings). Briefly, a significant memory disturbance was observed only in animals treated with multiple ECS compared with sham (Group D), as indicated by significant differences in all four measures of platform memory. No significant spatial memory disturbances were observed in animals treated with a single ECS and tested 24 h (Group A), 72 h (Group B), or 7 days (Group C) after the treatment. Top row dashed lines correspond to 25% chance performance. Asterisks indicate the statistical significant difference level (*<0.05, **<0.01, ***<0.001). ECS, electroconvulsive seizures.
Group B
Animals in Group B were given a single ECS or sham, and spatial retrograde memory was evaluated 72 h after the treatment. The independent samples t-tests revealed no significant differences between ECS- and sham-treated animals regarding any of the measures of platform memory ([p platform quadrant=0.952; meanECS=52.6±6.22%; meansham=52.0±5.62%], [p proximity=0.990; meanECS=39.4±5.28 cm; meansham=39.3±3.35 cm], [p first platform crossing=0.280; meanECS=6.99±0.744 s; meansham=9.26±2.00 s], [p crossings=0.134; meanECS=3.38±0.680 crossings; meansham=2.00±0.488 crossings]). Both ECS- and sham-treated animals spent significantly more time in the platform quadrant compared to chance levels (p=0.003, respectively), indicating a memory for the former platform location for both groups (Fig. 4, Group B).
Group C
Animals in Group C were given a single ECS or sham, and spatial retrograde memory was evaluated 7 days after the treatment. The independent samples t-tests revealed no significant differences between ECS and sham groups regarding any of the measures of platform memory ([p platform quadrant=0.817; meanECS=57.2±6.31%; meansham=59.4±7.01%], [p proximity=0.360; meanECS=38.5±4.38 cm; meansham=32.9±3.83 cm], [p first platform crossing=0.720; meanECS=11.1±4.30 s; meansham=14.3±8.21 s], [p crossings=0.869; meanECS=3.13±0.549 crossings; meansham=3.29±0.808 crossings]). Both ECS- and sham-treated animals spent significantly more time in the platform quadrant compared to chance levels (pECS=0.001, psham=0.0027), indicating a memory for the former platform location for both groups (Fig. 4, Group C).
Group D
Animals in Group D were given multiple (four) ECS or sham treatments and spatial retrograde memory was evaluated 7 days after the first treatment (i.e. the same day that animals in Group C were evaluated). The independent samples t-tests revealed significant differences between ECS and sham groups regarding all four measures of platform memory ([p platform quadrant=0.002; meanECS=27.1±4.31%; meansham=59.9±5.57%], [p proximity<0.001; meanECS=62.4±5.17 cm; meansham=33.4±3.11 cm], [p first platform crossing=0.007; meanECS=23.1±4.85 s; meansham=8.78±1.26 s], [p crossings=0.0023; meanECS=1.00±0.316 crossings; meansham=3.57±0.481 crossings]). The sham group spent significantly more time in the platform quadrant compared to chance levels (p<0.001), while the ECS group did not (p=0.645). These results indicate a significant spatial retrograde memory disturbance in animals receiving multiple ECS treatments (Fig. 4, Group D).
Analysis of total distance swum and swim velocity in the retention tests
The independent samples t-tests revealed no group differences in total distance swum ([p GroupA=0.398; meanECS=2235±64.4 cm; meansham=2104± 133 cm], [p GroupB=0.162; meanECS=2193±111 cm; meansham=2420±102 cm], [p GroupC=0.629; meanECS=2303±114 cm; meansham=2225±108 cm], [p GroupD=0.234; meanECS=2440±72.1 cm; meansham=2307±72.2 cm]) or swim velocity ([p GroupA=0.418; meanECS=24.9± 0.710 cm/s; meansham=23.5± 1.46 cm/s], [p GroupB=0.169; meanECS=24.5±1.24 cm/s; meansham=27.0 ± 1.15 cm/s], [p GroupB=0.636; meanECS= 25.6±1.26 cm/s; meansham=24.8± 1.19 cm/s],[p Group D=0.237; meanECS=27.2± 0.771 cm/s; meansham=25.7±0.823 cm/s]) between ECS- and sham-treated animals in Group A–D.
Analysis of thigmotaxis behaviour
The two-way mixed-design ANOVA revealed a significant effect of Time (F(1,25)=909, p<0.001), demonstrating a difference in thigmotaxis behaviour of both ECS- and sham-treated animals in Group D over time. However, no significant effect of Treatment (F(1,25)=0.297, p=0.591) or significant interaction between Time×Treatment were observed (F(1,25)=0.396, p=0.535), indicating no difference in thigmotaxis behaviour over time between ECS and sham (Fig. 5). Both ECS- and sham-treated animals spent significantly more time (p<0.001, respectively) along the sidewall during the first learning trial (ECS: 90.8±4.50%, sham: 87.4±1.70%) compared with the retention test (ECS: 10.4±2.80%, sham: 7.34±1.15%).

Fig. 5 The mean swimming paths of ECS-treated animals in Group D during the first learning trial (a) and during the retention test (b). The dashed line (15 cm from the sidewall of the pool) was used to analyse thigmotaxis behaviour. The thigmotaxis ratio was calculated by dividing the time spent within 15 cm from the arena border with the total time. The white circle illustrates the former platform location. Red colour in the density plots indicates that more time was spent in that area while blue colour indicates less time. The animals spent significantly more time along the sidewall during the first learning trial compared with the retention test (p<0.001; c). No significant difference was observed between ECS- and sham-treated animals regarding thigmotaxis behaviour during the first learning trial and the retention test. Asterisks indicate the statistical significant difference level (*<0.05, **<0.01, ***<0.001). ECS, electroconvulsive seizures.
Discussion
Here we show that a series of four ECS treatments causes retrograde amnesia in the MWM, as demonstrated by a group difference between animals receiving multiple ECS or sham treatments in the four measures of platform memory used in this study (platform quadrant, proximity, first platform crossing, and crossings, Fig. 4, Group D). This is in line with a recent study by Kyeremanteng et al., demonstrating that a series of five consecutive ECS treatments causes retrograde amnesia in MWM. They investigated retrograde memory impairment one and 7 days after the last ECS treatment in two rat strains (Wistar–Kyoto and Wistar). Both strains displayed retrograde amnesia 24 h after the last ECS, and the memory deficit was still present after 7 days (Reference Kyeremanteng, MacKay and James7). In the current study, we observed retrograde amnesia after multiple ECS in the Lister Hooded rat strain. We previously found similar results in the Long Evans and in the albino Sprague Dawley rat strains (unpublished observations), suggesting that the effect of ECS on memory is not strain-specific.
Although multiple ECS treatments disrupted spatial memory, the animals did not behave as naive animals during the retention test (Fig. 5). Instead, ECS-treated animals in Group D searched the entire pool area for the platform (demonstrating an intact memory for the task) but failed to locate it.
We observed no difference in tonic seizure duration over the treatment course, which is in line with other studies (Reference Svensson, Grahm, Ekstrand, Movahed-Rad, Johansson and Tingström29). Nonetheless, both a slight increase (Reference Jansson, Wennström, Johanson and Tingström28) and decrease (Reference Andrade, Suresh, Krishnan and Venkataraman30, Reference Svensson, Grahm, Ekstrand, Höglund, Johansson and Tingström31) in ECS seizure duration over administration days have been observed. Our interpretation is that several factors such that age, weight, stimulus parameters used, and rat strain might affect the seizure duration. In clinical settings, seizure duration commonly decreases over a treatment course, whereas seizure threshold (i.e. the minimal electrical dosage needed to elicit generalised seizures) increases (Reference Sackeim, Decina, Portnoy, Neeley and Malitz32). Suprathreshold stimulus doses have been suggested to result in shorter seizure durations (Reference Sackeim, Devanand and Prudic33). It is not known whether seizure duration correlates with cognitive dysfunction or antidepressant effect (Reference Miller, Faber, Hatch and Alexander34).
The mechanism(s) behind ECS-induced amnesia are not well understood. However, ECS has been shown to induce sprouting of the mossy fibre pathway (axons projecting from DG granule cells to the CA3 area) in the hippocampus (Reference Vaidya, Siuciak, Du and Duman35), and this has been suggested to be a possible mechanism for both the antidepressant and the amnestic effects (for review, see (Reference Gregory-Roberts, Naismith, Cullen and Hickie36)). Interestingly, ketamine (an NMDA-receptor antagonist), which has both a memory-protecting role when used as an anaesthetic agent during ECT (Reference Krystal, Weiner and Dean37, Reference McDaniel, Sahota, Vyas, Laguerta, Hategan and Oswald38) and an antidepressant effect by itself (Reference Berman, Cappiello and Anand39), attenuates ECS-induced mossy fibre sprouting (Reference Chen, Shin, Duman and Sanacora40).
Intriguingly, the integration of newborn neurons in pre-existing neuronal networks might affect stored memories (Reference Frankland, Köhler and Josselyn24, Reference Meltzer, Yabaluri and Deisseroth41). Indeed, a recent study showed that the retention of previously acquired memories was impaired when hippocampal neurogenesis was increased (Reference Akers, Martinez-Canabal and Restivo25). Thus, it appears that new hippocampal neurons could potentially disrupt existing memories (and cause forgetting) (Reference Frankland, Köhler and Josselyn24). It is known that ECS increases neurogenesis in the SGZ of the hippocampal DG in a dose-dependent manner (Reference Madsen, Treschow, Bengzon, Bolwig, Lindvall and Tingström8). It is unclear whether the neurons formed in response to ECS are indistinguishable from neurons continuously born in the adult hippocampus under normal physiological conditions. However, it has been shown that neurons proliferating after ECS in the SGZ survive and display a normal granule cell morphology (Reference Malberg, Eisch, Nestler and Duman9), suggesting that ECS does not result in a pathological hippocampal neurogenesis.
The peak of BrdU-labelled (proliferating) neurons is seen three to five days after a single ECS (Reference Madsen, Treschow, Bengzon, Bolwig, Lindvall and Tingström8). Several weeks are then required for these cells to differentiate, mature, and become functionally integrated into the hippocampal neuronal network (for review, see (Reference Tanti and Belzung42)). However, it has been shown that ECS affects several stages of the neurogenic process and this may explain how ECS-induced neurogenesis affects memory. For example, in a study by Zhao et al., ECS increased spine density in mature neurons and also promoted the maturation of dendritic spines in newborn cells (Reference Zhao, Warner-Schmidt, Duman and Gage43). In addition, other studies have shown that multiple ECS treatments promote dendritic arborization (Reference Yanpallewar, Barrick, Palko, Fulgenzi and Tessarollo44), induce synaptogenesis, and remodels synapses in the rat hippocampus (Reference Chen, Madsen, Wegener and Nyengaard45).
We have previously demonstrated that a single ECS induces a transient regulation of key molecules important for neuronal plasticity and for stabilisation of synaptic structure (Reference Nordgren, Karlsson and Svensson46). In the current study, we did not observe retrograde amnesia after a single ECS treatment (Figs 3 and 4, Group A–C). This is in line with ECS studies on retrograde amnesia using another memory test (the passive avoidance task, taxing both hippocampus-dependent contextual memory and amygdala-dependent emotional memory (Reference Phillips and LeDoux47)), where retrograde amnesia was seen 24 h after three, but not after one or two, ECS treatments (Reference Andrade, Thyagarajan, Vinod, Srikanth, Rao and Chandra48). Other researchers using the passive avoidance task have also failed to observe retrograde amnesia after one ECS, but observed a significant memory loss after multiple (seven) ECS treatments (Reference Lerer, Stanley, Keegan and Altman49). We conclude that an extended delay (7 days) after a single ECS is not in itself sufficient to cause retrograde amnesia. Rather the disrupting effect of ECS on spatial memory depends on an accumulated effect of consecutive treatments. The molecular processes behind such a cumulative effect are not clear, and it is not known whether this effect is dependent on the number of treatments alone or also on the frequency of treatments. Maybe there is a threshold, above which these processes lead to memory disruption. A single ECS would, according to this reasoning, be below the threshold and therefore not cause retrograde amnesia. Perhaps a single ECS could cause memory impairment if the electrical dose significantly exceeds seizure threshold (Reference Sackeim, Devanand and Prudic33), or if it is administered before a memory is properly consolidated or reconsolidated (Reference Alberini, Milekic and Tronel50). In our study, the memory for platform position was not reactivated before testing. This would be interesting to investigate further, since a recent clinical study (Reference Kroes, Tendolkar, van Wingen, van Waarde, Strange and Fernández51), as well as older preclinical studies (Reference Misanin, Miller and Lewis52, Reference Lewis, Bregman and Mahan53) suggest that reactivated memories can be disrupted by a single treatment.
To conclude, a single ECS treatment does not cause spatial retrograde amnesia in the MWM task when memory is evaluated 24 h, 72 h, or 7 days after the treatment. Our findings support the notion that the retrograde amnesia observed after multiple ECS might be a cumulative effect of repeated treatments, but the molecular mechanisms behind this effect is still unclear. By understanding the mechanisms behind the amnestic effects of ECT, new methods to counteract these adverse effects could be developed.
Acknowledgement
The authors thank Prof. Peter Höglund for excellent assistance with statistical analyses.
Authors Contributions: M.S. has contributed to the manuscript by designing the study, collecting the behavioural data, analysing and interpreting the data and drafting the manuscript. T.H. has contributed by collecting the behavioural data and critically revising the manuscript. J.B. has contributed by analysing and interpreting the data and critically revising the manuscript. J.E. has contributed by designing the study and critically revising the manuscript. A.T. has contributed by designing the study, analysing and interpreting the data, drafting the manuscript and critically revising it. All authors met the following authorship criteria: (1) substantial contributions to conception and design of, or acquisition of data or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be published.
Financial Support
‘Kungliga Fysiografiska Sällskapet i Lund’ and ‘OM Perssons donationsfond’ provided funding for this study. The study sponsors had no further role in study design, collection, analysis or interpretation of data or in writing the report.
Conflicts of Interest
None.
Animal Welfare
This study is in compliance with the ARRIVE (Animal Research Reporting In Vivo Experiments) guidelines.
Ethical Standards
All experiments were carried out according to guidelines set by the Malmö-Lund Ethical Committee for the use and care of laboratory animals.








