Significant outcomes
• Levels of lipids, corticosterone, and conjugated dienes (CD) in serum and thiobarbituric acid-reactive substance (TBARS) in serum and brain were found to be higher in all stress groups than in controls.
• Furthermore, in all cases, the elevations were reversed in Vit E-treated stress groups compared with their respective control groups. Serum paraoxonase-1 (PON1) activity was not altered among the groups.
• The mean latencies of visual evoked potentials (VEPs) were prolonged in all stress groups and these prolongations were reversed in the Vit E-treated stress groups compared with their respective control groups.
Limitations
• This study was not designed to examine the central or peripheral mechanisms modulating the stress-induced response of the hypothalamic–pituitary–adrenal (HPA) axis.
Introduction
Evidence is accumulating indicating that stress can stimulate numerous pathways, leading to increased production of oxygen radicals that can attack proteins, nucleic acids, and lipid membranes, thereby disrupting cellular function and integrity (Reference Kovacs, Juranek, Stankovicova and Svec1). The brain is particularly sensitive to the effects of oxygen radicals because of its very high rate of oxygen consumption and the non-regenerative nature of its neurons (Reference Hacioglu, Agar and Yargicoglu2). Because brain and retina tissues have rich oxygen supplies and contain large quantities of polyunsaturated fatty acids, they are ideal substrates for lipid peroxidation (Reference Yaras, Yargicoglu, Agar, Gumuslu, Abidin and Ozdemir3). Thus, it seems likely that stress-induced lipid peroxidation may affect the nervous system, particularly the visual system.
Previous data demonstrated the effects of immobilisation and/or cold stress on VEP components, with significant prolongation in the amplitudes and latencies (Reference Yaras, Yargicoglu, Agar, Gumuslu, Abidin and Ozdemir3). VEPs, which consist of several components arising from the retina, optic pathway, subcortex, and cortex, are known to be sensitive and highly reliable indicators of visual system changes (Reference Halliday, McDonald and Mushin4).
In this study, levels of TBARS and CD were used as indicators of lipid peroxidation, and PON1 activity was assessed as an antioxidant determinant in serum. Stress and its effects on organisms have been investigated in several tissues in earlier studies (Reference Sahin and Gumuslu5–Reference Sahin and Gumuslu7) but no detailed study has examined whether serum lipid peroxidation markers are correlated with those in the brain during stress, which would avoid the need to perform invasive procedures. We sought to investigate the correlations between serum levels of lipid peroxidation markers and those in brain samples in different stress models.
Methods
Preparation of animals
In total, 96 healthy male albino rats aged 3 months were used. Animals were housed in groups of four to five rats in stainless steel cages under standard conditions (24 ± 2°C and 50 ± 5% humidity) with 12/12-h light/dark cycle. They were divided equally into eight groups: a control group (C) and groups treated with vitamin E (Vit E); exposed to immobilisation stress (IS); exposed to immobilisation stress and treated with vitamin E (IS + Vit E); exposed to cold stress (CS); exposed to cold stress and treated with vitamin E (CS + Vit E); exposed to both immobilisation and cold stress (IS + CS); and a final group exposed to both immobilisation and cold stress and treated with vitamin E (IS + CS + Vit E).
Vit E (30 mg/kg/day) (Reference Yargicoglu, Yaras, Agar, Gumuslu, Bilmen and Ozkaya8) was injected intramuscularly in the Vit E, IS + Vit E, CS + Vit E, and IS + CS + Vit E groups. Physiological saline (0.9% NaCl) was injected intraperitoneally in the C, IS, CS, and IS + CS groups.
Experimental procedures
The study protocol was approved by the Akdeniz University Animal Care and Use Committee. The experiments were performed between 09:00 and 12:00 h in the morning. Immobilisation and cold stress models were performed according to methods described by Finlay (Reference Finlay, Jedema, Rabinovic, Mana, Zigmon and Sved9) and Inoue (Reference Inoue, Koyoma, Muraki and Yamashita10), respectively.
Immobilisation stress
The rats were exposed to 180 min of immobilisation stress daily for 15 days by fixing their limbs to appropriate shelves using sticky tape.
Cold stress
The rats were placed in a cold room (ambient temperature, 4–5°C) for 15 min daily for 15 days.
Immobilisation and cold stress
To examine the psychological and physical stress effects on the brain oxidative parameters, both immobilisation and cold stress were applied at the same time. Animals were exposed to cold stress during the last 15 min of immobilisation stress. The daily food and water consumption of each cage and the weekly weights of individual rats were recorded during the feeding period. The mean daily food and water consumption were estimated from the recorded values. At the end of the experimental period, rats were deprived of food for 24 h and then prepared for the experimental procedure.
VEP recordings
VEPs were recorded with stainless steel subdermal electrodes (Nihon Kohden NE 223 S, Nihon Kohden Corporation, Tokyo 161, Japan) under ether anaesthesia. The reference and active electrodes were placed 0.5 cm in front of (reference electrode) and behind bregma (active electrode), as described previously (Reference Hacioglu, Agar, Ozkaya, Yargicoglu and Gumuslu11). A ground electrode was placed on the tail of the animal. After 5 min of dark adaptation, a photic stimulator (Nova-Strobe AB, Biopac System Inc., Santa Barbara, CA 93117, USA) at the lowest intensity setting was used to provide a flash stimulus at a distance of 15 cm, which allowed lighting of the entire pupilla from the temporal visual field. The repetition rate of the flash stimulus was 1 Hz, and the flash energy was 0.1 J. VEP recordings from both eyes were obtained, and throughout the experiments, the eye not under investigation was occluded by appropriate black carbon paper and cotton. Body temperature was maintained between 37.5°C and 38°C using a heating pad (Reference Hetzler, Boyes, Creason and Dyer12). One hundred responses were averaged with the averager in the Biopac MP100 data acquisition equipment (Biopac System Inc., Santa Barbara, CA 93117, USA). The analysis time was 300 ms, the frequency bandwidth of the amplifier was 1–100 Hz, and the gain was set at 20 μV/div. The microprocessor was programmed to reject any sweeps contaminated with larger artifacts, and at least two averages were obtained to ensure response reproducibility. Peak latencies of the components were measured from the stimulus artifact to the peak (in ms). Amplitudes were measured as the voltage between successive peaks.
Sample collection
After the VEP recordings, animals were anesthetised with diethyl ether, and their abdomens were opened by a midline incision. Blood samples were taken, and the animals were sacrificed by cardiac puncture. The brain tissues were removed. Brain tissue homogenates were used for TBARS analysis, and blood samples were used for the analysis of serum TBARS, corticosterone, CD, lipid levels, and PON1 activity.
Thiobarbituric acid-reactive substance assay
TBARS levels were measured by a fluorometric method, described by Wasowicz (Reference Wasowicz, Nève and Peretz13), using 1,1,3,3-tetramethoxypropane as a standard, and the results are given as nmol/g protein for brain TBARS levels. Samples (50 μl) were added to a tube containing 1 ml of distilled water. After addition of 1 ml of a solution containing 29 mmol/l 2-thiobarbituric acid in acetic acid (8.75 mol/l), samples were placed in a water bath and heated for 1 h at 95–100°C. After the samples had cooled, 25 μl of 5 mol/l HCl was added, and the reaction mixture was extracted by agitation for 5 min with 3.5 ml of n-butanol. After centrifugation, the butanol phase was separated, and the fluorescence of the butanol extract was measured in a spectrofluorometer (Shimadzu RF-5000, Kyoto, Japan) using wavelengths of 525 nm for excitation and 547 nm for emission. The values are expressed as μmol MDA/l for serum TBARS levels.
Corticosterone assay
To determine the corticosterone level, Gamma-B 125I, a specific RIA kit for rats and mice (IDS Systems Limited, Bolden, UK, Code AA-13F1), was used (Reference Yargicoglu, Yaras, Agar, Gumuslu, Bilmen and Ozkaya8). A sample of 100 μl was taken from the plasma of rats incubated with 125I-corticosterone and corticosterone antiserum for 18 h at 4°C. Then, after the addition of 100 μl secondary antibody and incubation for 1 h at room temperature, 1 ml isotonic solution was added and centrifuged (1500 g, 4°C, 15 min). The supernatant was removed, and the radioactivity of the pellet was measured for 1 min using a gamma counter (GC-20) (Biopac System Inc., Santa Barbara, CA 93117, USA). The amount of corticosterone was then calculated from the standard curve obtained from corticosterone solutions of differing concentrations (ng/ml).
Conjugated diene assay
CD levels were measured by the method of Recknagel (Reference Recknagel and Glende14). Lipids were extracted with 2 : 1 (v/v) chloroform–methanol. The extract was evaporated to dryness under a stream of nitrogen and then redissolved in cyclohexane. The cyclohexane solution was assayed at 234 nm. The results are expressed as μmol/l using εmax = 25.200/M/cm.
Lipid assay
Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured enzymatically using commercial kits (Aeroset system, Abbott Laboratories, Abbott Park, IL, USA). The low-density lipoprotein cholesterol (LDL-C) fraction was calculated indirectly using the Friedewald equation (Reference Friedewald, Levy and Fredrickson15). The factor [triglyceride]/5 was used to estimate the very low-density lipoprotein cholesterol (VLDL-C) concentration (Reference Friedewald, Levy and Fredrickson15).
Paraoxonase-1 activity assay
Serum PON1 activity was assayed spectrophotometrically as described previously (Reference Gocmen, Gumuslu and Semiz16). Briefly, the assay mixture consisted of 1500 μl of 6 mmol/l paraoxon substrate solution in 0.1 mol/l Tris-HCl buffer, pH 8.0, containing 1 mmol/l CaCl2 and 60 μl of fresh serum specimen. The absorbance was monitored photometrically at 405 nm and at 37°C at 1-min intervals on a spectrophotometer (photometer 4010, Boehringer Mannheim GmbH, Mannheim, Germany). One unit of PON1 activity was defined as 1 μmol of p-nitrophenol formed per minute, and the activity is expressed as U/l of serum.
Statistical analysis
Differences between groups were analysed by one-way analysis of variance. Post-hoc comparisons of the means were carried out using Tukey's test. The number of rats was 12 in each group. The level of significance was set at p < 0.05.
Results
At the end of the study, body weight and food consumption did not differ significantly between the experimental groups (p > 0.05). Water intake was less in the CS group than in controls; this was reversed in the CS + Vit E group compared with the other stress groups (p < 0.05; Table 1).
Table 1 Baseline characteristics in control and stress groups in rats
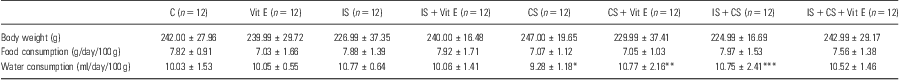
C, control group; CS, cold stress; IS, immobilisation stress; Vit E, vitamin E.
Results are given as mean ± SEM.
*p < 0.05 for CS versus IS, **p < 0.05 for CS + Vit E versus CS, ***p < 0.05 for IS + CS versus CS.
Serum levels of TC, LDL-C, VLDL-C, and TG were significantly higher in all stress groups than in controls (IS, CS, IS + CS; p < 0.05). These elevations were reversed in Vit E-treated stress groups compared with their respective control groups (IS + Vit E, CS + Vit E, IS + CS + Vit E; p < 0.05). However, HDL levels were lower in all stress groups compared with the controls (IS, CS, IS + CS; p < 0.05), and these reduced levels were reversed in the CS + Vit E and IS + CS + Vit E groups (p < 0.05; Table 2). Corticosterone levels were significantly higher in all stress groups than in controls (IS, CS, IS + CS; p < 0.05); these elevations were reversed in the Vit E-treated stress groups compared with their respective control groups (IS + Vit E, CS + Vit E, IS + CS + Vit E; p < 0.001; Table 3). Serum PON1 activity did not differ among the groups (p > 0.05; Table 3). Serum CD and brain TBARS levels were also significantly higher in all stress groups than in controls (IS, CS, IS+CS; p < 0.05), and these levels were reversed in Vit E-treated stress groups compared with their respective control groups (IS + Vit E, CS + Vit E, IS + CS + Vit E; p < 0.001). Similarly, serum TBARS levels were significantly elevated in all stress groups (IS, CS, IS + CS; p < 0.05) but this elevation was only reversed in the IS + CS + Vit E group (p < 0.001; Table 3).
Table 2 Effects of different stress models on serum lipid levels in rats

C, control group; CS, cold stress; HDL-C, high-density lipoprotein cholesterol; IS, immobilisation stress; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; Vit E, vitamin E; VLDL, very low-density lipoprotein cholesterol.
Results are given as mean ± SEM.
*p < 0.05 for Vit E versus C, **p < 0.001 for IS versus C, ***p < 0.05 for IS + Vit E versus IS.
αp < 0.001 for CS versus C.
βp < 0.05 for CS + Vit E versus CS.
γp < 0.05 for IS + CS versus C.
θp < 0.05 for IS + CS + Vit E versus IS + CS.
Table 3 Effects of different stress models on the levels of serum corticosterone, lipid peroxidation markers and antioxidant enzyme activities in rats

C, control group; CD, conjugated dienes; CS, cold stress; IS, immobilisation stress; PON1, paraoxonase-1; TBARS, thiobarbituric acid-reactive substance; Vit E, vitamin E.
Results are given as mean ± SEM.
*p < 0.05 for Vit E versus C, **p < 0.05 for IS versus C, ***p < 0.001 for IS + Vit E versus IS.
αp < 0.05 for CS versus C.
βp < 0.001 for CS + Vit E versus CS.
γp < 0.05 for IS + CS versus C.
θp < 0.001 for IS + CS + Vit E versus IS + CS.
Regarding VEP recordings, the mean latencies of P1, N1, P2, N2, and P3 components were prolonged significantly in all stress groups compared with controls (IS, CS, IS + CS; p < 0.001); all of the prolongations were reversed in the Vit E-treated stress groups compared with their respective control groups (IS + Vit E, CS + Vit E, IS + CS + Vit E, p < 0.05; Table 4).
Table 4 Effects of different stress models on the values of VEP latencies in rats
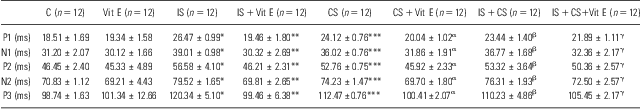
C, control group; CS, cold stress; IS, immobilisation stress; VEP, visual evoked potential; Vit E, vitamin E.
Results are given as mean ± SEM.
*p < 0.001 for IS versus C, **p < 0.001 for IS + Vit E versus IS, ***p < 0.001 for CS versus C.
αp < 0.001 for CS + Vit E versus CS.
βp < 0.001 for IS + CS versus C.
γp < 0.05 for IS + CS + Vit E versus IS + CS.
Correlation coefficients among lipid peroxidation markers, lipids, and VEPs are summarised in Table 5.
Table 5 Correlation coefficients among lipid peroxidation markers, lipids and VEP (n = 96)

CD, conjugated dienes; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ns, non-significant; PON1, paraoxonase-1; TBARS, thiobarbituric acid-reactive substance; TC, total cholesterol; TG, triglycerides; VEP, visual evoked potentials; VLDL, very low-density lipoprotein cholesterol.
Discussion
In the present study, body weight and food intake did not differ significantly with exposure to stress. Water intake was less in rats exposed to CS than in those experiencing the other stressors. This may be attributable to the negative impact of cold temperature exposure on performance (Reference Pilcher, Nadler and Busch17). Earlier reports demonstrated that hypercholesterolaemia was associated with increased lipid peroxidation, leading to tissue damage (Reference Hong, Zhao and Wu18). Similarly, serum lipid levels were found to be higher in stress groups, and this elevation was reversed in Vit E-treated stress groups.
Plasma corticosterone levels were higher in stress groups than in the control group. Our results are consistent with previous studies that concluded that plasma corticosterone levels were an important indicator of stress (Reference Finlay, Jedema, Rabinovic, Mana, Zigmon and Sved9). However, we did not observe a significant difference among the groups, in contrast to the results of Sahin et al. (Reference Sahin and Gumuslu5). There was also a significant decrease in corticosterone levels in Vit E-treated stress groups compared with the respective control groups.
There have been many reports of enhanced levels of CD and TBARS in brain tissues of rats in various stress exposure models (Reference Yaras, Yargicoglu, Agar, Gumuslu, Abidin and Ozdemir3,Reference Yargicoglu, Yaras, Agar, Gumuslu, Bilmen and Ozkaya8,Reference Hacioglu, Agar, Ozkaya, Yargicoglu and Gumuslu11). However, we studied these oxidative markers in serum as well as brain samples. Consistent with recent studies (Reference Sahin and Gumuslu5–Reference Sahin and Gumuslu7), we found significant increases in serum CD and in serum and brain TBARS levels in all stress groups; we found no difference among the groups, in contrast to a previous study published by Sahin et al. (Reference Sahin and Gumuslu5), who reported that immobilisation + cold stress resulted in higher lipid peroxidation markers than did immobilisation or cold alone stress. This could be attributable to the similar effects of the experimental stress models on these markers. The enhancement of lipid peroxidation products might be due to increased free radical production and/or decreased hydrolysis of lipid peroxides (Reference Silambarasan and Raja19). Because we saw no change in serum antioxidant PON1 activity among the groups, we suggest that this increase was mainly due to serum oxidative content as a generating factor rather than to insufficient elimination of these toxic products. In fact, in correlation analyses, serum levels of CD and TBARS were correlated positively with serum TC, LDL-C, VLDL-C, TG, and brain TBARS. This may also provide a valuable method for clinicians to measure lipid peroxidation markers easily in serum instead of using invasive methods. Additionally, the enhancement of these markers was almost reversed in Vit E-treated stress groups.
Another prominent effect of stress was observed in VEP latencies, all components of which were prolonged significantly in all stress groups compared with controls. This is consistent with previous findings (Reference Yaras, Yargicoglu, Agar, Gumuslu, Abidin and Ozdemir3,Reference Yargicoglu, Yaras, Agar, Gumuslu, Bilmen and Ozkaya8). This result probably indicates that stress markedly affects the visual system through a mechanism that has not yet been fully defined. However, one hypothesis suggests the formation of toxic lipid products as a cause of oxidative damage in the membrane structures of the brain and retina due to their high lipid contents (Reference Matsumoto, Yobimoto, Huong, Abdel-Fattah, Van Hien and Watanabe20). Similarly, we found serum levels of CD and TBARS to be positively correlated with VEP latencies in a correlation analysis. Another important finding supports the argument that vitamin E reversed all prolongations of VEP components, which returned to values similar to those of the controls in all stress models. This finding indicates that vitamin E has potential to prevent VEP changes caused by stress. Therefore, it may be reasonable to suggest antioxidant treatment for stress-induced oxidative injury in clinical practice.
Consistent with previous studies, we found that the higher levels of corticosterone and lipid peroxidation markers, together with the prolongations of VEPs in response to stress, were largely reversed by vitamin E administration (Reference Yargicoglu, Yaras, Agar, Gumuslu, Bilmen and Ozkaya8,Reference Siu, Reiter and To21). This may be explained by the antioxidant properties of vitamin E, which prevent free radical production and lipid peroxidation, thus preventing the accumulation of toxic metabolites during stress. Consequently, vitamin E may be a useful agent for the prevention of VEP alterations caused by stress-induced lipid peroxidation.
Our study has some limitations. Prolonged stress of similar intensity is known to cause stress-induced adaptation of the hypothalamic–HPA axis (Reference Gądek-Michalska, Tadeusz, Rachwalska, Spyrka and Bugajski22). However, we were not able to measure other HPA hormones simultaneously, particularly adrenocorticotropic hormone. Thus, post-stress corticosterone levels alone do not allow us to identify a clear pattern of HPA response because of factors such as complex feedback mechanisms as well as issues related to accurate sampling times or different characteristics of stressors (Reference García, Martí, Vallès, Dal-Zotto and Armario23). This study also was not designed to determine central or peripheral mechanisms modulating the stress-induced HPA axis responses during prolonged stress.
In conclusion, the present study suggests that serum levels of lipid peroxidation markers, accompanied by VEP changes, can be determined readily and may be used as an indicator to evaluate the effects of oxidative stress representing inner neurochemical alterations in the brain without the need for invasive methods. Further studies are needed to clarify the exact mechanism of stress-dependent free radical generation and to develop appropriate therapies in different stress models.
Acknowledgements
This research was funded by Akdeniz University Research Project Unit.
Conflict of Interest
None.







